Abstract
Breast cancer is a highly prevalent disease affecting women. The association of IQ motif containing GTPase-activating protein 3 (IQGAP3) and breast cancer is poorly defined. Here we reported that IQGAP3 is a key regulator of cell proliferation and metastasis during breast cancer progression. The expression of IQGAP3 was significantly increased in breast tissues compared to nontumor tissues at both protein and mRNA levels. Furthermore, IQGAP3 had a high expression level in ZR-75-30 and BT474 compared to other breast cancer cell lines. Depletion of IQGAP3 through RNA interference in ZR-75-30 and BT474 significantly inhibited cell proliferation. More importantly, IQGAP3 silencing in breast cancer cells notably repressed cell migration and invasion. Further analysis suggested that inhibition of cell proliferation and metastasis was associated with some proteins, including p53, MMP9, Snail, CDC42, p-ERK1/2, KIF2C, KIF4A, PCNA, and Twist. Since expression of IQGAP3 seems to be associated with the pathogenesis of breast cancer and suppression of it can inhibit cancer cell growth and metastasis, IQGAP3 may be a potential therapeutic target in human breast cancer.
Key words: IQ motif containing GTPase-activating protein 3 (IQGAP3), Breast cancer, Proliferation, Migration, Invasion
INTRODUCTION
Breast cancer is a highly prevalent and heterogeneous disease affecting women. Because of high incidence rates and high death rates, breast cancer is a difficult problem and needs to be solved (1). The primary modality for breast cancer therapy remains surgery and radiotherapy. Chemotherapy, hormone therapy, and biologic therapy are additional available therapeutic methods in the clinic at present (2). Despite recent progress in breast tumor therapy, breast cancer remains one of the leading causes of cancer. Current therapies fall short of treating breast cancer, and they fail to prevent recurrence, underpinning the high mortality rate of breast cancer. In this context, gene therapy might prove a useful substitute for conventional treatment modalities. Recent advances in molecular technologies have facilitated genes that could serve as novel targets for cancer. The small interfering RNA (siRNA) is used to determine whether proteins are overexpressed and are potential targets for therapy.
The IQ motif containing GTPase-activating protein (IQGAP) family of proteins is well conserved in organisms from yeast to mammals (3). It comprises three members: IQGAP1, IQGAP2, and IQGAP3 (4). The function of IQGAPs can be corrupted during oncogenesis and is usurped by microbial pathogens (5). IQGAP1 has been suggested to function in the regulation of the actin cytoskeleton and cell migration (6,7). IQGAP2 seems to act as a tumor suppressor when coupled to the activation of the Wnt/β-catenin signaling pathway (8). IQGAP3, an effector of Rac1 and Cdc42, is a new member to this family (9). In cultured Eph4 mouse epithelial cells, IQGAP3 was found at cell–cell junctions, and IQGAP3 expression is reported to be confined to proliferating cells (10). However, its role in tumorigenesis remains to be determined.
In the present study, we demonstrate that IQGAP3 is highly overexpressed in breast cancer. In addition, we provide evidence that silencing IQGAP3 expression decreases the proliferation, migration, and invasion potential of breast cancer cell lines in vitro. On the basis of the results of this study, we conclude that IQGAP3 may be a potential novel target for breast cancer therapy.
MATERIALS AND METHODS
Tissue Samples Cell Culture
Tissue specimens were obtained from breast cancer patients undergoing surgical therapy in the Department of General Surgery, Shanghai Traditional Chinese Medicine-Integrated Hospital. Normal breast tissue specimens were also obtained from patients. IQGAP3 expression in the tissues was evaluated by Western blot and RT-PCR analysis.
The human breast cancer cell lines ZR-75-1, MCF-7, ZR-75-30, MDA-MB-231, BT474, and SK-BR-3 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were maintained at 37°C in 5% CO2 atmosphere.
Silencing of IQGAP by Small Interfering RNA
The siRNA targeting position 5′-CAGCUGUGGUCCUGAUUAA-3′ of human IQGAP mRNA was synthesized. A nonspecific scramble siRNA was used as negative control (NC). The siRNAs were transiently transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Assays were performed 48 h after transfection.
CCK-8 Assay
Cell proliferation was measured using Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s protocol. Briefly, treated and untreated cells were seeded into 96-well plates at a density of 5 × 103 cells in 200 μl of medium per well. At the indicated time point (0, 24, 48, and 72 h), the CCK-8 solution was added to each well and incubated for 1 h. The absorbance rate at 450 nm was read using a microplate reader (Bio-Rad Laboratories). All experiments were performed in triplicate and repeated at least three times.
Migration and Invasion Assay
Cell migration and invasion assays were performed using 12-well Boyden chambers containing polycarbonate filters with a pore size of 8 μm. For Transwell migration assay, treated and untreated cells were serum starved for 24 h. Cells (5 × 104) were plated into the upper well of the Boyden chambers with serum-free medium in the top chamber and medium containing 10% FBS in the lower chamber. After 24 h of incubation, cells on the top of the membrane were removed. Cells that migrated to the bottom surface of the membrane were washed with PBS, fixed in 4% paraformaldehyde, and stained with 0.2% crystal violet. The migrated cells were observed under a Leica inverted microscope. Cell number was counted in 10 random fields for each condition.
For in vitro invasion assay, the upper well of the Boyden chamber was coated with 10 mg/ml Matrigel (BD Biosciences). The Matrigel was allowed to harden at 37°C in a 5% CO2 incubator for 1 h. The rest of the assay was performed as described above.
Real-Time PCR
Total RNA was isolated from fresh tissue or cell using TRIzol reagent (Life Technologies), and an aliquot of 1 μg of total RNA was reverse transcripted using a SuperScript II (Life Technologies) reverse transcriptase. qRT-PCR was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green Master Mix. The primers used are listed below: IQGAP3, 5′-GCAGAATGTTGCCTATCAG-3′ (forward) and 5′-CGGAAATGTAAGCCAGTTG-3′ (reverse); GADPH, 5′-CACCCACTCCTCCACCTTTG-3′ (forward) and 5′-CCACCACCCTGTTGCTGTAG-3′ (reverse).
Western Blot Analysis
For Western blot analysis, protein from each sample of fresh tissue or cells was lysed in lysis buffer. Equal amounts of protein were separated via gel electrophoresis, transferred onto a nitrocellulose membrane, and blotted with primary antibodies. The incubation antibodies include IQGAP3 (Ab88353; Abcam), Twist (Ab175430; Abcam), Snail (#3879s; Cell Signaling Technology), KIF2C (Ab205026; Abcam), KIF4A (Ab3815; Abcam), CDC42 (Ab155940; Abcam), proliferating cell nuclear antigen PCNA; #13110; Cell Signaling Technology), p53 (#9282; Cell Signaling Technology), matrix metallopeptidase 9 (MMP9) (Ab119906; Abcam), ERK1/2 (#4695; Cell Signaling Technology), p-ERK1/2 (#4376; Cell Signaling Technology), and glyceraldehyde 3-phosphate dehydrogenase GADPH; #5174; Cell Signaling Technology). Blots were then incubated with goat anti-mouse secondary antibody (cat. #A0208; Beyotime) or goat anti-rabbit secondary antibody (cat. #A0216; Beyotime). GADPH served as a loading control. Values under each blot are shown as the ratios of target protein/GADPH and represent the mean of three independent assays.
Statistical Analysis
Experimental values are expressed as mean ± standard deviation (SD) of the mean if not otherwise indicated. Statistical significance was analyzed using GraphPad Prism 5.0 software (GraphPad, La Jolla, CA, USA) and determined by unpaired Student’s t-tests and one-way analysis of variance. A value of p < 0.05 was considered significant. All results were reproduced in at least three independent experiments.
RESULTS
IQGAP3 Was Highly Expressed in Breast Cancer
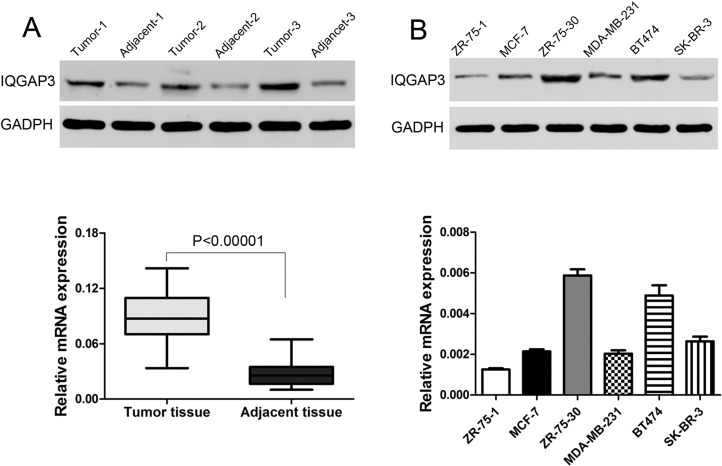
Western blot and RT-PCR were performed to verify IQGAP3 expression in breast tumor tissues. IQGAP3 was upregulated at the protein and mRNA levels in 35 breast cancer tissues compared to adjacent nontumor tissues (Fig. 1A). We then examined the mRNA and protein levels of IQGAP3 in six breast cancer cell lines—ZR-75-1, MCF-7, ZR-75-30, MDA-MB-231, BT474, and SK-BR-3—by Western blot and RT- PCR, respectively. Protein and mRNA levels of IQGAP3 were higher in ZR-75-30 and BT474 cells than in other four cell lines (Fig. 1B). The two cell lines were then selected for the following assays.
Figure 1.
Overexpressed IQGAP3 in breast cancer. (A) The protein and mRNA levels in breast cancer and adjacent tissues. (B) Western blot and RT-PCR were used to choose the highly expressed IQGAP3 cells in breast cancer cells.
shIQGAP3 Inhibited Breast Cancer Cell Proliferation
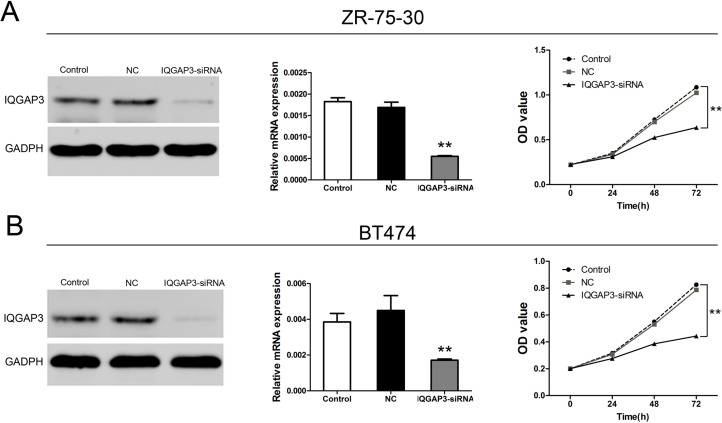
To inhibit IQGAP3 expression in ZR-75-30 and BT474 breast cancer cells, cells were transfected with shIQGAP3 lentivirus. Protein and mRNA expression of IQGAP3 was detected following 48 h of incubation. As shown in Figure 2A and B, two cell lines were successfully transfected with shIQGAP3 lentivirus, and protein and mRNA expression was obviously decreased.
Figure 2.
Effects of IQGAP3 siRNA on cell viability in breast cancer cells. (A) Expression of IQGAP3 and cell viability of ZR-75-30 were analyzed after siRNA transfection. (B) After siRNA IQGAP3 transfection, IQGAP3 expression and cell viability of BT474 cells were analyzed after siRNA transfection. **p < 0.01 compared to the Control group.
To investigate the function of IQGAP3 knockdown in breast cancer, we analyzed cell growth in cancer cell lines (Fig. 2A and B). When cells were transfected with shIQGAP3, cell proliferation was significantly depressed in ZR-75-30 and BT474 cells compared to the control group.
shIQGAP3 Decreased Cell Migration and Invasion in Breast Cancer Cells
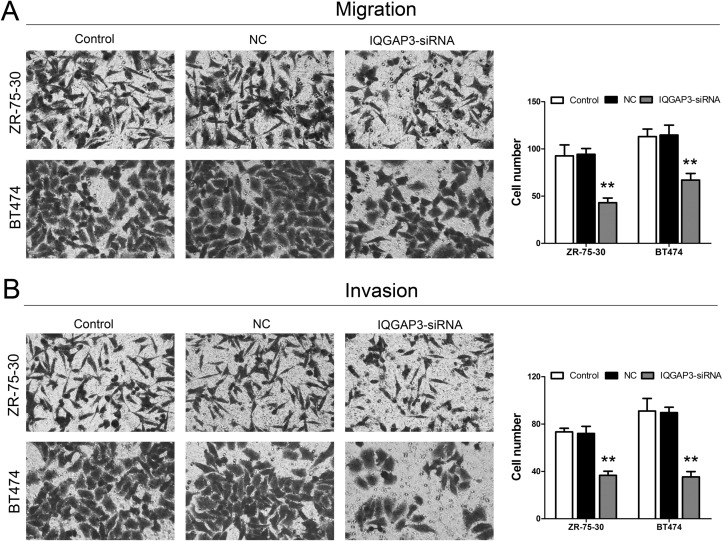
We attempted to investigate the ability of IQGAP3 siRNA treatment to affect cell migration and invasion. Cell migration and invasion studies were performed using the Matrigel matrix assays. Following treatment with shIQGAP3, the results showed that IQGAP3 siRNA treatment resulted in a dramatic inhibition of the migration and invasion capacity of ZR-75-30 and BT474 cells when compared to the control group (Fig. 3A and B).
Figure 3.
Effects of IQGAP3 siRNA on the migration and invasion of breast cancer cells. (A) The migration ability of ZR-75-30 and BT474 cells was identified after siRNA IQGAP3 transfection. (B) The invasion ability of ZR-75-30 and BT474 cells was identified after siRNA IQGAP3 transfection. **p < 0.01 compared to the Control group.
shIQGAP3 Related to Some Proteins in Breast Cancer Cells
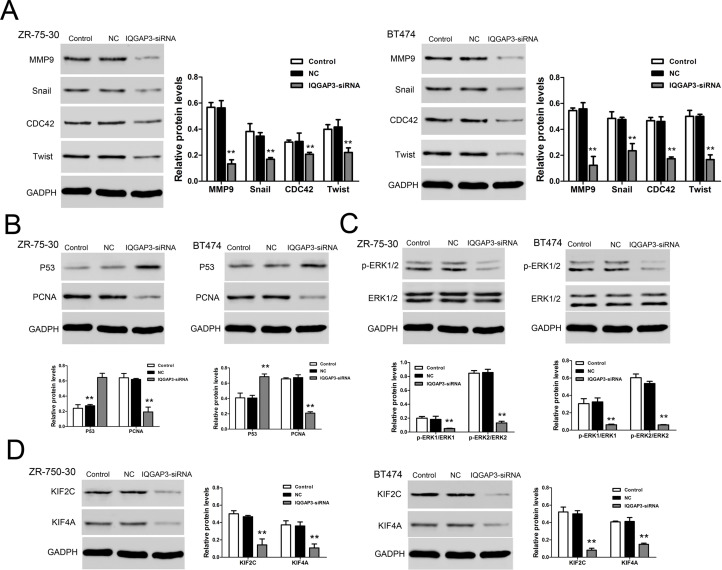
We detected the expression of proteins using Western blot. IQGAP3 siRNA significantly upregulated p53 protein level (Fig. 4B) and remarkably decreased the levels of MMP9, Snail, CDC42, Twist (Fig. 4A), PCNA (Fig. 4B), p-ERK1/2 (Fig. 4C), KIF2C, and KIF4A (Fig. 4D). These data indicated that IQGAP3 siRNA inhibited cell proliferation and metastasis via regulating these proteins.
Figure 4.
Relative protein levels in ZR-75-30 and BT474 were analyzed by Western blot. (A) A representative data showed the related protein expression levels of MMP9, Snail, CDC42, and Twist in ZR-75-30 and BT474 cell lines. (B) Protein expression levels of p53 and PCNA in ZR-75-30 and BT474 cell lines. (C) Protein expression levels of p-ERK1/2 and ERK1/2 in ZR-75-30 and BT474 cell lines. (D) Protein expression levels of KIF2C and KIF4A in ZR-75-30 and BT474 cell lines. **p < 0.01 compared to the Control group.
DISCUSSION
Breast cancer remains the most common cancer among women, and the mortality of breast cancer is still high. Additional treatment methods are required to improve the cure of this disease. Recently, three members of the IQGAP family have been described (11). Here we demonstrated that IQGAP3 is highly expressed in a large proportion of breast cancer samples. While IQGAP3 knockdown inhibited proliferation and metastasis of breast cancer cells, suppression of its expression caused a reduction in tumorigenic potential.
IQGAP3, located at 1q21.3, has been reported to be linked with gastroesophageal carcinoma and infiltrating ductal carcinoma of the breast (12,13). In the present study, we reported that IQGAP3 protein and mRNA expression were elevated in breast cancer tissues compared to nontumor breast tissues. We then investigated the function of IQGAP3 on breast cancer by knocking down its expression in two breast cancer cell lines: ZR-75-30 and BT474. We found that knockdown of IQGAP3 in breast cancer cells significantly impaired cell proliferation. Migration and invasion are the essential processes toward the metastasis of cancer. Depletion of IQGAP3 in breast cancer cells remarkably decreased their migration and invasion abilities, which suggested a role for IQGAP3 in breast cancer metastasis.
The mechanism by which IQGAP3 enhances proliferation and increases metastasis potential is still not fully understood. p53 is a tumor suppressor gene that can suppress the formation of tumors (14). MMP9 is a matrix that is involved in the degradation of extracellular matrix in normal physiological processes and is related to tumor metastasis (15). Snail and Twist genes may have a role in the recurrence of breast cancer by downregulating E-cadherin and inducing an epithelial-to-mesenchymal transition (16). CDC42 is a small GTPase of the Rho family, which regulates the signaling pathway that controls functions, including cell migration, morphology, and cell cycle. Activated ERK1/2 regulates growth and cell cycle progression of cells (17). KIF2C acts to regulate microtubule dynamics in cells and move chromosomes during cell division. KIF4A encodes a member of the kinesin 4 subfamily of kinesin-related proteins and is involved in maintaining chromosome integrity during mitosis (18). Proliferating cell nuclear antigen (PCNA) is a DNA clamp, which is involved in DNA and DNA repair and interacts with DNA polymerase (19). Data from this study show that downregulation of IQGAP3 expression increases p53 expression and decreases MMP9, Snail, Twist, CDC42, p-ERK1/2, KIF2C, KIF4A, and PCNA. We believe that this is the mechanism responsible for the decrease in proliferation and invasion potential after IQGAP3 knockdown in breast cancer cells.
In summary, our study provides, for the first time, that IQGAP3 was overexpressed in breast cancer tissues. In addition, our data indicate that IQGAP3 plays a key role in the proliferation and metastasis of breast cancer cells. Whether IQGAP3 can be used as a therapeutic target for breast cancer remains to be further investigated.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Chung C.; Lee S.; Hwang S.; Park E. C. Systematic review of exercise effects on health outcomes in women with breast cancer. Asian Nurs. Res. 7(3):149–159; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Osta W. A.; Chen Y.; Mikhitarian K.; Mitas M.; Salem M.; Hannun Y. A.; Cole D. J.; Gillanders W. E. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 64(16):5818–5824; 2004. [DOI] [PubMed] [Google Scholar]
- 3. Briggs M. W.; Sacks D. B. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 542(1):7–11; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Weissbach L.; Settleman J.; Kalady M. F.; Snijders A. J.; Murthy A. E.; Yan Y.-X.; Bernards A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J. Biol. Chem. 269(32):20517–20521; 1994. [PubMed] [Google Scholar]
- 5. Hedman A. C.; Smith J. M.; Sacks D. B. The biology of IQGAP proteins: Beyond the cytoskeleton. EMBO Rep. e201439834; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noritake J.; Watanabe T.; Sato K.; Wang S.; Kaibuchi K. IQGAP1: A key regulator of adhesion and migration. J. Cell Sci. 118(10):2085–2092; 2005. [DOI] [PubMed] [Google Scholar]
- 7. Kim H.; White C. D.; Sacks D. B. IQGAP1 in microbial pathogenesis: Targeting the actin cytoskeleton. FEBS Lett. 585(5):723–729; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt V. A.; Chiariello C. S.; Capilla E.; Miller F.; Bahou W. F. Development of hepatocellular carcinoma in Iqgap2-deficient mice is IQGAP1 dependent. Mol. Cell. Biol. 28(5):1489–1502; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang S.; Watanabe T.; Noritake J.; Fukata M.; Yoshimura T.; Itoh N.; Harada T.; Nakagawa M.; Matsuura Y.; Arimura N. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J. Cell Sci. 120(4):567–577; 2007. [DOI] [PubMed] [Google Scholar]
- 10. Nojima H.; Adachi M.; Matsui T.; Okawa K.; Tsukita S.; Tsukita S. IQGAP3 regulates cell proliferation through the Ras/ERK signalling cascade. Nat. Cell Biol. 10(8):971–978; 2008. [DOI] [PubMed] [Google Scholar]
- 11. Brill S.; Li S.; Lyman C. W.; Church D. M.; Wasmuth J. J.; Weissbach L.; Bernards A.; Snijders A. J. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol. Cell. Biol. 16(9):4869–4878; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawthorn L.; Luce J.; Stein L.; Rothschild J. Integration of transcript expression, copy number and LOH analysis of infiltrating ductal carcinoma of the breast. BMC Cancer 10(1):460; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koon N.; Zaika A.; Moskalukt C. A.; Friersont H. F.; Knuutilat S.; Powell S. M. Clustering of molecular alterations in gastroesophageal carcinomas. Neoplasia 6(2):143–149; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan K. M.; Phillips A. C.; Vousden K. H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13(3):332–337; 2001. [DOI] [PubMed] [Google Scholar]
- 15. Egeblad M.; Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2(3):161–174; 2002. [DOI] [PubMed] [Google Scholar]
- 16. De Herreros A. G.; Peiró S.; Nassour M.; Savagner P. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J. Mammary Gland. Biol. 15(2):135–147; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meloche S.; Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26(22):3227–3239; 2007. [DOI] [PubMed] [Google Scholar]
- 18. Taniwaki M.; Takano A.; Ishikawa N.; Yasui W.; Inai K.; Nishimura H.; Tsuchiya E.; Kohno N.; Nakamura Y.; Daigo Y. Activation of KIF4A as a prognostic biomarker and therapeutic target for lung cancer. Clin. Cancer Res. 13(22):6624–6631; 2007. [DOI] [PubMed] [Google Scholar]
- 19. Malkas L. H.; Herbert B. S.; Abdel-Aziz W.; Dobrolecki L. E.; Liu Y.; Agarwal B.; Hoelz D.; Badve S.; Schnaper L.; Arnold R. J. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc. Natl. Acad. Sci. USA 103(51):19472–19477; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]