Abstract
The SASH1 (SAM- and SH3-domain containing 1) gene, a member of the SLY (SH3 domain containing expressed in lymphocytes) family of signal adapter proteins, has been implicated in tumorigenesis of many types of cancers. However, the role and mechanism of SASH1 in the invasion and metastasis of hepatocarcinoma are largely unknown. In this study, we investigated the role and mechanism of SASH1 in the invasion and metastasis of hepatocarcinoma. Our results showed that SASH1 was lowly expressed in hepatocarcinoma cell lines. The in vitro experiments showed that overexpression of SASH1 inhibited the proliferation and migration/invasion of hepatocarcinoma cells, as well as the epithelial–mesenchymal transition (EMT) progress. Furthermore, overexpression of SASH1 suppressed the expression of Shh as well as Smo, Ptc, and Gli-1 in hepatocarcinoma cells. Taken together, these results suggest that overexpression of SASH1 inhibited the proliferation and invasion of hepatocarcinoma cells through the inactivation of Shh signaling pathway. Therefore, these findings reveal that SASH1 may be a potential therapeutic target for the treatment of hepatocarcinoma.
Key words: SAM- and SH3-domain containing 1 (SASH1), Hepatocarcinoma, Invasion, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Hepatocarcinoma, one of the most common malignancies and leading causes of cancer-related deaths in the world, is a significant health problem due to its high incidence and mortality (1). There are about 600,000 new-onset patients with this disease every year, and its incidence has a rising trend (2). Although various treatments for hepatocarcinoma, such as chemotherapy, radiation, and hormone therapy, have been used and improved recently, the clinical outcome of patients remains unsatisfactory (3–5). This is largely because of a lack of understanding of the molecular mechanisms behind hepatocarcinoma. So further studies on mechanisms of hepatocarcinoma and the identification of new and effective gene therapy targets are of great significance to the development of new treatment strategies and the improvement in patient survival.
The SASH1 gene, a member of the SLY family of signal adapter proteins, encodes a protein containing sterile a module (SAM) and Src homology domain 3 (SH3) domains that are predominantly seen in signaling molecules, adapters, and scaffold proteins (6). Previous studies revealed that SASH1 is critical during tumorigenesis of several cancer types (6–8). SASH1 overexpression in lung cancer cells inhibits the migration/invasion and the protein expression of cyclin D1, matrix metalloproteinase-1 (MMP-1), and MMP-2 (9). Recently, one study reported that the expression levels of SASH1 were strongly reduced in liver cancer tissues compared with adjacent normal tissues, and increase of DNA methylation degree in the promoter region of SASH1 gene, particularly CpG_26.27 sites, may inhibit SASH1 expression in liver cancer (10). However, the role and mechanism of SASH1 in the invasion and metastasis of hepatocarcinoma are largely unknown. In this study, we investigated the role and mechanism of SASH1 in the invasion and metastasis of hepatocarcinoma.
MATERIALS AND METHODS
Cell Culture
Hepatocarcinoma cell lines (HepG2, 97H, Hep3B, and HCCLM3) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin in 5% CO2 at 37°C.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from hepatocarcinoma cells using the RNeasy Fibrous Tissue mini kit (Qiagen, Valencia, CA, USA) and reverse transcribed into cDNA using a PrimeScript™ 1st Strand cDNA Synthesis kit (Takara, Dalian, China). RT-PCR was performed in a final volume of 10 µl, which contained 5 µl of SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories), 1 µl of cDNA (1:50 dilution), and 2 µl each of the forward and reverse primers (1 mM). The primer sequences used for PCR amplification were SASH1 forward 5′-TCCCGTCACAGGAAGAAACG-3′ and reverse 5′-GATACCCATCACGTCGGTCC-3′; β-actin was used as an internal standard and the primers were forward 5′-GTCCACCGCAAATGCTTCTA-3′ and reverse 5′-TGCTGTCACCTTCACCGTTC-3′. Cycling conditions for amplification were: 95°C for 1 min; 35 cycles at 95°C for 45 s, 60°C for 30 s, and 72°C for 30 s; finally, 72°C for 5 min. Quantitative measurements were determined using the comparative ΔCt method.
Western Blot Analysis
Proteins were extracted from hepatocarcinoma cells using cell lysis buffer, and the protein concentration in the lysates was determined using a BCA protein assay kit (Beyotime, Nantong, China). A total of 20 µg of protein was separated by 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes (Amersham, Little Chalfont, UK). After blocking with Tween-Tris-buffered saline (T-TBS) containing 5% nonfat milk powder, the membranes were incubated with primary antibodies (SASH1, E-cadherin, vimentin, N-cadherin, Shh, Smo, Ptc, Gli-1, and GAPDH; from Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Subsequently, they were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. The target protein was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Construction of the pcDNA3.1-SASH1 Vector and Cell Transfection
The full-length coding sequence of human SASH1 gene was amplified by RT-PCR and ligated into the pGEM-T vector (Clontech, Cambridge, UK) at the EcoRI and KpnI restriction sites. After sequence confirmation, positive clones were subcloned into the pcDNA3.1 expression vector to construct the recombinant expression vector pcDNA3.1-SASH1.
For in vitro transfection, the cells were seeded in six-well plates at a density of 1.0 × 105 cells/well and cultured overnight. When the cells grew to 70–80% confluence, pcDNA3.1-SASH1 and empty vector were transfected using Lipofectamine 2000™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Stable transfectants were clonally selected in complete medium containing 1 µg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA).
Cell Proliferation Assay
Cells transfected with pcDNA3.1-SASH1 or pcDNA3.1 were seeded into 96-well plate in a density of 1 × 104 cells/well in 200 µl medium. At each time point, the culture medium was replaced with fresh medium containing MTT (5 mg/ml in PBS, 200 µl/well) and incubated with the cells for an additional 4 h. Following incubation, medium was discarded and DMSO (150 µl/well; Sigma-Aldrich) was added to each well for 10 min. The results were analyzed by an automated plate reader at 490 nm.
Migration and Invasion Assays
Cell migration was measured by Transwell assay. In brief, cells transfected with pcDNA3.1-SASH1 or pcDNA3.1 were added to the upper compartment with an 8-µm microporous filter. Then 500 µl of DMEM containing 10% FBS was added to the bottom chamber. After incubation at 37°C for 24 h, the cells on the upper surface of the filters were removed with a cotton swab, and cells that had migrated to the lower surface were washed, fixed, stained with crystal violet, and counted using a light microscope.
Cell invasion was measured by Matrigel-precoated Transwell inserts (8.0 mm pore size with polyethylene tetraphthalate membrane) according to the manufacturer’s protocol. Briefly, cells transfected with pcDNA3.1-SASH1 or pcDNA3.1 were seeded in the upper chamber, and DMEM medium with 10% FBS was added into the lower chamber. After 24 h of incubation, cells that passed through the lower side of the membrane were stained with hematoxylin and eosin, and quantified by counting six high-powered fields in the center of each well. The number of cells that invaded the lower side of the membrane was determined by counting cells in a minimum of four randomly selected areas.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance or paired t-tests. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
SASH1 Is Lowly Expressed in Human Hepatocarcinoma Cell Lines
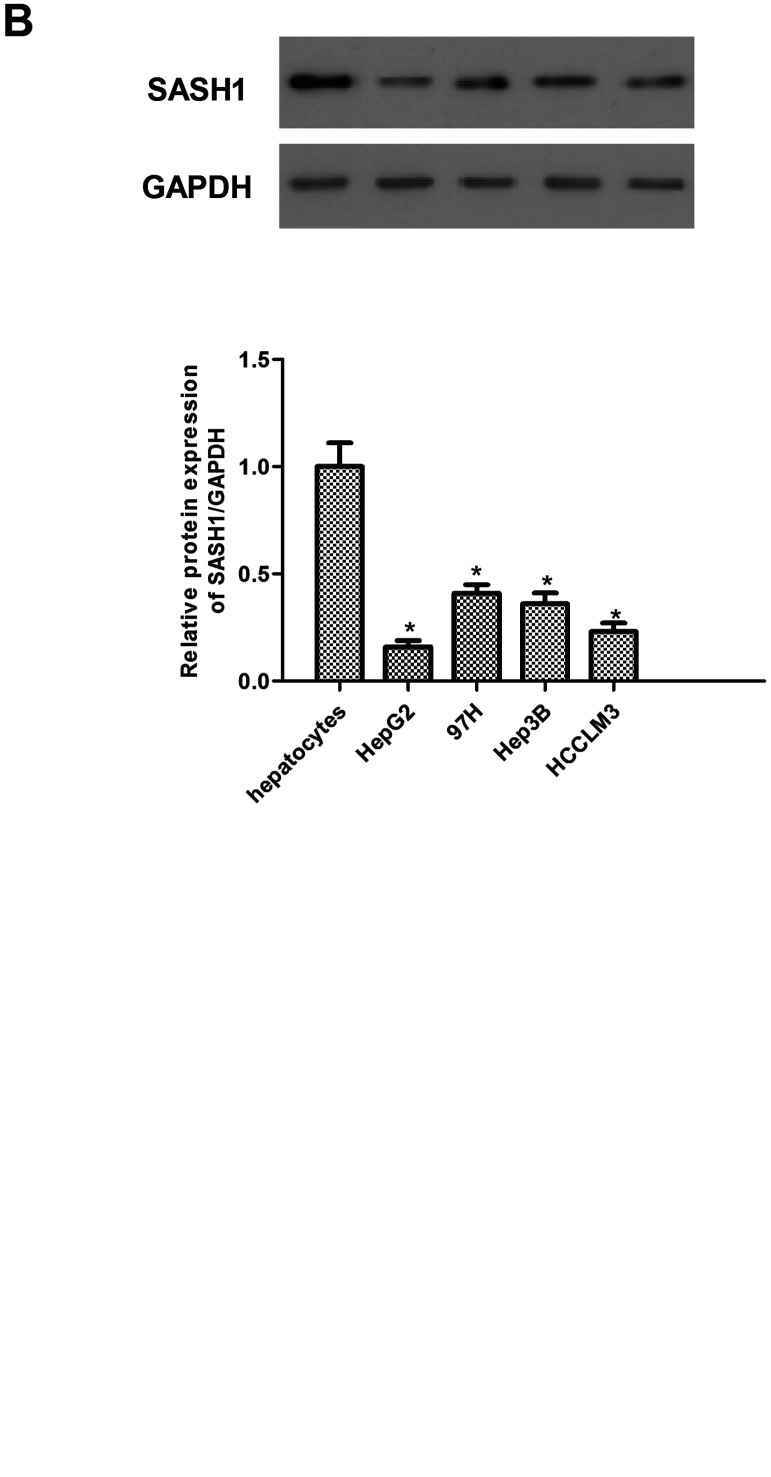
To explore the role of SASH1 in hepatocarcinoma tumorigenesis, we first performed qRT-PCR analyses of human hepatocarcinoma cell lines (HepG2, 97H, Hep3B, and HCCLM3) and hepatocytes. The results demonstrated that the expression of SASH1 mRNA was obviously lower in hepatocarcinoma cell lines compared with hepatocytes (Fig. 1A). Similarly, Western blot analysis demonstrated that the expression of SASH1 protein was also decreased in human hepatocarcinoma cell lines (Fig. 1B).
Figure 1.
SASH1 is lowly expressed in human hepatocarcinoma cell lines. The mRNA (A) and protein (B) levels of SASH1 were determined by qRT-PCR and Western blot in human hepatocarcinoma cell lines, respectively. The values shown represent the mean ± SD. *p < 0.05 versus control group.
SASH1 Inhibits Proliferation of Hepatocarcinoma Cells
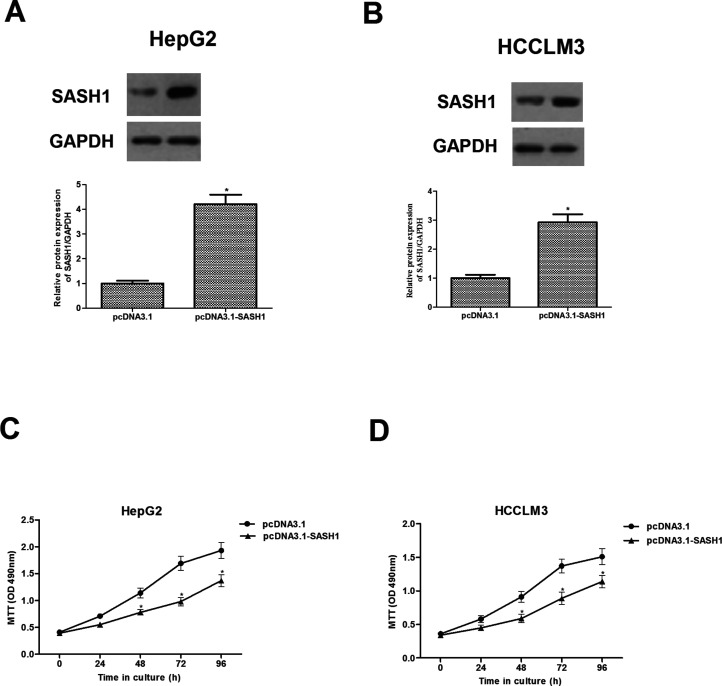
To further investigate the effect of SASH1 on hepatocarcinoma tumorigenesis, we constructed the recombinant expression vector pcDNA3.1-SASH1. The efficiency of SASH1 transfection was detected by Western blot. The results showed that SASH1 protein was significantly increased in HepG2 and HCCLM3 cells after transfection with pcDNA3.1-SASH1, respectively (Fig. 2A, B). We then investigated the effect of SASH1 on hepatocarcinoma cell proliferation. As shown in Figure 2C and D, SASH1 obviously inhibited the proliferation of HepG2 and HCCLM3 cells, respectively, in a time-independent manner.
Figure 2.
SASH1 inhibits proliferation of hepatocarcinoma cells. HepG2 (A) and HCCLM3 (B) cells were transiently transfected with pcDNA3.1-SASH1 or pcDNA3.1 for 48 h. Western blot was performed to detect the transfection efficiency. (C, D) Cell proliferation was examined by the MTT assay at the indicated time. The values shown represent the mean ± SD, n = 3 per group. *p < 0.05 versus pcDNA3.1 group.
SASH1 Inhibits Migration and Invasion of Hepatocarcinoma Cells
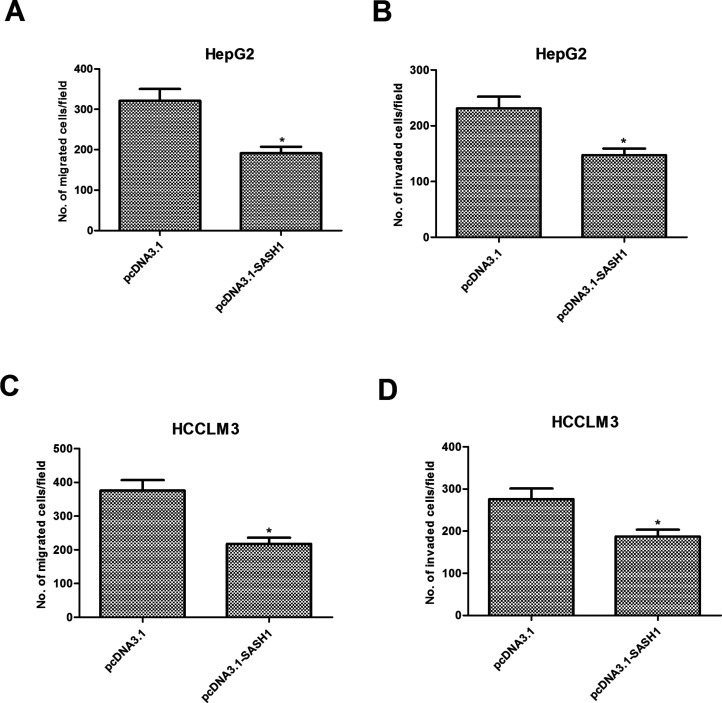
We next determined the potential impact of SASH1 on hepatocarcinoma cell migration and invasion. As shown in Figure 3A, the number of migrated HepG2 cells was reduced to 191 ± 15 after transfection with pcDNA3.1-SASH1 when compared with the pcDNA3.1 group (321 ± 23). Furthermore, overexpression of SASH1 significantly inhibited the invasion of HepG2 cells (Fig. 3B). Similar results were obtained in HCCLM3 cells transfected with pcDNA3.1-SASH1 (Fig. 3C, D).
Figure 3.
SASH1 inhibits migration and invasion of hepatocarcinoma cells. HepG2 and HCCLM3 cells were transiently transfected with pcDNA3.1-SASH1 or pcDNA3.1 for 48 h. The migratory potential of HepG2 (A) and HCCLM3 (C) cells was detected by Transwell assay. The invasive ability of HepG2 (B) and HCCLM3 (D) cells was detected by Transwell assay with Matrigel. The values shown represent the mean ± SD, n = 3 per group. *p < 0.05 versus pcDNA3.1 group.
SASH1 Prevents Epithelial–Mesenchymal Transition (EMT) Process in Hepatocarcinoma Cells
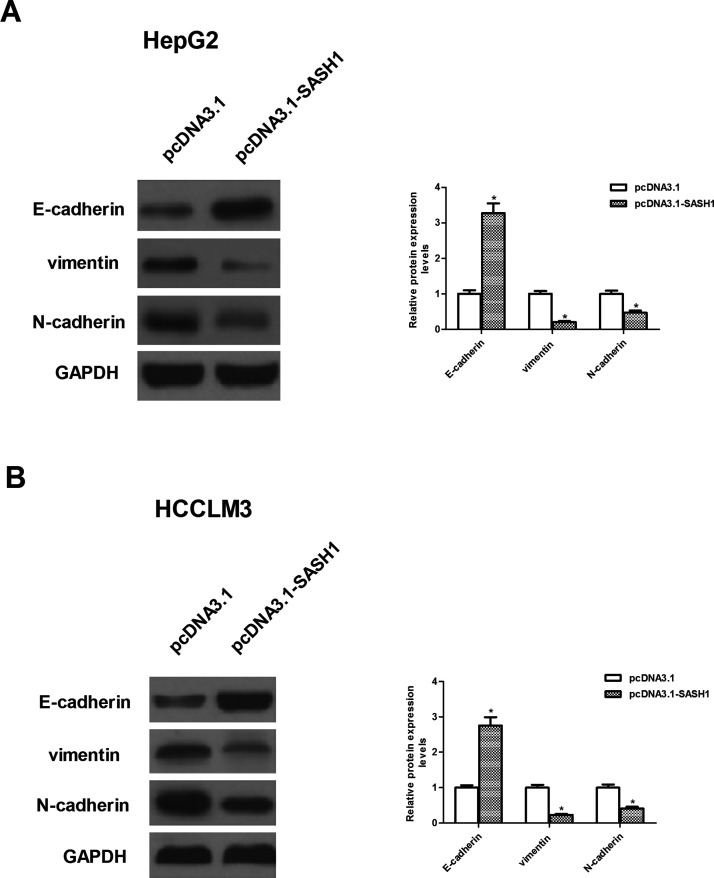
We investigated the effect of SASH1 on the EMT progression of hepatocarcinoma cells. As shown in Figure 4A and B, Western blot analysis showed that the protein expression levels of E-cadherin were higher in HepG2 and HCCLM3 cells after transfection with pcDNA3.1-SASH1, respectively. However, the protein expression levels of vimentin and N-cadherin were significantly decreased by SASH1 overexpression in HepG2 and HCCLM3 cells, respectively.
Figure 4.
SASH1 prevents epithelial–mesenchymal transition (EMT) process in hepatocarcinoma cells. HepG2 and HCCLM3 cells were transiently transfected with pcDNA3.1-SASH1 or pcDNA3.1 for 48 h. (A, B) Western blot analysis for N-cadherin, E-cadherin, and vimentin in pcDNA3.1-SASH1-transfected HepG2 and HCCLM3 cells, respectively. The values shown represent the mean ± SD. *p < 0.05 versus pcDNA3.1 group.
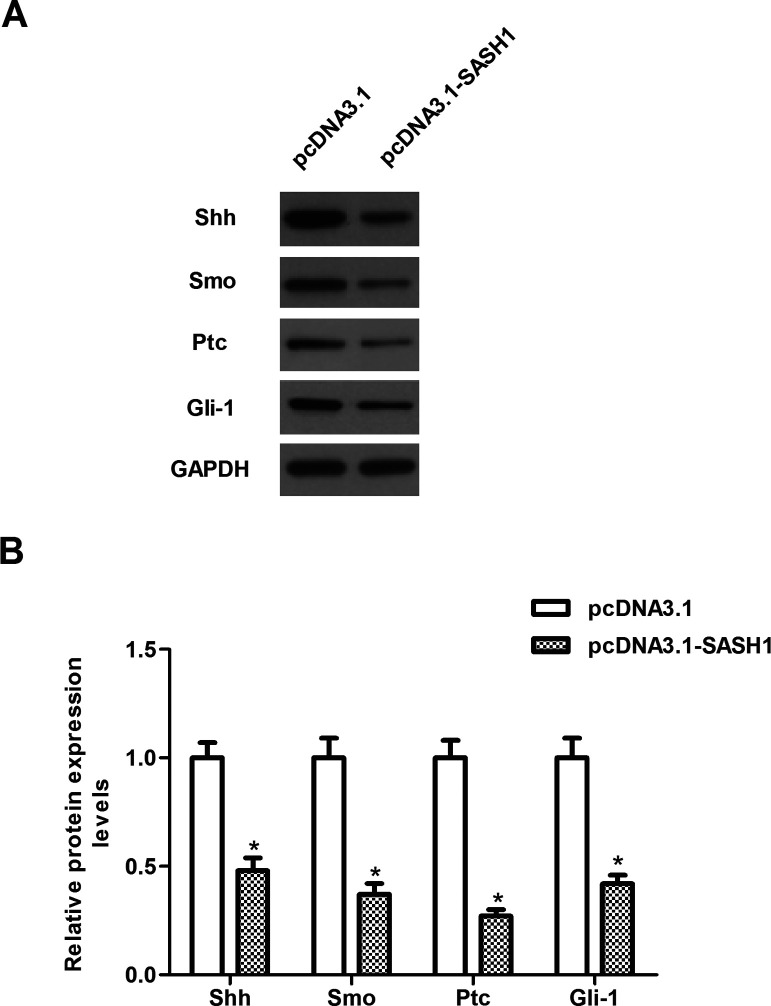
SASH1 Inhibits the Metastatic Process of Hepatocarcinoma Cells Through Suppressing the Sonic Hedgehog Signaling Pathway
To further investigate the underlying mechanism of SASH1-inhibited proliferation and invasion of hepatocarcinoma cells, we investigated the effect of SASH1 on the activity of the Shh pathway. The results demonstrated that SASH1 overexpression significantly decreased the protein expression of Shh as well as Smo, Ptc, and Gli-1 in HepG2 cells, compared with the pcDNA3.1 group (Fig. 5).
Figure 5.
SASH1 inhibits the metastatic process of hepatocarcinoma cells through suppressing the Sonic hedgehog signaling pathway. HepG2 cells were transiently transfected with pcDNA3.1-SASH1 or pcDNA3.1 for 48 h. (A) The protein levels of Shh, Smo, Ptc, and Gli-1 were determined by Western blot. (B) Quantitative analysis of protein expression levels of Shh, Smo, Ptc, and Gli-1 using Image-Pro Plus 6.0 software and normalized to GAPDH. The values shown represent the mean ± SD. *p < 0.05 versus pcDNA3.1 group.
DISCUSSION
In the present study, we provided evidence that SASH1 expression was downregulated in hepatocarcinoma cell lines, and SASH1 overexpression inhibits cell proliferation and invasion, as well as EMT progress in hepatocarcinoma cells. We further demonstrated that SASH1 overexpression significantly decreased the protein expression of Shh as well as Smo, Ptc, and Gli-1 in hepatocarcinoma cells.
Several studies have reported that SASH1 is downregulated in tumors and that this expression is correlated with tumor grade and prognosis. Sun et al. confirmed that SASH1 is downregulated in thyroid cancer cell lines (11). A study by Zeller et al. showed that SASH1 expression is decreased in human breast cancer cell lines (6). Consistent with the above-mentioned results, in the present study we found that SASH1 mRNA and protein expression were both decreased in human hepatocarcinoma cell lines, which suggested that SASH1 may be a candidate tumor suppressor gene in hepatocarcinoma.
EMT is a process by which the mesenchymal phenotype is acquired by epithelial cells, which plays an important role in the metastasis of many types of carcinomas, including hepatocarcinoma (12–14). In general, increased motility and invasion are positively associated with EMT, which is characterized by repression of epithelial markers and induction of mesenchymal markers (15). In the present study, we observed that SASH1 overexpression inhibits hepatocarcinoma cell migration and invasion. SASH1 also increased the level of the epithelial marker (E-cadherin) and suppressed the levels of the mesenchymal markers (N-cadherin and vimentin) in hepatocarcinoma cells. These results suggest that SASH1 could inhibit the migration and invasion by preventing EMT through downregulating EMT-inducing genes in hepatocarcinoma cells.
Multiple lines of evidence support that the sonic hedgehog (SHh) pathway plays an important role in the development and invasion of hepatocarcinoma. Blockade of the Hh signaling pathway may be a potential target of new therapeutic strategy for hepatocarcinoma (16–19). Hh protein binds to its receptor human Ptch1, and relieves Ptch1 inhibition on smoothened (SMO). Smo translocates into the primary cilium, and leads to the activation of transcription factor Gli-1, which induces the expression of numerous target genes, such as Ptch1, Hip, Gli2, and Wnt, regulating proliferation and invasion (18,20,21). The transcription of components of the Hh signaling pathway and related molecules has been reported to be increased in the development and progression of hepatocarcinoma (17). Research has indicated that overexpression of the Smo proto-oncogene mediates the expression of c-myc, which plays an important role in hepatocarcinogenesis (22). Furthermore, it was reported that knockdown of Gli-1 obviously inhibits HCC cell migration and invasion likely through inhibiting expressions and activations of matrix metalloproteinase (MMP)-2 and MMP-9 and blocking EMT (23). In the present study, we observed that that SASH1 overexpression greatly decreased the protein expression of Shh as well as Smo, Ptc, and Gli-1 in HepG2 cells. These data suggest that SASH1 inhibits hepatocarcinoma cell proliferation and invasion through the inactivation of Shh signaling pathway.
In conclusion, this study demonstrates that SASH1 is lowly expressed in human hepatocarcinoma cell lines and provides the first evidence that SASH1 inhibits hepatocarcinoma cell proliferation and invasion through the inactivation of Shh signaling pathway. Thus, these results suggest that SASH1 may be a novel potential therapeutic target for hepatocarcinoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Abdel-Rahman O. Revisiting oxaliplatin-based regimens for advanced hepatocellular carcinoma. Curr. Oncol. Rep. 16:1–3; 2014. [DOI] [PubMed] [Google Scholar]
- 2. El–Serag H. B.; Rudolph K. L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576; 2007. [DOI] [PubMed] [Google Scholar]
- 3. Cahill G. T. L.; Baker R. A.; Brown A.; Huang Y. C.; Ou C. W. Principles of radiation oncology. Asian Pac. J. Surg. Oncol. 1:27–38; 2015. [Google Scholar]
- 4. Mellotte G. M. V.; Devitt P. G.; Shin V. Y.; Leung C. P. Minimally invasive surgical oncology: State of the art. Asian Pac. J. Surg. Oncol. 1:101–112; 2015. [Google Scholar]
- 5. Bruix J.; Llovet J. M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35:519–524; 2002. [DOI] [PubMed] [Google Scholar]
- 6. Zeller C.; Hinzmann B.; Seitz S.; Prokoph H.; Burkhard-Goettges E.; Fischer J.; Jandrig B.; Schwarz L. E.; Rosenthal A.; Scherneck S. SASH: A candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 22:2972–2983; 2003. [DOI] [PubMed] [Google Scholar]
- 7. Meng Q.; Zheng M.; Liu H.; Song C.; Zhang W.; Yan J.; Qin L.; Liu X. SASH1 regulates proliferation, apoptosis, and invasion of osteosarcoma cell. Mol. Cell. Biochem. 373:201–210; 2013. [DOI] [PubMed] [Google Scholar]
- 8. Yang L.; Liu M.; Gu Z.; Chen J.; Yan Y.; Li J. Overexpression of SASH1 related to the decreased invasion ability of human glioma U251 cells. Tumor Biol. 33:2255–2263; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Chen E. G.; Chen Y.; Dong L. I.; Zhang J. S. Effects of SASH1 on lung cancer cell proliferation, apoptosis, and invasion in vitro. Tumor Biol. 33:1393–1401; 2012. [DOI] [PubMed] [Google Scholar]
- 10. Peng L.; Wei H.; Liren L. Promoter methylation assay of sash1 gene in hepatocellular carcinoma. J. BUON 19:1041–1047; 2013. [PubMed] [Google Scholar]
- 11. Sun D.; Zhou R.; Liu H.; Sun W.; Dong A.; Zhang H. SASH1 inhibits proliferation and invasion of thyroid cancer cells through PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 8:12276–12283; 2015. [PMC free article] [PubMed] [Google Scholar]
- 12. Van Zijl F.; Zulehner G.; Petz M.; Schneller D.; Kornauth C.; Hau M.; Machat G.; Grubinger M.; Huber H.; Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 5:1169–1179; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee T. K.; Poon R. T.; Yuen A. P.; Ling M. T.; Kwok W. K.; Wang X. H.; Wong Y. C.; Guan X. Y.; Man K.; Chau K. L. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin. Cancer Res. 12:5369–5376; 2006. [DOI] [PubMed] [Google Scholar]
- 14. Jou J.; Diehl A. M. Epithelial-mesenchymal transitions and hepatocarcinogenesis. J. Clin. Invest. 120:1031–1034; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huber M. A.; Kraut N.; Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558; 2005. [DOI] [PubMed] [Google Scholar]
- 16. Cheng W. T.; Xu K.; Tian D. Y.; Zhang Z. G.; Liu L. J.; Chen Y. Role of hedgehog signaling pathway in proliferation and invasiveness of hepatocellular carcinoma cells. Int. J. Oncol. 34:829–836; 2009. [DOI] [PubMed] [Google Scholar]
- 17. Patil M. A.; Zhang J.; Ho C.; Cheung S. T.; Fan S. T.; Chen X. Hedgehog signaling in human hepatocellular carcinoma. Cancer Biol. Ther. 5:111–117; 2006. [DOI] [PubMed] [Google Scholar]
- 18. Katoh Y.; Katoh M. Hedgehog target genes: Mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 9:873–886; 2009. [DOI] [PubMed] [Google Scholar]
- 19. Philips G. M.; Chan I. S.; Swiderska M.; Schroder V. T.; Guy C.; Karaca G. F.; Moylan C.; Venkatraman T.; Feuerlein S.; Syn W. K. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One 6:e23943; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen M. M. The hedgehog signaling network. Am. J. Med. Genet. A 123:5–28; 2003. [DOI] [PubMed] [Google Scholar]
- 21. Chari N. S.; McDonnell T. J. The sonic hedgehog signaling network in development and neoplasia. Adv. Anat. Pathol. 14:344–352; 2007. [DOI] [PubMed] [Google Scholar]
- 22. Sicklick J. K.; Li Y. X.; Jayaraman A.; Kannangai R.; Qi Y.; Vivekanandan P.; Ludlow J. W.; Owzar K.; Chen W.; Torbenson M. S. Dysregulation of the hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 27:748–757; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Chen J. S.; Li H. S.; Huang J. Q.; Zhang L. J.; Chen X. L.; Wang Q.; Lei J.; Feng J. T.; Liu Q.; Huang X. H. Down-regulation of Gli-1 inhibits hepatocellular carcinoma cell migration and invasion. Mol. Cell. Biochem. 393:283–291; 2014. [DOI] [PubMed] [Google Scholar]