Abstract
Extracellular signal-regulated kinase (ERK)1/2 signaling pathway plays a critical role in regulating tumor angiogenesis. Our previous studies have demonstrated that HPV-16 oncoproteins enhanced hypoxia-inducible factor-1α (HIF-1α) protein accumulation and vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) expression in non-small cell lung cancer (NSCLC) cells, thus contributing to angiogenesis. In this study, we further investigated the role of ERK1/2 signaling pathway in HPV-16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in NSCLC cells. Our results showed that HPV-16 E6 and HPV-16 E7 oncoproteins promoted the activation of ERK1/2 signaling pathway in A549 and NCI-H460 cells. Moreover, PD98059, a specific inhibitor of ERK1/2, blocked in vitro angiogenesis stimulated by HPV-16 E6 but not E7 oncoprotein. Additionally, HIF-1α protein accumulation and VEGF and IL-8 expression in NSCLC cells induced by HPV-16 E6 but not E7 oncoprotein were significantly inhibited by PD98059. Taken together, our results suggest that ERK1/2 signaling pathway is involved in HPV-16 E6 but not E7 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression in NSCLC cells, leading to the enhanced angiogenesis in vitro.
Key words: Extracellular signal-regulated kinases (ERK) 1/2, Human papillomavirus, Non-small cell lung cancer, Angiogenesis, Hypoxia-inducible factor-1α, Vascular endothelial growth factor (VEGF), Interleukin-8
INTRODUCTION
Lung cancer is one of the most common cancers worldwide and is predominantly caused by cigarette smoking (1). However, approximately 25% of all lung cancer cases are observed in never smokers. Moreover, never smoking-associated non-small cell lung cancer (NSCLC) is suggested to be a distinct disease due to its unique clinical characteristics (1,2). The pathogenesis of never smoking-associated NSCLC is complex, and its risk factors are uncertain (3). Recently, a growing body of epidemiological evidence has shown that human papillomavirus (HPV), especially HPV-16, infection rate in NSCLC was higher than those in benign lung tumors, especially in the Asian region (4,5). Furthermore, our previous studies found that overexpression of HPV-16 oncoproteins promoted invasion, migration, and angiogenesis in NSCLC cells (6,7). Noticeably, the malignant transformation of NSCLC induced by HPV is mainly attributed to its oncoproteins, E6 and E7 (4–8). A series of studies have made it clear now that HPV-16 E6 oncoprotein binds a tumor-suppressor gene, namely p53, and subsequently triggers its degradation via interaction with E6AP protein, thus leading to cell cycle disruption, proliferation promotion, and apoptosis inhibition (9,10). E7 has been demonstrated to bind and degrade retinoblastoma proteins (pRb, p107, and p130), resulting in the displacement of E2F transcription factors, consequently leading to the loss of the checkpoint control at G1/S transition and resulting in an unbounded cell proliferation (11,12).
Angiogenesis is essential for tumor growth and metastasis and has a pivotal role in the development and progression of a variety of cancers including NSCLC (13–16). Vascular endothelial growth factor (VEGF), one of the best recognized angiogenic factors, plays a critical role in tumor angiogenesis, metastasis, and overall survival of NSCLC patients (14,17). Hypoxia-inducible factor-1α (HIF-1α), the master transcription factor in response to hypoxia, is suggested to be a crucial upstream molecule mediating VEGF expression and angiogenesis, and the increase of HIF-1α expression is associated with a poor prognosis in NSCLC patients (18). In addition, interleukin-8 (IL-8), a proinflammatory chemokine, has been implicated in a wide variety of processes, including angiogenesis and metastasis in NSCLC (19). On the whole, HIF-1α, VEGF, and IL-8 are closely related to angiogenesis and prognosis in NSCLC patients. Interestingly, our previous studies have demonstrated that HPV-16 E6 and HPV-16 E7 oncoproteins promoted angiogenesis via enhancing the accumulation of HIF-1α protein and the expression of VEGF and IL-8 in NSCLC cells (6,7).
Extracellular signal-regulated kinases (ERK) 1/2 signaling pathway, a member of mitogen-activated protein kinase (MAPK) family, has been shown to be a critical regulator of various cellular processes such as proliferation, differentiation, development, and angiogenesis (20–22). The abnormal activation of ERK1/2 has been implicated in a variety of malignant tumors including NSCLC (21,23). Accumulating evidence has shown that overexpression of HPV-16 E6 and E7 oncoproteins leads to an increased level of phosphorylated ERK1/2 in different types of cells (24,25). Moreover, we have demonstrated previously that ERK1/2 signaling pathway regulated HIF-1α, VEGF, and IL-8 expression induced by HPV-16 E6 and E7 oncoproteins in two types of cervical cancer cell lines, C-33A and HeLa cells (26). However, whether ERK1/2 signaling pathway is involved in HPV-16 oncoprotein-induced HIF-1α, VEGF, and IL-8 protein expression in NSCLC cells remains unclear. In the present study, we investigated the role of ERK1/2 signaling pathway in the expression of HIF-1α, VEGF, and IL-8 and in vitro angiogenesis induced by HPV-16 E6 and HPV-16 E7 oncoproteins in A549 and NCI-H460 cells. We found for the first time to our knowledge that ERK1/2 signaling pathway is involved in HPV-16 E6 but not E7 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression in NSCLC cells, consequently leading to angiogenesis in vitro.
MATERIALS AND METHODS
Plasmids and Antibodies
Enhanced green fluorescence protein (EGFP) plasmid vectors harboring HPV-16 E6 (p-EGFP-N1-HPV-16 E6) or E7 (p-EGFP-N1-HPV-16 E7) were from our lab (Institute of Biochemistry and Molecular Biology, Guangdong Medical University). Rabbit anti-human total and phosphorylated ERK1/2 (Thr202/Thr204) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Mouse anti-human HIF-1α antibody was purchased from BD Transduction Laboratories (San Diego, CA, USA). Mouse anti-human β-actin antibody and streptavidin/horseradish peroxidase (HRP)-conjugated secondary antibodies (streptavidin HRP-goat anti-mouse IgG and streptavidin HRP-goat anti-rabbit IgG) were purchased from Beijing Biosynthesis Biotechnology Co., LTD (Beijing, China).
Cell Culture
Human lung adenocarcinoma cell line A549 and human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA). Human NSCLC cell line NCI-H460 was obtained from Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS; Shanghai, China). A549 and NCI-H460 cells were grown in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 µg/ml) (Invitrogen). HUVEC cells were cultured in DEME media containing 10% FBS. All cells were maintained in a 5% CO2 incubator at 37°C.
Establishment of Stable-Transfected NSCLC Cells
The method has been described in our previous study (7). The plasmids (p-EGFP-N1-HPV-16 E6 and p-EGFP-N1-HPV-16 E7) were respectively transfected into A549 and NCI-H460 cells using Lipofectamine™ 2000 (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions, wherein transfection with empty vector served as negative controls. The cells exposed to transfection reagent alone served as mock transfection controls. The cells were selected by 400 µg/ml G418 in RPMI-1640 and visualized with fluorescence microscopy.
Western Blot Analysis
Protein extraction was performed as described previously (6). BCA methods were used to determine protein concentrations. The proteins were separated on 10% polyacrylamide-SDS gel and then electroblotted onto polyvinylidene difluoride (PVDF) membrane. After blocking with TBS or 5% skim milk, the membranes were incubated overnight at 4°C with corresponding primary antibodies at 1:1,000. After washing with TBST, the membranes were incubated with specific HRP-conjugated secondary antibodies at a 1:2,000 dilution or a 1:20,000 dilution, and the signals were detected using enhanced chemiluminescence reagent. Protein levels were normalized to β-actin.
Quantitative Real-Time PCR
The mRNA levels of VEGF and IL-8 were detected by quantitative real-time PCR using One Step SYBR PrimeScript RT-PCR (TaKaRa, China) according to the manufacturer’s instructions. The method was as described previously (7). Total RNA was extracted from treated and untreated NSCLC cells with TRIzol reagent (Invitrogen). The following specific primers were used: human VEGF (forward 5′-TCTACCTCCACCATGCCAAGT-3′ and reverse 5′-GATGATTCTGCCCTCCTCCTT-3′) (Genbank: NM_001025366.2); IL-8 (forward 5′-TTGCCAAGGAGTGCTAAAGAA-3′ and reverse 5′-GCCCTCTTCAAAAACTTCTCC-3′) (Genbank: NM_000584.3); β-actin (forward 5′-TCAAGATCATTGCTCCTCCTG-3′ and reverse 5′-CTGCTTGCTGATCCACATCTG-3′) (Genbank: NM_001017992.3). All the primers were produced by TaKaRa Biotechnology Co., Ltd. (Dalian, China). The thermocycling conditions for real-time PCR were as follows: 42°C for 5 min, 95°C for 10 s, followed by the PCR process (40 cycles at 95°C for 5 s, and 60°C for 31 s). The relative mRNA levels were normalized to β-actin. The experiment was repeated in triplicate.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentration of VEGF and IL-8 in the conditioned media derived from treated and untreated cells was respectively determined by human VEGF ELISA Development kit and human IL-8 ELISA reagent kit (Wuhan Boster Bio-Engineering Limited Company) according to the manufacturer’s instructions. Results were normalized to cell number (2 × 105). The experiment was repeated in triplicate.
In Vitro Angiogenesis Assay
An in vitro angiogenesis assay kit (ECM625; Millipore, Temecula, CA, USA) was employed to analyze the formation of capillary tube-like structures according to the manufacturer’s instructions. The method was as described previously (7). Briefly, HUVECs were seeded at a density of 5 × 103 cells per well onto the surface of 96-well cell culture plates precoated with polymerized ECMatrix™. Subsequently, the conditioned media, derived from transfected A549 cells pretreated with different concentrations of PD98059, were respectively added into each HUVEC-containing well. The tubule formation was observed under the phase-contrast microscope, and Scion image software was used to analyze the total tube length in three random view fields per well, and the average value was calculated. The experiment was repeated in triplicate.
Statistical Analysis
For all experiments, data are presented as the mean ± standard deviation (SD) for three independent experiments. One-way ANOVA and LSD test were performed for statistical analysis using SPSS 19.0 for windows software. A value of p < 0.05 was considered to be statistically significant.
RESULTS
HPV-16 E6 and HPV-16 E7 Oncoproteins Promoted the Activation of ERK1/2 Signaling Pathway in A549 and NCI-H460 Cells
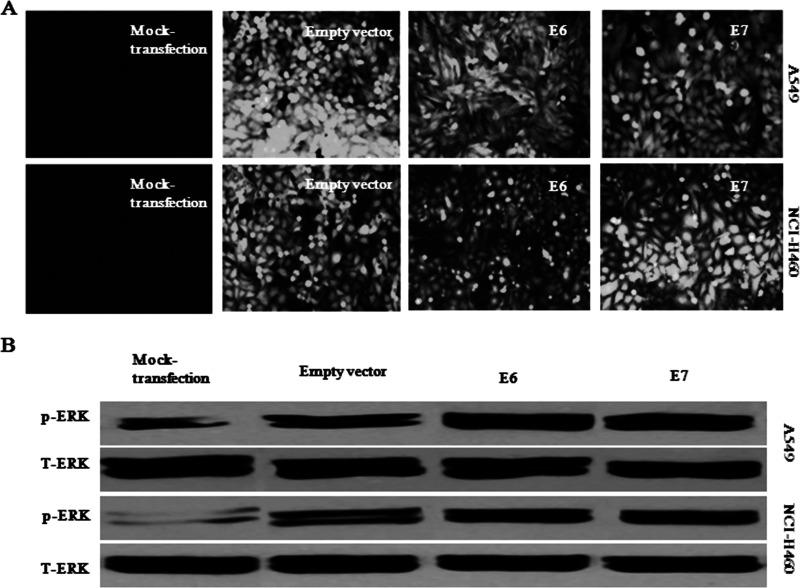
We have successfully established stable-transfected A549 and NCI-H460 cells overexpressing HPV-16 E6 or E7 oncoprotein in our previous study (7). Obvious green fluorescence signals were observed in stable-transfected NSCLC cells but not in cells with mock transfection (Fig. 1A). The expression of HPV-16 E6 and E7 proteins in the stable-transfected cells was confirmed by our previous studies (27). We have demonstrated previously that the expression of HPV-16 E6 and E7 oncoproteins led to an increased level of phosphorylated-ERK1/2 (p-ERK1/2) in cervical cancer cells (26). In order to clarify whether HPV-16 E6 and E7 oncoproteins promote the activation of ERK1/2 in A549 and NCI-H460 cells, we determined the expression levels of p-ERK1/2 protein in stable-transfected NSCLC cells. Our results showed that NSCLC cells overexpressing HPV-16 oncoproteins increased p-ERK1/2 protein levels compared with mock transfection and empty vector controls (Fig. 1B). These results indicated that HPV-16 E6 and HPV-16 E7 enhanced the activation of ERK1/2 signaling pathway in NSCLC cells.
Figure 1.
Overexpression of HPV-16 oncoproteins promoted the activation of ERK1/2 signaling pathway in NSCLC cells. (A) The green fluorescence signals derived from stable-transfected A549 and NCI-H460 cells were observed under a fluorescence microscope (10×). (B) p-ERK protein levels were determined by Western blot analysis in stable-transfected A549 and NCI-H460 cells.
ERK1/2 Signaling Pathway Was Involved in HPV-16 E6 but not E7 Oncoprotein-Induced In Vitro Angiogenesis in NSCLC Cells
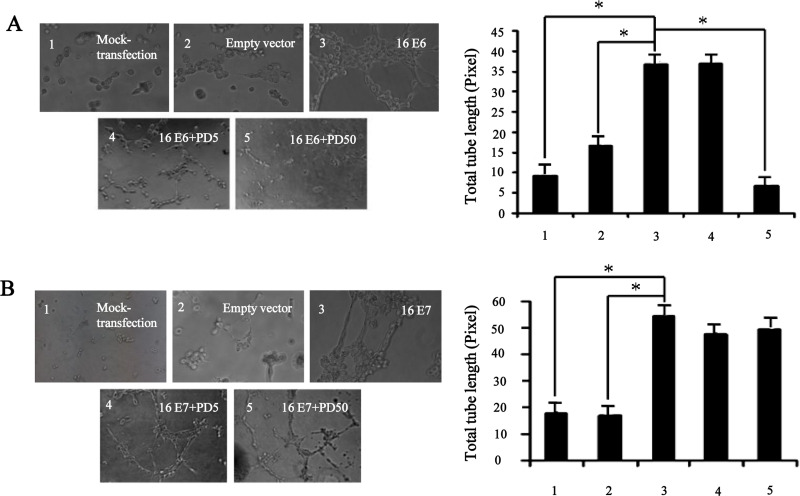
It has been reported that ERK1/2 signaling pathway played an important role in angiogenesis of NSCLC (21,22,24). Therefore, we further investigated the effects of ERK1/2 signaling pathway on angiogenesis induced by HPV-16 E6 and HPV-16 E7 in NSCLC cells. To this end, we employed an in vitro angiogenesis model to evaluate the capillary tube formation of HUVECs induced by the conditioned media derived from stable-transfected NSCLC cells in the presence or absence of PD98059, a specific pharmacologic inhibitor of ERK1/2. Our results showed that the transfected NSCLC cells overexpressing HPV-16 E6 or HPV-16 E7 promoted HUVECs to form capillary tube-like structures compared with mock or empty vector transfection controls, which were consistent with our previous findings (6,7). Moreover, the formation of capillary tube-like structures stimulated by HPV-16 E6 oncoprotein was significantly abrogated by PD98059, which was further confirmed by quantification of the total tube length pixel values (p < 0.05) (Fig. 2A). However, such inhibitory effects of PD98059 on angiogenesis in vitro were not observed in stable-transfected cells overexpressing HPV-16 E7 (Fig. 2B). These results suggested that ERK1/2 signaling pathway mediated HPV-16 E6 but not E7 oncoprotein-stimulated in vitro angiogenesis in NSCLC cells.
Figure 2.
PD98059 blocked HPV-16 E6 but not E7 oncoprotein-induced angiogenesis in vitro. NSCLC cells overexpressing HPV-16 oncoproteins were treated with different concentrations of PD98059 (5 µg/ml, 50 µg/ml) for 24 h. Left: the formation of capillary tube was observed under a phase-contrast microscope (20×). Right: the quantification of the total tube length pixel value in three random view fields per well. All data are presented as mean ± SD of three separate experiments. *p < 0.05.
ERK1/2 Signaling Pathway Regulated HPV-16 E6 but not E7 Oncoprotein-Induced HIF-1α Protein Accumulation in NSCLC Cells
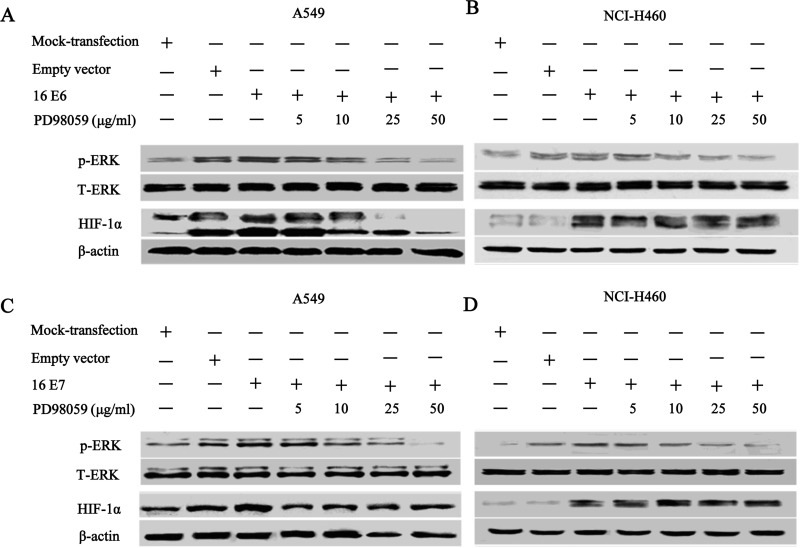
Our previous studies have demonstrated that the enhanced in vitro capillary tube formation induced by HPV-16 oncoproteins in NSCLC cells was, at least in part, in a HIF-1α-dependent manner (6,7). Here we further analyzed the potential mechanisms by which ERK1/2 signaling pathway mediated angiogenesis in NSCLC cells overexpressing HPV-16 oncoproteins. To this purpose, we explored whether ERK1/2 signaling pathway contributed to an elevated expression of HIF-1α in NSCLC cells. The accumulation of HIF-1α protein in stable-transfected NSCLC cells pretreated with various concentrations of PD98059 was examined. As shown in Figure 3, NSCLC cells overexpressing HPV-16 E6 or HPV-16 E7 oncoprotein exhibited an increase in HIF-1α protein expression, while control cells showed the minimal HIF-1α expression. Moreover, pretreatment with various concentrations of PD98059 inhibited HPV-16 E6 oncoprotein-induced HIF-1α protein accumulation in NSCLC cells, especially in A549 cells (Fig. 3A). However, PD98059 had no apparent inhibitory effects on HPV-16 E7 oncoprotein-stimulated HIF-1α protein accumulation in both A549 (Fig. 3C) and NCI-H460 cells (Fig. 3D). These results indicated that ERK1/2 signaling pathway was involved in the regulation of HIF-1α protein accumulation induced by HPV-16 E6 but not E7 oncoprotein in NSCLC cells.
Figure 3.
PD98059 inhibited HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein accumulation in NSCLC cells. Stable-transfected A549 and NCI-H460 cells overexpressing HPV-16 E6 or HPV-16 E7 oncoprotein were pretreated with different concentrations of PD98059 for 24 h. Western blot analysis was performed to detect the expression of HIF-1α protein in NSCLC cells. (A and B) HIF-1α protein levels in NSCLC cells overexpressing HPV-16 E6 oncoprotein. (C and D) HIF-1α protein levels in NSCLC cells overexpressing HPV-16 E7 oncoprotein.
ERK1/2 Signaling Pathway Regulated VEGF and IL-8 mRNA Expression and Protein Secretion Induced by HPV-16 E6 but not E7 Oncoprotein in NSCLC Cells
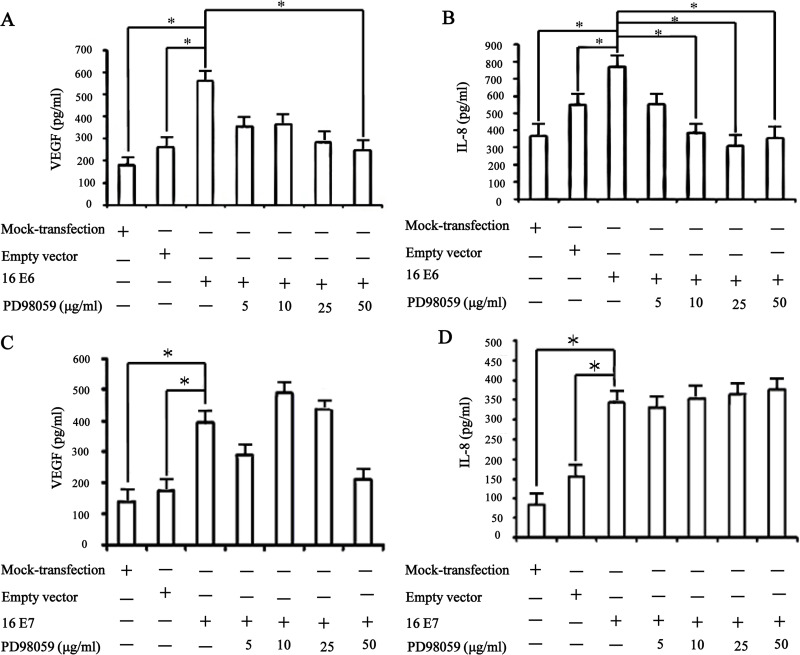
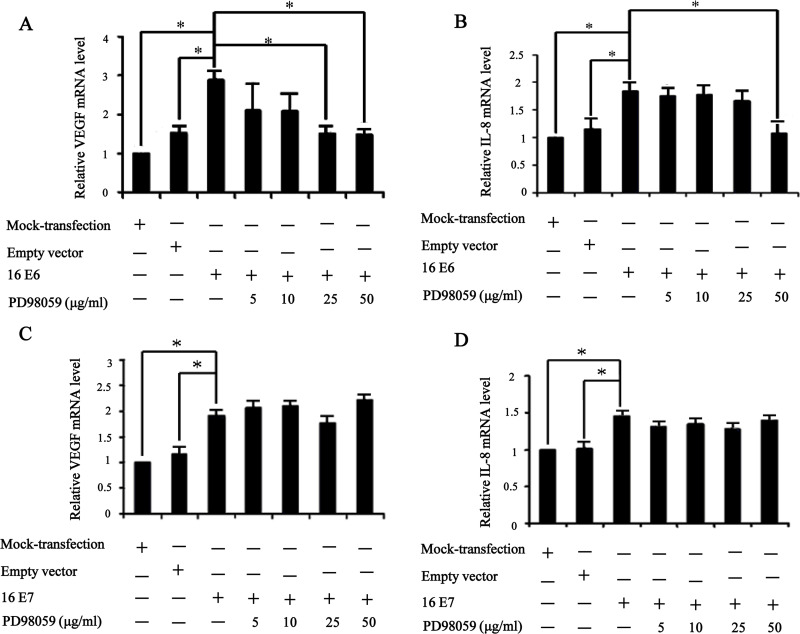
VEGF and IL-8, the key proangiogenic factors, play a critical role in tumor angiogenesis, metastasis, and overall survival of NSCLC patients (14,17,19). Our previous studies have well established that overexpression of HPV-16 E6 and E7 oncoproteins significantly promoted HIF-1α-dependent VEGF and IL-8 protein secretion in NSCLC cells (6,7). To investigate whether ERK1/2 signaling pathway plays a role in VEGF and IL-8 mRNA expression and protein secretion stimulated by HPV-16 oncoproteins, real-time PCR and ELISA assays were performed to determine the concentration of VEGF and IL-8 in the conditioned media derived from stable-transfected NSCLC cells pretreated for 24 h with difference concentrations of PD98059. As expected, a significant increase in VEGF and IL-8 mRNA expression and protein secretion was observed in cells overexpressing HPV-16 E6 and HPV-16 E7. Moreover, pretreatment with PD98059 inhibited VEGF and IL-8 protein secretion (Fig. 4A and B) and mRNA expression (Fig. 5A and B) induced by HPV-16 E6 in NSCLC cells. However, PD98059 had no notably inhibitory effect on HPV-16 E7 oncoprotein-stimulated VEGF and IL-8 protein secretion (Fig. 4C and D) and mRNA expression (Fig. 5C and D) in NSCLC cells. Taken together, these results suggested that the enhanced VEGF and IL-8 protein secretion and mRNA expression stimulated by HPV-16 E6 but not E7 oncoprotein in NSCLC cells were, at least in part, mediated by ERK1/2 signaling pathway, which were consistent with the effects of ERK1/2 signaling pathway on HIF-1α protein accumulation induced by HPV-16 E6 but not E7 oncoprotein.
Figure 4.
PD98059 inhibited HPV-16 E6 but not E7 oncoprotein-induced VEGF and IL-8 protein secretion. A549 cells, transfected with HPV-16 E6 or HPV-16 E7, were exposed to various concentrations of PD98059 for 24 h. (A and B) VEGF (A) and IL-8 (B) protein concentration in the conditioned media derived from A549 cells overexpressing HPV-16 E6 oncoprotein was determined by ELISA. (C and D) VEGF (C) and IL-8 (D) protein concentration in the conditioned media derived from A549 cells overexpressing HPV-16 E7 oncoprotein was determined by ELISA. *p < 0.05.
Figure 5.
PD98059 downregulated the mRNA levels of VEGF and IL-8 induced by HPV-16 E6 but not E7 oncoprotein. Real-time PCR was performed to determine VEGF and IL-8 mRNA levels in transfected A549 cells treated with different concentrations of PD98059. (A and B) The mRNA levels of VEGF (A) and IL-8 (B) in A549 cells overexpressing HPV-16 E6 oncoprotein. (C and D) The mRNA levels of VEGF (C) and IL-8 (D) in A549 cells overexpressing HPV-16 E7 oncoprotein. *p < 0.05.
DISCUSSION
Angiogenesis is a fundamental prerequisite for progressive expansion of tumors including NSCLC; therefore, the inhibition of tumor angiogenesis serves as an important strategy for cancer therapy (13–16,20). It is well known that ERK1/2 signaling pathway plays an important regulatory role in tumor angiogenesis, prognosis, and survival of cancers including NSCLC (23,24). Accumulating evidence has also shown that ERK1/2 signaling pathway was involved in the upregulation of multiple proangiogenic factors such as HIF-1α, VEGF, and IL-8 (20–22). Recently, various antiangiogenic drugs were supported as promising candidates for the inhibition of tumor progression via blocking the ERK1/2 signaling pathway (23,24). Interestingly, our previous studies have revealed that ERK1/2 signaling pathway was involved in HIF-1α protein accumulation and HIF-1α-dependent VEGF protein secretion induced by HPV-16 oncoproteins in human cervical cancer cell lines, thus contributing to the formation of capillary tube (26). However, the role of ERK1/2 signaling pathway in HIF-1α, VEGF, and IL-8 expression induced by HPV-16 oncoproteins in NSCLC was unknown. In this study, we detected the effects of HPV-16 oncoproteins on the activation of ERK1/2 signaling pathway in two types of NSCLC cell lines, A549 and NCI-H460 cells. Our results showed that HPV-16 E6 and E7 oncoproteins activated ERK1/2 signaling pathway in NSCLC cells. Furthermore, we also explored the role of ERK1/2 in the expression of HIF-1α, VEGF, and IL-8 stimulated by HPV-16 E6 and E7 oncoproteins in A549 and NCI-H460 cells. We first found that PD98059, a specific ERK1/2 inhibitor, disturbed HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein accumulation, VEGF and IL-8 expression, and the capillary tube formation in NSCLC cells. Our results suggested that ERK1/2 signaling pathway may be, at least in part, involved in HPV-16 E6 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression in NSCLC cells, thus regulating angiogenesis in vitro. However, HPV-16 E7 oncoprotein induced HIF-1α protein accumulation, VEGF and IL-8 expression, and angiogenesis in vitro via ERK1/2-independent pathway in NSCLC cells. These conflicting results may be due to the different mechanisms for tumor angiogenesis between HPV-16 E6 and HPV-16 E7 oncoprotein in NSCLC cells.
A growing body of epidemiological evidence suggested that HPV infection has been hypothesized to be a possible causal agent of a wide variety of human cancers including anogenital and oral cancers, head and neck cancers, and cervical cancer (28–30). It is worth noting that the oncogenic properties of high-risk HPV are mainly mediated by two virus proteins, E6 and E7, which can mediate various regulators at cell cycle and genome stability to perturb the fundamental processes including proliferation, survival, transcription, and angiogenesis (4–12). It was well established that high-risk HPV E6 and E7 oncoproteins are responsible for the degradation of p53 and pRb, respectively (9–12). E6 oncoprotein promotes ubiquitin-dependent conjugation and degradation of p53, an important mediator of DNA damage and cell apoptosis (9,10). An increasing body of evidence has indicated that p53 promotes Mdm2-mediated ubiquitination and proteasomal degradation of HIF-1α, and the loss of p53 in tumor cells enhances HIF-1α levels and augments HIF-1α-dependent transcriptional activation of the VEGF gene, thus contributing to angiogenesis (31,32). Therefore, according to previous reports and our results, it can be hypothesized that HPV-16 E6 oncoprotein may upregulate VEGF through p53-HIF-1α pathway. Moreover, an E6-responsive region is observed in the VEGF promoter between -194 and -50 bp, suggesting E6 may improve tumor angiogenesis by the direct stimulation of VEGF promoter (33). Taken together, HPV-16 E6 oncoprotein may promote VEGF expression through p53-dependent and p53-independent manner in NSCLC cells, hence contributing to angiogenesis in vitro. On the other hand, HPV E7 oncoprotein binds to cullin2 ubiquitin ligase complex, resulting in the ubiquitination and degradation of pRb family members, leading to the release of E2F transcription factor and the activation of E2F target genes, consequently contributing to an uncontrolled cell proliferation and the malignant transformation of tumors (11,12). Interestingly, the ability of HPV-16 E7 oncoprotein to induce VEGF is pRb independent and may be dependent upon degradation of pRb family member p107 and/or p130 (34,35). E7 oncoprotein could also enhance the level of VEGF through transactivating AP-1 sites on the VEGF promoter (36). Furthermore, E7 oncoprotein is responsible for the enhanced HIF-1α activity and the enhanced HIF-1-mediated transcription by inducing the dissociation of histone deacetylases from HIF-1α (37). Additionally, HPV-16 E7 oncoprotein HIF-1α dependently increases the levels of VEGF and IL-8 in NSCLC, thus resulting in angiogenesis (6,7). Therefore, it can be hypothesized that the regulation mechanisms for tumor angiogenesis between HPV-16 E6 and HPV-16 E7 may be different in NSCLC. This hypothesis may help to explain our present results that ERK1/2 signaling pathway mediated HIF-1α protein accumulation and VEGF and IL-8 expression induced by HPV-16 E6 but not E7 oncoprotein. These results also suggested the possibility that there may be some potential links between p53 and ERK1/2 in NSCLC cells, which need to be investigated in future studies.
Summarily, in the present study, we found to our knowledge for the first time that ERK1/2 signaling pathway was involved in HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein accumulation and VEGF and IL-8 expression in both A549 and NCI-H460 cells, contributing to angiogenesis in vitro.
ACKNOWLEDGMENTS
This work was supported by the grants from National Natural Science Foundation of China, 81372511 and 30872944 (to X. Tang), Guangdong Natural Science Foundation, S2012010008232 (to X. Tang), Science and Technology of Guangdong Province, 2013B031100002 (to X. Tang), and Zhanjiang Municipal Governmental Specific Financial Fund Allocated for Competitive Scientific and Technological Projects, 2012C0303-56 (to X. Tang).
REFERENCES
- 1. Muallaoglu S.; Karadeniz C.; Mertsoylu H.; Ayberk Besen A.; Sezer A.; Murat Sedef A.; Kose F.; Ozyilkan O. The clinicopathological and survival differences between never and ever smokers with non-small cell lung cancer. J. BUON. 19:453–458; 2014. [PubMed] [Google Scholar]
- 2. Kawaguchi T.; Takada M.; Kubo A.; Matsumura A.; Fukai S.; Tamura A.; Saito R.; Kawahara M.; Maruyama Y. Gender, histology, and time of diagnosis are important factors for prognosis: Analysis of 1499 never-smokers with advanced non-small cell lung cancer in Japan. J. Thorac. Oncol. 5:1011–1017; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Yano T.; Haro A.; Shikada Y.; Maruyama R.; Maehara Y. Non-small cell lung cancer in never smokers as a representative ‘non-smoking-associated lung cancer’: Epidemiology and clinical features. Int. J. Clin. Oncol. 16:287–293; 2011. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y.; Wang A.; Jiang R.; Pan H.; Huang B.; Lu Y.; Wu C. Human papillomavirus type 16 and 18 infection is associated with lung cancer patients from the central part of China. Cancer 121(Suppl. 17):3069–3079; 2015. [PubMed] [Google Scholar]
- 5. Fei Y.; Yang J.; Hsieh W. C.; Wu J. Y.; Wu T. C. Different human papillomavirus 16/18 infection in Chinese non-small cell lung cancer patients living in Wuhan, China. Jpn. J. Clin. Oncol. 36:274–279; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Li G.; He L.; Zhang E.; Shi J.; Zhang Q.; Le A. D.; Zhou K.; Tang X. Overexpression of human papillomavirus (HPV) type 16 oncoproteins promotes angiogenesis via enhancing HIF-1α and VEGF expression in non-small cell lung cancer cells. Cancer Lett. 311:160–170; 2011. [DOI] [PubMed] [Google Scholar]
- 7. Zhang E.; Feng X.; Liu F.; Zhang P.; Liang J.; Tang X. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS One 9:e103440; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muñoz J. P.; González C.; Parra B.; Corvalán A. H.; Tornesello M. L.; Eizuru Y.; Aguayo F. Functional interaction between human papillomavirus type 16 E6 and E7 oncoproteins and cigarette smoke components in lung epithelial cells. PLoS One 7:e38178; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanier K.; Stutz C.; Kintscher S.; Reinz E.; Sehr P.; Bulkescher J.; Hoppe-Seyler K.; Travé G.; Hoppe-Seyler F. The E6AP binding pocket of the HPV16 E6 oncoprotein provides a docking site for a small inhibitory peptide unrelated to E6AP, indicating druggability of E6. PLoS One 9:e112514; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chlon T. M.; Hoskins E. E.; Mayhew C. N.; Wikenheiser-Brokamp K. A.; Davies S. M.; Mehta P.; Myers K. C.; Wells J. M.; Wells S. I. High-risk human papillomavirus E6 protein promotes reprogramming of Fanconi anemia patient cells through repression of p53 but does not allow for sustained growth of induced pluripotent stem cells. J. Virol. 88:11315–11326; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Branca M.; Giorgi C.; Santini D.; Di Bonito L.; Ciotti M.; Benedetto A.; Paba P.; Costa S.; Bonifacio D.; Di Bonito P.; Accardi L.; Favalli C.; Syrjänen K.; HPV-Pathogen ISS Study Group. DNA vaccine encoding HPV-16 E7 with mutation in L-Y-C-Y-E pRb-binding motif induces potent anti-tumor responses in mice. J. Virol. Methods 206:12–18; 2014. [DOI] [PubMed] [Google Scholar]
- 12. Todorovic B.; Hung K.; Massimi P.; Avvakumov N.; Dick. F. A.; Shaw G. S.; Banks L.; Mymryk J. S. Conserved region 3 of human papillomavirus 16 E7 contributes to deregulation of the retinoblastoma tumor suppressor. J. Virol. 86:13313–13323; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badillo-Almaraz I.; Zapata-Benavides P.; Saavedra-Alonso S.; Zamora-Avila D.; Reséndez-Pérez D.; Tamez-Guerra R.; Herrera-Esparza R.; Rodríguez-Padilla C. Human papillomavirus 16/18 infections in lung cancer patients in Mexico. Intervirology 56:310–315; 2013. [DOI] [PubMed] [Google Scholar]
- 14. Han Y.; Zhang Y.; Jia T.; Sun Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol. 36:1385–1394; 2015. [DOI] [PubMed] [Google Scholar]
- 15. Zhuo X.; Chang A.; Huang C.; Yang L.; Xiang Z.; Zhou Y. Expression and clinical significance of microvessel density and its association with TWIST in nasopharyngeal carcinoma. Int. J. Clin. Exp. Med. 8:1265–1270; 2015. [PMC free article] [PubMed] [Google Scholar]
- 16. Pasquier E.; Tuset M. P.; Sinnappan S.; Carnell M.; Macmillan A.; Kavallaris M. γ-Actin plays a key role in endothelial cell motility and neovessel maintenance. Vasc. Cell 7:2; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen H.; Feng J.; Zhang Y.; Shen A.; Chen Y.; Lin J.; Lin W.; Sferra T. J.; Peng J. Pien Tze Huang inhibits hypoxia-induced angiogenesis via HIF-1 α/VEGF-A pathway in colorectal cancer. Evid. Based Complement. Alternat. Med. 2015:454279; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jamison S.; Lin Y.; Lin W. pancreatic endoplasmic reticulum kinase activation promotes medulloblastoma cell migration and invasion through induction of vascular endothelial growth factor A. PLoS One 10:e0120252; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rafrafi A.; Chahed B.; Kaabachi S.; Kaabachi W.; Maalmi H.; Hamzaoui K.; Sassi F. H. Association of IL-8 gene polymorphisms with non small cell lung cancer in Tunisia: A case control study. Hum. Immunol. 74:1368–1374; 2013. [DOI] [PubMed] [Google Scholar]
- 20. Kachroo P.; Lee M. H.; Zhang L.; Baratelli F.; Lee G.; Srivastava M. K.; Wang G.; Walser T. C.; Krysan K.; Sharma S.; Dubinett S. M.; Lee J. M. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. J. Exp. Clin. Cancer Res. 32:97; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Young R. J.; Tin A. W.; Brown N. J.; Jitlal M.; Lee S. M.; Woll P. J. Analysis of circulating angiogenic biomarkers from patients in two phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br. J. Cancer 106:1153–1159; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He L.; Zhang E.; Shi J.; Li X.; Zhou K.; Zhang Q.; Le A. D.; Tang X. (-)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α. Cancer Chemother. Pharmacol. 71:713–725; 2013. [DOI] [PubMed] [Google Scholar]
- 23. Qu Y.; Wu X.; Yin Y.; Yang Y.; Ma D.; Li H. Antitumor activity of selective MEK1/2 inhibitor AZD6244 in combination with PI3K/mTOR inhibitor BEZ235 in gefitinib-resistant NSCLC xenograft models. J. Exp. Clin. Cancer Res. 33:52; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C.; Fan S.; Owonikoko T. K.; Khuri F. R.; Sun S. Y.; Li R. Oncogenic role of EAPII in lung cancer development and its activation of the MAPK-ERK pathway. Oncogene 30:3802–3812; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y.; Wang L.; Zhang M.; Jin M.; Bai C.; Wang X. Potential mechanism of interleukin-8 production from lung cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J. Cell. Physiol. 227:35–43; 2012. [DOI] [PubMed] [Google Scholar]
- 26. Tang X.; Zhang Q.; Nishitani J.; Brown J.; Shi S.; Le A. D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Cancer Res. 13:2568–2576; 2007. [DOI] [PubMed] [Google Scholar]
- 27. Zhang E. Y.; Li G.; He L.; Shi J. L.; Tang X. D. Construction and application of human papillomavirus-16 oncoproteins E6 and E7 plasmids with double-selection markers. Prog. Modern Biomed. 12: 6045–6050; 2012. [In Chinese] [Google Scholar]
- 28. Jackowska J.; Bartochowska A.; Karlik M.; Wichtowski M.; Tokarski M.; Wierzbicka M. The knowledge of the role of papillomavirus-related head and neck pathologies among general practitioners, otolaryngologists and trainees. A survey-based study. PLoS One 10(10):e0141003; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ajila V.; Shetty H.; Babu S.; Shetty V.; Hegde S. Human papilloma virus associated squamous cell carcinoma of the head and neck. J. Sex. Transm. Dis. 2015:791024; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doorbar J. Model systems of human papillomavirus-associated disease. J. Pathol. doi: 10.1002/path.4656; 2015. [DOI] [PubMed] [Google Scholar]
- 31. Choi K. S.; Bae M. K.; Jeong J. W.; Moon H. E.; Kim K. W. Hypoxia-induced angiogenesis during carcinogenesis. J. Biochem. Mol. Biol. 36:120–127; 2003. [DOI] [PubMed] [Google Scholar]
- 32. Ravi R.; Mookerjee B.; Bhujwalla Z. M.; Sutter C. H.; Artemov D.; Zeng Q.; Dillehay L. E.; Madan A.; Semenza G. L.; Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 14:34–44; 2000. [PMC free article] [PubMed] [Google Scholar]
- 33. López-Ocejo O.; Viloria-Petit A.; Bequet-Romero M.; Mukhopadhyay D.; Rak J.; Kerbel R. S. Oncogenes and tumor angiogenesis: The HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 19:4611–4620; 2000. [DOI] [PubMed] [Google Scholar]
- 34. Walker J.; Smiley L. C.; Ingram D.; Roman A. Expression of human papillomavirus type 16 E7 is sufficient to significantly increase expression of angiogenic factors but is not sufficient to induce endothelial cell migration. Virology 410(2):283–290; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanseverino F.; Santopietro R.; Torricelli M.; D’Andrilli G.; Russo G.; Cevenini G.; Bovicelli A.; Leoncini L.; Scambia G.; Petraglia F.; Claudio P. P.; Giordano A. pRb2/p130 and VEGF expression in endometrial carcinoma in relation to angiogenesis and histopathologic tumor grade. Cancer Biol. Ther. 5(1):84–88; 2006. [DOI] [PubMed] [Google Scholar]
- 36. Antinore M. J.; Birrer M. J.; Patel D.; Nader L.; McCance D. J. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 15:1950–1960; 1996. [PMC free article] [PubMed] [Google Scholar]
- 37. Bodily J. M.; Mehta K. P.; Laimins L. A. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 71(3):1187–1195; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]