Abstract
In order to improve therapeutic efficacy, it is a current emergency to better know the mechanisms underlying cisplatin resistance in lung cancer cells. In this study, we aim to investigate the role of Krüppel-like factor 4 (KLF4) in cisplatin-resistant lung cancer cells. We developed cisplatin-resistant lung cancer cell line A549/DDP, and then a battery of experiments was used to analyze the effects of KLF4 in cisplatin resistance of lung cancer. We found that KLF4 was significantly downregulated in cisplatin-resistant A549 cells and forced KLF4 expression inhibited cell growth and induced apoptosis. Further, we found that overexpression of KLF4 was able to inhibit cell migration and invasion, to inhibit the expression of Slug, Twist, and vimentin, and to increase the expression of E-cadherin and subsequent inhibition of the EMT process. Thus, overexpression of KLF4 may be a potential strategy for lung cancer treatment, especially for cisplatin-resistant cases.
Key words: Non-small lung cancer, Resistance, Cisplatin, Krüppel-like factor 4 (KLF4), Epithelial-to-mesenchymal transition
INTRODUCTION
Lung cancer is the leading cause of cancer mortality worldwide. The incidence of non-small lung cancer (NSCLC) is increasing every year with almost 1.3 million new cases diagnosed in the world each year (1). Perioperative sequential or concurrent chemoradiotherapy is the standard treatment for patients with NSCLC, but locally advanced patients still have a quite poor prognosis (2). The standard of care for advanced NSCLC is cisplatin (DDP) in combination with drugs including paclitaxel, gemcitabine, vinorelbine, and pemetrexed (3). Previous studies have shown several pathways associated with the effect of cisplatin on NSCLC, including nucleotide excision repair (NER), copper transporters, and glutathione S-transferases (GSTs) (4–6). Cisplatin is able to enter the cell. Once in the cell, cisplatin can interact with and intercalate tumor DNA, resulting in DNA damage and inhibition of DNA replication with subsequent apoptosis and necrosis (7). However, due to intrinsic or acquired nonresponse for neoadjuvant chemotherapy, the 5-year survival rate for NSCLC over all stages is still very low. Thus, in order to improve therapeutic efficacy, it is a current emergency to better know the mechanisms underlying cisplatin resistance in lung cancer cells.
Krüppel-like factor 4 (KLF4), a member of the Sp1/KLF family, functions as a regulator in diverse cell processes of cell proliferation and differentiation (8). It seems that KLF4 might act as a tumor suppressor, as it is an antiproliferative factor in differentiated epithelia (9). KLF4 is able to inhibit both EMT and invasion. Loss of KLF4 function induces EMT-like morphological changes, while forced expression of KLF4 is sufficient to restore E-cadherin expression and suppress migration and invasion in breast cancer cells (10). On the other hand, KLF4 also seems to act as an oncogene in head and neck cancer and pancreatic cancer (11,12). This indicates that KLF4 can be a tumor suppressor or an oncogene depending on tumor type. However, whether KLF4 plays a role in cisplatin resistance of lung cancer remains unknown.
In this study, we aim to investigate the role of KLF4 in cisplatin-resistant lung cancer cells. We developed cisplatin-resistant lung cancer cell line A549/DDP, and then a battery of experiments was used to analyze the effects of KLF4 in cisplatin resistance of lung cancer.
MATERIALS AND METHODS
Cell Culture
Human lung cancer cell line A549 was obtained from ATCC. Cisplatin (DDP)-resistant A549 (A549/DDP) (single clone) cells were developed from A549 cells by treatment with gradually increasing concentrations of DDP in cell culture medium. All the cells were cultured in DMEM medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 unit/ml penicillin/streptomycin (Invitrogen) at 37°C in a humidified 5% CO2 incubator.
Quantitative PCR (qPCR) Analysis
TRIzol reagent (Invitrogen) was used to extract total RNA from the indicated cells according to the manufacturer’s instructions. SYBR Green qRCR Mix (TOYOBO, Japan) was used for real-time PCR to detect mRNA levels. The primers are as follows: KLF4, sense, GCCGCTCCATTACCAAGAG and antisense, ATCCACAGCCGTCCCAGTC; β-actin, sense, AGGGGCCGGACTCGTCATACT and antisense, GGCGGCACCACCATGTACCCT. The expression of target genes was normalized by β-actin, and 2−ΔΔCT method was used to process the data.
Western Blot Analysis
The protein was extracted by RIPA lysis buffer (Auragene, Changsha, China) from indicated cells, and Bradford Protein Assay Kit (Beyotime Biotechnology, Shanghai, China) was used to measure the protein concentration. After being separated on 10% SDS-PAGE gels, the protein was transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk and incubated with primary antibodies [rabbit polyclonal anti-KLF4 (Cat. No. ab72543; dilution: 1:500), rabbit polyclonal anti-Slug (Cat. No. ab27568; dilution: 1:500), rabbit polyclonal anti-Twist1 (Cat. No. ab49254; dilution: 1:1,000), mouse monoclonal anti-vimentin (Cat. No. ab8978; dilution: 1:1,000), and rabbit monoclonal anti-E-cadherin (Cat. No. ab133597; dilution: 1:500) from Abcam, Cambridge, UK; mouse monoclonal anti-GAPDH (1:3,000) from Sigma- Aldrich, St. Louis, MO, USA] overnight at 4°C. The membranes were washed with TBST and then incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibody. Enhanced chemiluminescence (ECL) reagent was used to detect the signal on the membrane.
MTT Assay
Cell growth and inhibition of viability were measured by MTT assay. Three thousand indicated cells were seeded in each 96-well plate for 12 h and further incubated for 0 h and 24 h. One hour before the ending of incubation, 100 µl of MTT at 5 mg/ml final concentration was added to each well for 4 h at 37°C. Then the supernatant was removed, and 150 µl of DMSO was added to each well for 15 min. After that, OD 570 nm value in each well was determined by an enzyme immunoassay analyzer.
Flow Cytometric Analysis of Apoptosis
Cells from each group were trypsinizated and washed with cold PBS, and Annexin V-FITC-PI (Jiancheng, Nanjing, China) then was used to stain the apoptosis cells according to the manufacturer’s instructions. The apoptosis rate was analyzed by flow cytometry (BD). The experiments were independently performed in triplicate.
Scratch Assay
Cells in each group were collected and resuspended in DMEM medium. Each well of a six-well plate was seeded with 1 × 105 cells and cultured for 36 h to 90–100% confluence. The cells were scratched with the head of a 200-µl tip and washed with serum-free medium. These cells were further cultured for 0, 24, or 48 h in DMEM medium containing 3% FBS, and these cells in each group were photographed, and the width of the gap was calculated.
Transwell Assay
The indicated cells were starved for 24 h and then resuspended in serum-free medium and added to the upper chamber of the Transwell. The lower chamber was filled with completed medium containing 10% FBS. Following 48 h of culture, cells attached to the bottom were fixed and stained with crystal violet for 30 min.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 software (Graphpad Software, Inc., La Jolla, CA, USA), and the data are presented as the mean ± standard deviation. An unpaired two-tailed Student’s t-test was used to analyze the data. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
KLF4 Was Significantly Decreased in DDP-Resistant A549 Cells
To investigate the mechanism underlying chemotherapy resistance in lung cancer cells, we developed DDP-resistant A549 cells by treatment with gradually increasing concentrations of DDP. Our data indicated that the cell viability assay showed that A549/DDP cells could tolerate much higher concentrations of DDP compared with A549 cells, with their IC50 concentrations found to be more than eightfold higher than those of parental A549 cells (17 vs. 1.9 µg/ml) (Fig. 1A). We then analyzed the expression of KLF4 in A549 and A549/DDP cells and found that the mRNA levels of KLF4 were significantly downregulated in A549/DDP cells compared with parental A549 cells (Fig. 1B). These results indicate that KLF4 may play an important role in the development of drug resistance in lung cancer cells.
Figure 1.
The expression of KLF4 in A549/DDP cells. (A) MTT assay was used to calculate the IC50 values in DDP-resistant A549 cells and its parental A549 cells. (B) qPCR analysis for KLF4. Data are presented as means ± SD. ***p < 0.001.
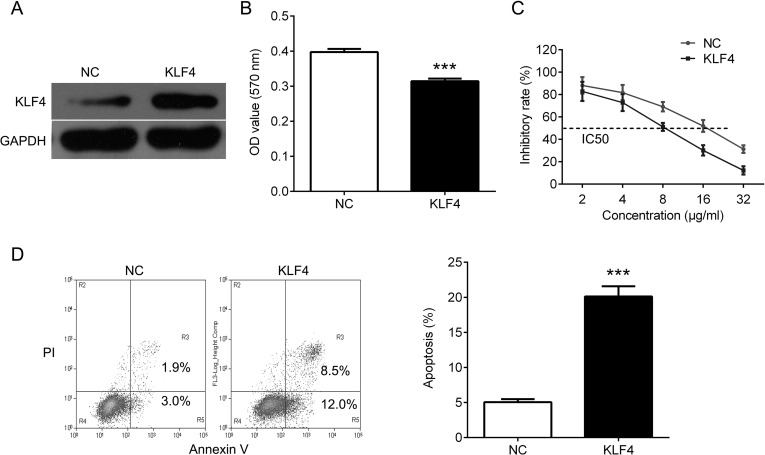
Upregulation of KLF4 Inhibits Cell Proliferation and Induces Apoptosis
To further investigate the role of KLF4 in DDP resistance, we overexpressed KLF4 in A549/DDP cells (Fig. 2A). We found that overexpression of KLF4 significantly inhibited cell proliferation, and DDP sensitivities were significantly increased after forced overexpression of KLF4, as the IC50 values were decreased in A549/DDP cells (Fig. 2B, C). In addition, upregulation of KLF4 significantly increased apoptosis (Fig. 2D).
Figure 2.
Overexpression of KLF4 inhibits cell proliferation and induces apoptosis in A549/DDP cells. (A) Western blot analysis for KLF4 in A549/DDP cells after transfection with KLF4. (B) MTT assay was used to measure cell proliferation in A549/DDP cells after transfection with KLF4. (C) The impact of KLF4 on drug sensitivity at different DDP doses (2, 4, 8, 16, 32 µg/ml) was determined by MTT assay. (D) Flow cytometry analysis was used to measured cell apoptosis in A549/DDP cells after transfection with KLF4. Data are presented as means ± SD. ***p < 0.001.
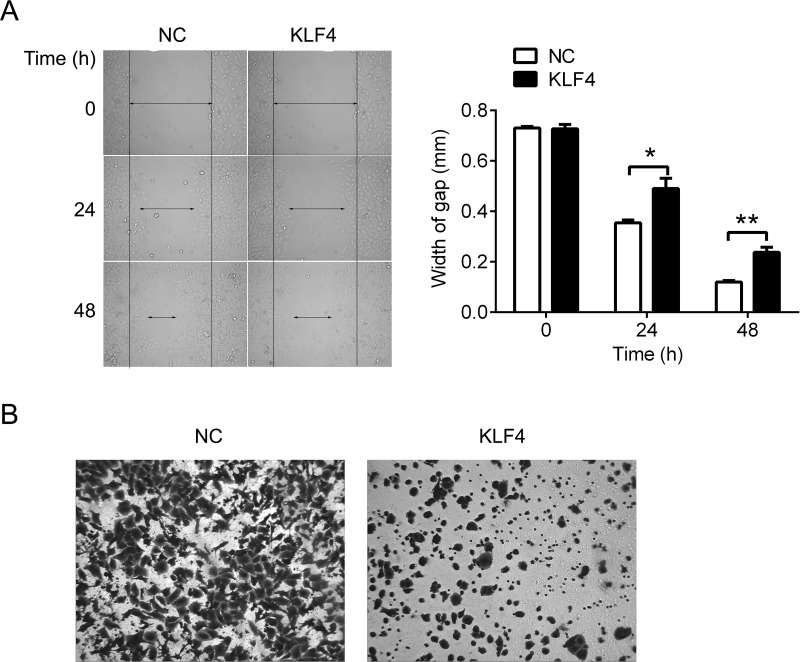
Upregulation of KLF4 Represses the Ability of Migration and Invasion in A549/DPP Cells
Furthermore, we assessed the role of KLF4 in cell migration and invasion. Scratch assay was used to evaluate the ability of migration. Upregulation of KLF4 significantly increased the width of gap compared with the negative control, indicating that KLF4 was able to inhibit the movement of A549/DDP cells (Fig. 3A). Upregulation of KLF4 significantly reduced invasion ability compared with the negative control in A549/DDP cells (Fig. 3B). Thus, KLF4 is able to repress the ability of migration and invasion in A549/DPP cells.
Figure 3.
Overexpression of KLF4 represses the ability of migration and invasion of A549/DDP cells. (A) Scratch assay was used to measure cell migration in A549/DDP cells after transfection with KLF4. (B) Transwell assay was used to analyze cell invasion in A549/DDP cells after transfection with KLF4. Data are presented as means ± SD. *p < 0.05, **p < 0.01.
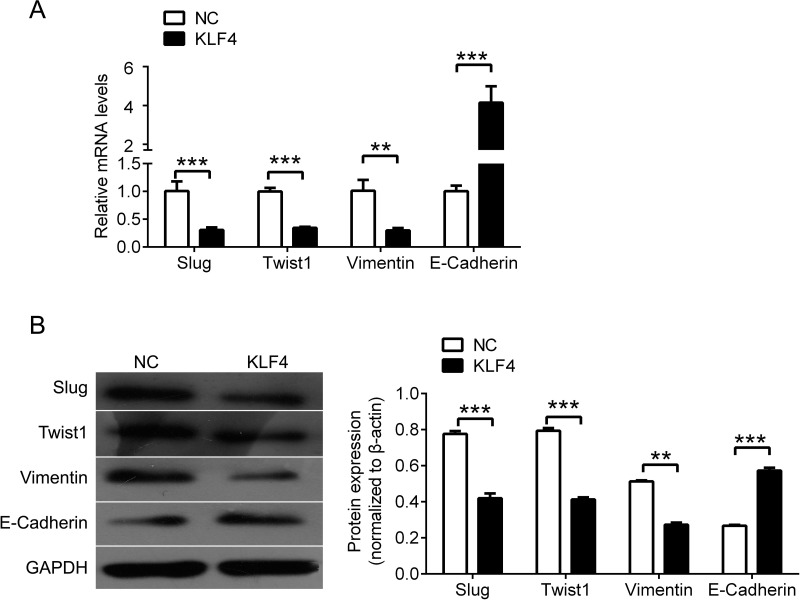
KLF4 Regulates the Expression of EMT-Related Genes
Furthermore, we tested the expression of EMT-related genes, including Slug, Twist1, vimentin, and E-cadherin by qPCR (Fig. 4A) and Western blot (Fig. 4B) in A549/DPP cells after KLF4 overexpression. A significant decrease in Slug, Twist1, and vimentin was observed in A549 cells with upregulated KLF4, while E-cadherin expression was significantly increased by KLF4 overexpression.
Figure 4.
KLF4 regulates EMT-related genes. (A) qPCR analysis for Slug, Twist, vimentin, and E-cadherin in A549/DDP cells after transfection with KLF4. (B) Western blot analysis for Slug, Twist, vimentin, and E-cadherin in A549/DDP cells after transfection with KLF4, and quantification. Data are presented as means ± SD. **p < 0.01, ***p < 0.001.
DISCUSSION
In this study, we found that KLF4 was significantly downregulated in cisplatin-resistant A549 cells and forced KLF4 expression inhibited cell growth and induced apoptosis. It is conceivable that KLF4 is able to enhance the sensitivity of cisplatin to lung cancer cells. KLF4 is a transcription factor expressed in a wide variety of tissues in humans and is important in many different physiologic processes, including development, proliferation, differentiation, and apoptosis (13). KLF4 can either activate or repress transcription depending on the target gene by utilizing different mechanisms (14). In addition, KLF4 can function as an oncogene or a tumor suppressor depending on the type of cancer (15). It was supported that KLF4 functioned as a tumor suppressor in the human and mouse intestinal epithelium at an early stage (16). KLF4 is downregulated in adenomas from mice with expression inversely related to the size of the tumor (17). In human colon cancer cell lines, several point mutations have been found in the KLF4 gene, and loss of KLF4 may promote centrosome amplification and chromosomal instability after γ irradiation (18). KLF4 is also downregulated in gastric cancer, which may result in precancerous changes in the stomach (19). In addition to gastric and colorectal cancer, KLF4 is downregulated in esophageal cancer (20), bladder cancer (21), leukemia (22), and non-small cell lung carcinoma (23). Thus, taken together with our results, the evidence strongly supports the hypothesis that KLF4 functions as a tumor suppressor in lung cancer.
Further, we found that overexpression of KLF4 was able to inhibit cell migration and invasion, to inhibit the expression of Slug, Twist, and vimentin, and to increase the expression of E-cadherin. Epithelial-to-mesenchymal transition (EMT) is a process characterized by epithelial cells losing cell–cell adhesions and developing a fibroblastoid motile phenotype (24). EMT was found to be strongly associated with tumor cell proliferation, invasion, metastasis, and chemotherapy resistance (25). Suppression of E-cadherin makes cells lose cell–cell contacts and detach from each other, while induction of mesenchymal markers, such as N-cadherin and vimentin, makes cells acquire the ability to migrate and invade the extracellular matrices (26). E-cadherin is frequently lost in cancer cells, resulting in disruption of the cell–cell contacts, which is associated with the metastasis and escape from chemotherapy in many cancers, including lung cancer (27). E-cadherin can be transcriptionally repressed by a number of factors, such as Slug and Twist (28). Recently, KLF4 also seems to function as a transcriptional activator of epithelial genes and as a suppressor of mesenchymal genes during the process of EMT (29). Forced KLF4 expression repressed mesenchymal characteristics and then restrained cell migration and invasion activities, whereas downregulation of KLF4 enhanced mesenchymal features and cell migration (30). It was demonstrated that KLF4 was able to bind and suppress the activity of the Slug promoter (31). KLF4 is able to inhibit EMT by reducing the expression of Snail in a mouse model of breast cancer (32). In addition, KLF4 binds to the E-cadherin promoter in a region overlapping with a known ZEB2 binding site to enhance the activity of E-cadherin promoter (33). The direct transcriptional targets of KLF4 also included vimentin, β-catenin, and VEGF-A (33). EMT is also regulated by other factors, and it is a product of cross talk of signaling pathways. Thus, it is conceivable that KLF4 inhibits the ability of invasion and the escape from response to chemotherapy by repressing the EMT process, and overexpression of KLF4 may be a potential strategy for lung cancer treatment, especially for cisplatin-resistant cases.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Rose M. C.; Kostyanovskaya E.; Huang R. S. Pharmacogenomics of cisplatin sensitivity in non-small cell lung cancer. Genomics Proteomics Bioinformatics 12:198–209; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giovannetti E.; Toffalorio F.; De Pas T.; Peters G. J. Pharmacogenetics of conventional chemotherapy in non-small-cell lung cancer: A changing landscape? Pharmacogenomics 13:1073–1086; 2012. [DOI] [PubMed] [Google Scholar]
- 3. Murphy M.; Stordal B. Erlotinib or gefitinib for the treatment of relapsed platinum pretreated non-small cell lung cancer and ovarian cancer: A systematic review. Drug Resist. Updat. 14:177–190; 2011. [DOI] [PubMed] [Google Scholar]
- 4. Duan S.; Tsai Y.; Keng P.; Chen Y.; Lee S. O.; Chen Y. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget 6:27651–27660; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Z. H.; Qiu M. Z.; Zeng Z. L.; Luo H. Y.; Wu W. J.; Wang F.; Wang Z. Q.; Zhang D. S.; Li Y. H.; Xu R. H. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC). J. Transl. Med. 10:21; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagar R.; Khan A. R.; Poonia A.; Mishra P. K.; Singh S. Metabolism of cisplatin in the organs of Rattus norvegicus: Role of glutathione S-transferase P1. Eur. J. Drug Metab. Pharmacokinet. 40:45–51; 2015. [DOI] [PubMed] [Google Scholar]
- 7. Scarpace S. L. Metastatic squamous cell non-small-cell lung cancer (NSCLC): Disrupting the drug treatment paradigm with immunotherapies. Drugs Context 4:212289; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaira V.; Faversani A.; Martin N. M.; Garlick D. S.; Ferrero S.; Nosotti M.; Kissil J. L.; Bosari S.; Altieri D. C. Regulation of lung cancer metastasis by Klf4-Numb-like signaling. Cancer Res. 73:2695–2705; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris V. A.; Cummings C. L.; Korb B.; Boaglio S.; Oehler V. G. Deregulated KLF4 expression in myeloid leukemias alters cell proliferation and differentiation through microRNA and gene targets. Mol. Cell. Biol. 36:559–573; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H. K.; Lee D. S.; Park J. C. Nuclear factor I-C regulates E-cadherin via control of KLF4 in breast cancer. BMC Cancer 15:113; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrigo M.; Alvarez R.; Paparella M. L.; Calb D. E.; Bal D. K. J. E.; Gutkind J. S.; Raimondi A. R. Impairing squamous differentiation by Klf4 deletion is sufficient to initiate tongue carcinoma development upon K-Ras activation in mice. Carcinogenesis 35:662–669; 2014. [DOI] [PubMed] [Google Scholar]
- 12. Shi M.; Cui J.; Du J; Wei D.; Jia Z.; Zhang J.; Zhu Z.; Gao Y.; Xie K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin. Cancer Res. 20:4370–4380; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rowland B. D.; Peeper D. S. KLF4, p21 and context-dependent opposing forces in cancer. Nat. Rev. Cancer 6:11–23; 2006. [DOI] [PubMed] [Google Scholar]
- 14. Evans P. M.; Liu C. Roles of Krupel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim. Biophys. Sin. (Shanghai) 40:554–564; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghaleb A. M.; Nandan M. O.; Chanchevalap S.; Dalton W. B.; Hisamuddin I. M.; Yang V. W. Kruppel-like factors 4 and 5: The yin and yang regulators of cellular proliferation. Cell Res. 15:92–96; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei D.; Kanai M.; Huang S.; Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis 27:23–31; 2006. [DOI] [PubMed] [Google Scholar]
- 17. Dang D. T.; Bachman K. E.; Mahatan C. S.; Dang L. H.; Giardiello F. M.; Yang V. W. Decreased expression of the gut-enriched Kruppel-like factor gene in intestinal adenomas of multiple intestinal neoplasia mice and in colonic adenomas of familial adenomatous polyposis patients. FEBS Lett. 476:203–207; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoon H. S.; Ghaleb A. M.; Nandan M. O.; Hisamuddin I. M.; Dalton W. B.; Yang V. W. Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene 24:4017–4025; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang N.; Zhang J.; Shuai L.; Zha L.; He M.; Huang Z.; Wang Z. Kruppel-like factor 4 negatively regulates beta-catenin expression and inhibits the proliferation, invasion and metastasis of gastric cancer. Int. J. Oncol. 40:2038–2048; 2012. [DOI] [PubMed] [Google Scholar]
- 20. He H.; Li S.; Hong Y.; Zou H.; Chen H.; Ding F.; Wan Y.; Liu Z. Kruppel-like factor 4 promotes esophageal squamous cell carcinoma differentiation by up-regulating keratin 13 expression. J. Biol. Chem. 290:13567–13577; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohnishi S.; Ohnami S.; Laub F.; Aoki K.; Suzuki K.; Kanai Y.; Haga K.; Asaka M.; Ramirez F.; Yoshida T. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem. Biophys. Res. Commun. 308:251–256; 2003. [DOI] [PubMed] [Google Scholar]
- 22. Li W.; Jiang Z.; Li T.; Wei X.; Zheng Y.; Wu D.; Yang L.; Chen S.; Xu B.; Zhong M.; Jiang J.; Hu Y.; Su H.; Zhang M.; Huang X.; Geng S.; Weng J.; Du X; Liu P.; Li Y.; Liu H.; Yao Y.; Li P. Genome-wide analyses identify KLF4 as an important negative regulator in T-cell acute lymphoblastic leukemia through directly inhibiting T-cell associated genes. Mol. Cancer 14:26; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu W.; Hofstetter W. L.; Li H.; Zhou Y.; He Y.; Pataer A.; Wang L.; Xie K.; Swisher S. G.; Fang B. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 15:5688–5695; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muir K. R.; Lima M. J.; Docherty H. M.; McGowan N. W.; Forbes S.; Heremans Y.; Forbes S. J.; Heimberg H.; Casey J.; Docherty K. Kruppel-like factor 4 overexpression initiates a mesenchymal-to-epithelial transition and redifferentiation of human pancreatic cells following expansion in long term adherent culture. PLoS One 10:e140352; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H. F.; Huang C. H.; Liu C. J.; Hung J. J.; Hsu C. C.; Teng S. C.; Wu K. J. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat. Commun. 5:4697; 2014. [DOI] [PubMed] [Google Scholar]
- 26. Akalay I.; Tan T. Z.; Kumar P.; Janji B.; Mami-Chouaib F.; Charpy C.; Vielh P.; Larsen A. K.; Thiery J. P.; Sabbah M.; Chouaib S. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene 34:2261–2271; 2015. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka K.; Kumano K.; Ueno H. Intracellular signals of lung cancer cells as possible therapeutic targets. Cancer Sci. 106:489–496; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galvan J. A.; Astudillo A.; Vallina A.; Crespo G.; Folgueras M. V.; Gonzalez M. V. Prognostic and diagnostic value of epithelial to mesenchymal transition markers in pulmonary neuroendocrine tumors. BMC Cancer 14:855; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z.; Wang Y.; Liu W.; Zhao G.; Lee S.; Balogh A.; Zou Y.; Guo Y.; Zhang Z.; Gu W.; Li C.; Tigyi G.; Yue J. Doxycycline inducible Kruppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One 9:e105331; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui J.; Shi M.; Quan M.; Xie K. Regulation of EMT by KLF4 in gastrointestinal cancer. Curr. Cancer Drug Targets 13:986–995; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Z. S.; Chu H. C.; Yen Y. C.; Lewis B. C.; Chen Y. W. Kruppel-like factor 4, a tumor suppressor in hepatocellular carcinoma cells reverts epithelial mesenchymal transition by suppressing slug expression. PLoS One 7:e43593; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yori J. L.; Seachrist D. D.; Johnson E.; Lozada K. L.; Abdul-Karim F. W.; Chodosh L. A.; Schiemann W. P.; Keri R. A. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia 13:601–610; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tiwari N.; Meyer-Schaller N.; Arnold P.; Antoniadis H.; Pachkov M.; van Nimwegen E.; Christofori G. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8). PLoS One 8:e57329; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]