Abstract
Tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (TNFAIP8L2, TIPE2) is involved in the invasion and metastasis of human tumors. However, the functional role of TIPE2 in prostate cancer remains unclear. In the present study, we explored the role of TIPE2 in prostate cancer and cancer progression including the molecular mechanism that drives TIPE2-mediated oncogenesis. Our results showed that TIPE2 was lowly expressed in human prostate cancer tissues and cell lines. In addition, restored TIPE2 obviously inhibits proliferation in prostate cancer cells. TIPE2 overexpression also suppresses the epithelial–mesenchymal transition (EMT) process and migration/invasion in prostate cancer cells. Mechanistically, TIPE2 overexpression obviously inhibits the phosphorylation levels of phosphatidylinositol 3-kinase (PI3K) and Akt in prostate cancer cells. In conclusion, for the first time we demonstrated that TIPE2 overexpression may suppress proliferation, migration, and invasion in prostate cancer cells by inhibiting the PI3K/Akt signaling pathway. Therefore, TIPE2 might serve as a potential therapeutic target for human prostate cancer.
Key words: Tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (TNFAIP8L2, TIPE2); Prostate cancer; Epithelial–mesenchymal transition (EMT); PI3K/Akt signaling
INTRODUCTION
Prostate cancer is the most common malignancy and the second leading cause of cancer-related death in males (1). Despite advances in surgery and new chemotherapy regimens over the last few decades, the prognosis for patients with advanced prostate cancer remains poor (2–4). This high mortality rate is attributed to the aggressive migration and invasion of prostate cancer cells (5). Thus, a better understanding of molecular mechanisms and identification of tumor suppressors is essential for the development of diagnostic markers that aid novel therapeutic strategies for prostate cancer.
Tumor necrosis factor-α (TNF-α)-induced protein 8 (TNFAIP8) family, a new subfamily of death effector domain that contains proteins, consists of TNFAIP8, TIPE1, TIPE2, and TIPE3 (6). TIPE2 was originally identified as an inflammation-related gene (7); human TIPE2 shares approximately 53% identity and 78% similarity amino acid sequence with TNFAIP8 (8). In addition, TIPE2 plays an important role in maintaining immune homeostasis by negatively regulating T-cell receptor and Toll-like receptor (TLR) signaling (8). Recently, several studies have demonstrated that TIPE2 was involved in the development of cancers (9–11). One study showed that the expression level of TIPE2 was significantly decreased in lung cancer cell lines, and TIPE2 overexpression inhibited lung cancer cell proliferation, colony formation, and cell invasion in vitro (12). Zhang et al. reported that TIPE2 can inhibit TNF-α-induced hepatocellular carcinoma cell metastasis via ERK1/2 downregulation and NF-κB activation (13). However, the functional role of TIPE2 in prostate cancer remains unclear. In the present study, we explored the role of TIPE2 in prostate cancer and cancer progression including the molecular mechanism that drives TIPE2-mediated oncogenesis.
MATERIALS AND METHODS
Human Tissue Specimens
Tissue samples were collected from healthy individuals and patients with prostate cancer admitted to the Department of Urology, Hunan Provincial People’s Hospital (China). All tissues were classified according to the WHO criteria and staged according to the tumor–node–metastasis classification. Written informed consent was obtained from all patients, and the study was approved by the Institute Research Ethics Committee of Hunan Provincial People’s Hospital (China).
Cell Culture
Human prostate cancer cell lines (LNCaP, DU-145, and PC-3) and the normal prostate cell line (RWPE-1) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 µg/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Cells were seeded in cell culture plates and left to grow in a humidified incubator at 37°C, 5% CO2 atmosphere.
Construction of TIPE2-Overexpressing Cell Lines
Full-length human TIPE2 was generated from human peripheral blood mononuclear cell (PBMC) cDNA by polymerase chain reaction (PCR) and cloned into pcDNA3.1 vector. Twenty-four hours prior to transfection, cells were plated onto a 96-well plate (Invitrogen) at 40–60% confluence. Then the recombinant plasmid pcDNA3.1-TIPE2 and mock plasmid were transferred into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. The transfection efficiency was confirmed by Western blot.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from tissue samples or cells with TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized using the Reverse Transcription System (Promega, Madison, WI, USA). qRT-PCR was carried out in an Applied Biosystems 7500 System with Power SYBR Green PCR Master Mix (Applied Biosystems). The specific primers for TIPE2 were 5′-TCAGAAACATCCAAGGCCAGAC-3′ (sense) and 5′-CGGACCGACCAGCCATTTTAC-3′ (antisense), and for β-actin were 5′-GATCATTGCTCCTCCTGAGC-3′ (sense) and 5′-ACTCCTGCTTGCTGATCCAC-3′ (antisense). Amplification cycles were 94°C for 5 min, then 40 cycles at 94°C for 1 min, 59°C for 1 min, and 72°C for 1.5 min, followed by 72°C for 15 min. The products were analyzed on a 1.5% agarose gel containing 0.2 µg/ml ethidium bromide and visualized under an ultraviolet transilluminator. The relative expression levels were calculated by the 2−ΔΔCt method, and the target gene was normalized to the internal reference gene.
Western Blotting
Total protein was extracted using lysis buffer containing 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% TX-100, 1 mmol/L EDTA, pH 8.0, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). Protein concentration was assayed using a Micro BCA protein kit (Pierce, Rockford, IL, USA). Equal amounts of protein sample (30 µg) were separated by 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes (Millipore, Boston, MA, USA). Membranes were blocked with 5% defatted milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 for 1 h at room temperature. The membrane was then blocked with 5% nonfat dry milk for 1 h. Primary antibodies including anti-TIPE2, anti-E-cadherin, anti-vimentin, anti-N-cadherin, anti-Snail, anti-phosphatidylinositol 3-kinase (PI3K), anti-p-PI3K, p-Akt, p-p-Akt, and anti-GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). These antibodies were added and incubated overnight at 4°C. Protein bands were visualized using horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) followed by chemiluminescence (ECL; Amersham Biosciences).
Cell Proliferation Assay
Cell proliferation was measured using the MTT assay. In brief, cells at a density of 1 × 105 cells/well were incubated with pcDNA3.1-TIPE2 or mock for 24, 48, or 72 h. Then 20 µl of MTT (5 mg/ml; Sigma-Aldrich) was added to the cells and incubated for 4 h. Subsequently, the supernatant was discarded, and 200 µl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added to dissolve formazan production. The absorbance was read at 490 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Abcam, Cambridge, UK). All experiments were performed in duplicate.
Cell Migration and Invasion Assays
Cell migration was measured using sterile 6.5-mm Transwell with 8.0-µm pore polycarbonate membrane insert (Corning, Cambridge, MA, USA). In brief, cells transfected with TIPE2 were added to the top chamber of the Transwell, and the medium including 10% fetal bovine serum (FBS) was added into the lower compartment. After 24 h of incubation, nonmigrated cells were removed from the upper chamber using a cotton bud while cells that had migrated were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution for 15 min. Cells were enumerated by counting five random fields per Transwell.
For the invasion assay, the same procedures described above were used, except that the filters were precoated with Matrigel (BD Biosciences, San Jose, CA, USA) at a 1:4 dilution in DMEM to form a genuine reconstituted basement membrane.
Statistical Analysis
The values were expressed as mean ± SD. Statistical analysis involved use of the Student’s t-test for the comparison of two groups or one-way analysis of variance (ANOVA) for multiple comparisons. A value of p < 0.05 was considered statistically significant.
RESULTS
TIPE2 Was Lowly Expressed in Prostate Cancer Tissues and Cell Lines
We detected the TIPE2 mRNA and protein levels in primary prostate cancer tissues and normal prostate tissues using qRT-PCR and Western blot, respectively. The results indicated that both mRNA (Fig. 1A) and protein levels (Fig. 1B) were significantly lower in prostate cancer tissues compared with that in normal prostate tissues. Similarly, we observed that TIPE2 expression at both mRNA (Fig. 1C) and protein (Fig. 1D) was lower in the prostate cancer cell lines (LNCaP, DU-145, and PC-3) than in the normal prostate cell line (RWPE-1).
Figure 1.
TIPE2 was lowly expressed in prostate cancer tissues and cell lines. The TIPE2 mRNA and protein levels were detected by quantitative real-time polymerase chain reaction (qRT-PCR) (A) and Western blot assays (B) in primary prostate cancer tissues and normal prostate tissues. *p < 0.05 versus normal tissues. The TIPE2 mRNA and protein levels were detected by qRT-PCR (C) and Western blot assays (D) in human prostate cancer cell lines (LNCaP, DU-145, and PC-3) and the normal prostate cell line (RWPE-1). Data are mean ± SD values from three experiments, each performed in triplicate. *p < 0.05 versus RWPE-1 cells.
TIPE2 Inhibits the Proliferation in Prostate Cancer Cells
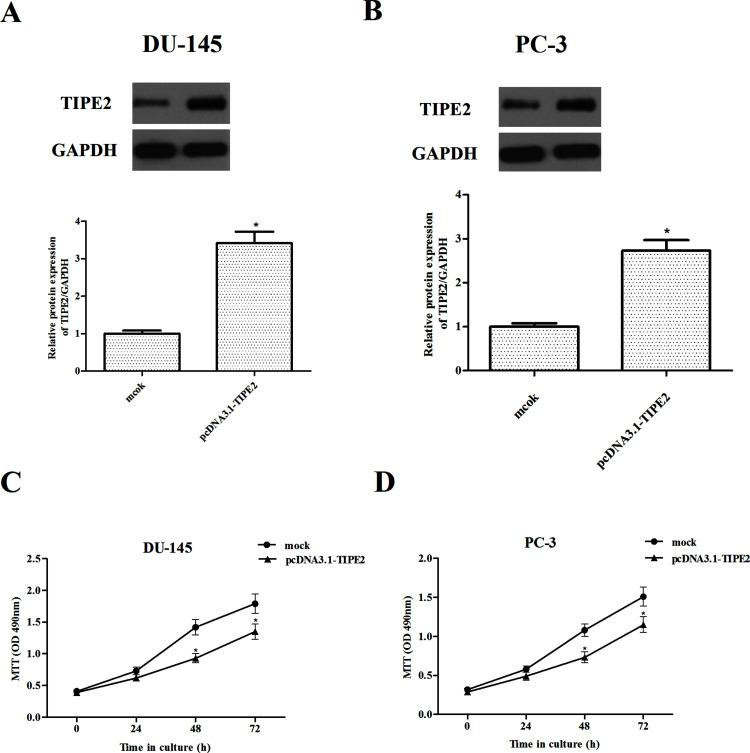
Because of the lower expression of TIPE2, the DU-145 and PC-3 cell lines were chosen for overexpressing the KIF3C gene. Elevated levels of TIPE2 in the DU-145 (Fig. 2A) and PC-3 cells (Fig. 2B) were confirmed by Western blot analysis, respectively. Then the impact of LRG1 overexpression on proliferation of prostate cancer cells was evaluated by the MTT assay. The results showed that overexpression of TIPE2 remarkably inhibited the proliferation in DU-145 (Fig. 2C) and PC-3 cells (Fig. 2D), respectively, as compared with the mock group.
Figure 2.
TIPE2 inhibits the proliferation in prostate cancer cells. (A, B) Western blot analysis of TIPE2 expression in stably overexpressed (TIPE2) and control (mock) cells (DU-145 and PC-3 cell lines). Cell proliferation after TIPE2 overexpression in DU-145 (C) and PC-3 cells (D) was measured using MTT assays. Data are mean ± SD values from three experiments, each performed in triplicate. Compared with vector control cells, *p < 0.05.
TIPE2 Suppresses Epithelial–Mesenchymal Transition (EMT) Process in Prostate Cancer Cells
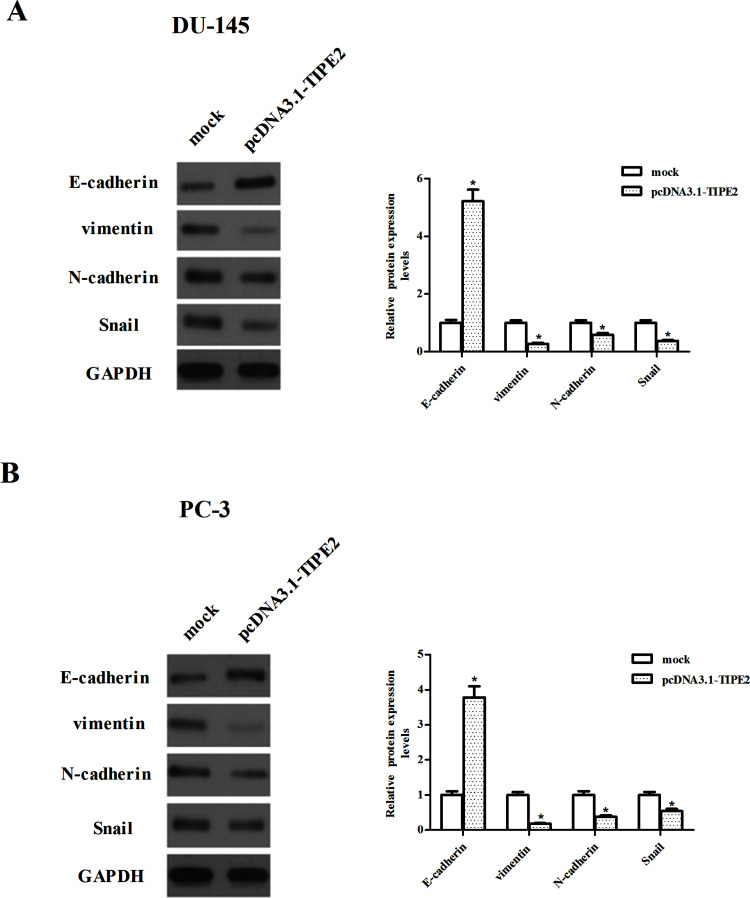
In order to examine the effect of TIPE2 on the EMT progression of prostate cancer cells, we identified the expression of EMT markers by Western blot. As indicated in Figure 3A, overexpression of TIPE2 resulted in the increased expression of epithelial marker and E-cadherin, and the reduced expression of various mesenchymal markers, namely, vimentin, N-cadherin, and Snail in DU-145 cells. Similar results were observed in the PC-3 cells (Fig. 3B).
Figure 3.
TIPE2 suppresses the epithelial–mesenchymal transition (EMT) process in prostate cancer cells. DU-145 (A) and PC-3 (B) cells were transfected with pcDNA3.1-TIPE2 or mock for 24 h. The expression of E-cadherin, vimentin, N-cadherin, and Snail proteins was analyzed via Western blotting. GAPDH served as a loading control. Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. Data are mean ± SD values from three experiments, each performed in triplicate. Compared with the mock group, *p < 0.05.
TIPE2 Inhibits the Migration and Invasion in Prostate Cancer Cells
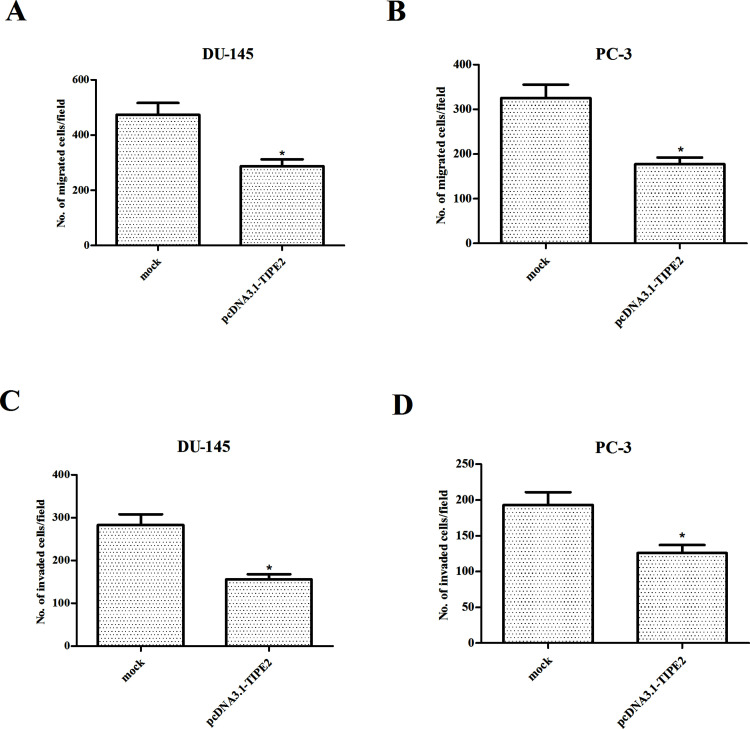
In order to investigate the role of TIPE2 in the migration and invasiveness of prostate cancer cells, we performed Transwell migration assay and Boyden chamber invasion assay. Transwell migration assay showed a significantly lower number of migrating cells in TIPE2-overexpressing DU-145 cells (Fig. 4A) or PC-3 cells (Fig. 4B), as compared with the mock group, respectively. In addition, TIPE2 overexpression resulted in less cell invasion of DU-145 cells (Fig. 4C) or PC-3 cells (Fig. 4D) compared with control cells.
Figure 4.
TIPE2 inhibits the migration and invasion in prostate cancer cells. DU-145 and PC-3 cells were transfected with pcDNA3.1-TIPE2 or mock for 24 h. (A, B) The migration of the indicated cells was evaluated by a Transwell assay. (C, D) The invasiveness of the indicated cells was evaluated by a Matrigel-coated Transwell assay. Data are mean ± SD values from three experiments, each performed in triplicate. Compared with the mock group, *p < 0.05.
TIPE2 Inhibits the PI3K/Akt Signaling Pathway in Prostate Cancer Cells
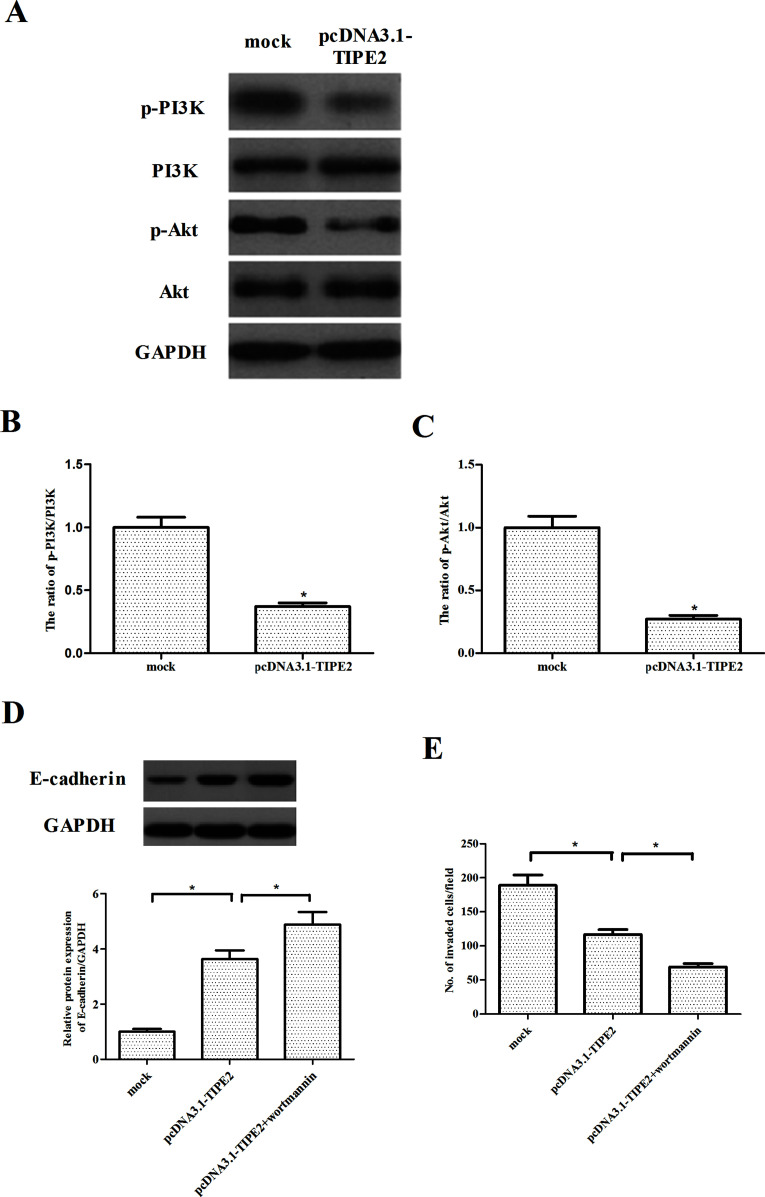
PI3K/Akt plays a critical role in tumorigenesis by regulating cell proliferation, EMT, and cell migration/invasion (14,15). To investigate the molecular mechanism for TIPE2-regulated cell migration and invasiveness, we assessed the activation of PI3K/Akt signaling pathway in prostate cancer cells. The results showed that phosphorylation levels of PI3K and Akt were reduced in TIPE2-overexpressing cells, while no change was found in total PI3K and Akt (Fig. 5A). Quantification analysis of p-PI3K/PI3K and p-Akt/Akt is shown in Figure 5B and C, respectively. Furthermore, we examined the effects of Akt inhibitor (wortmannin) on TIPE2-mediated EMT progression and cell proliferation in PC-3 cells. As shown in Figure 5D, wortmannin (100 nM) significantly increased the expression of E-cadherin in TIPE2-overexpressing PC-3 cells. The Transwell invasion assay indicated that wortmannin dramatically enhanced the inhibitory effect of TIPE2 on cell invasion in PC-3 cells (Fig. 5E).
Figure 5.
TIPE2 inhibits the PI3K/Akt signaling pathway in prostate cancer cells. PC-3 cells were transfected with pcDNA3.1-TIPE2 or mock for 24 h. (A) The expression of p-PI3K, PI3K, p-Akt, and Akt proteins was detected by Western blotting. GAPDH served as a loading control. (B, C) Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. (D) PC-3 cells were transfected with pcDNA3.1-TIPE2 or mock in the presence or absence of wortmannin (100 nM) for 24 h. The expression of E-cadherin was analyzed via Western blotting. (E) Cell invasion was evaluated by the Transwell invasion chamber assay. Data are mean ± SD values from three experiments, each performed in triplicate. Compared with the mock group, *p < 0.05.
DISCUSSION
In this study, we found that TIPE2 was lowly expressed in human prostate cancer tissues and cell lines. In addition, restored TIPE2 obviously inhibits proliferation in prostate cancer cells. TIPE2 overexpression also suppresses the EMT process and migration/invasion in prostate cancer cells. Mechanistically, TIPE2 overexpression obviously inhibits the phosphorylation levels of PI3K and Akt in prostate cancer cells.
Previous studies revealed that TIPE2 contributes significantly to tumor growth and progression. In one study, Zhao et al. found that TIPE2 expression was reduced in gastric cancer, and restoration of TIPE2 expression in gastric cells significantly suppressed cell proliferation. In another study, Liu et al. demonstrated that TIPE2 expression was lost in small cell lung cancer; overexpression of TIPE2 significantly inhibited the growth of lung cancer cell H446 in vitro (16). In this study, we found that the expression levels of TIPE2 at mRNA and protein were lowly expressed in human prostate cancer tissues and cell lines. Restored TIPE2 obviously inhibits proliferation in prostate cancer cells. These data suggest that TIPE2 may be a potential suppressor in the development of prostate cancer.
EMT is a process by which epithelial cells acquire a mesenchymal cell phenotype and has been involved in increased cell invasion and the metastatic potential of prostate cancer cells (17). Reduction or a loss of E-cadherin expression has a crucial role in the progression of tumors to invasive cancer and is also one of the well-established hallmarks of EMT (18). Moreover, it has been reported that forced expression of TIPE2 markedly suppressed the gastric cancer cell migration and invasion in vitro (19). TIPE2 overexpression also inhibited the migration and invasion in vitro and suppressed growth and metastasis of hepatocellular carcinoma in vivo (11). Consistent with these results, in this study we found that TIPE2 overexpression significantly increased expression of epithelial marker E-cadherin, but decreased the expression of mesenchymal marker vimentin. In addition, TIPE2 overexpression significantly increased cell motility and invasion ability in prostate cancer cells. These data suggest that TIPE2 overexpression may induce alterations in prostate cancer cells resembling that of the EMT procedure.
Previous studies have documented that activation of the PI3K/Akt signaling pathway was involved in the regulation of EMT and the promotion of metastasis in prostate cancer cells (20–22). It was reported that activation of Akt leads to a significant reduction in E-cadherin expression and nuclear localization of Snail in prostate cancer cells (23). Snail, a zinc-finger transcription factor, has been found to increase cell migration in LNCaP and 22Rv1 prostate cancer cells, as well as increase ERK and PI3K/Akt activity in 22Rv1 prostate cancer cells (24). Interestingly, Zhu et al. reported that adenovirus-mediated human TIPE2 overexpression significantly downregulated the expression levels of B-cell lymphoma (Bcl)-XL, p-Akt, and p-ERK1/2 in AGS gastric cancer cells (25). In the present study, we found that phosphorylation levels of PI3K and Akt were significantly decreased in TIPE2-overexpressing cells. All of these data suggest that TIPE2 overexpression may suppress proliferation, migration, and invasion in prostate cancer cells by inhibiting the PI3K/Akt signaling pathway.
In conclusion, for the first time we demonstrated that TIPE2 overexpression may suppress proliferation, migration, and invasion in prostate cancer cells by inhibiting PI3K/Akt signaling pathway. Therefore, TIPE2 might serve as a potential therapeutic target for human prostate cancer.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R. L.; Miller K. D.; Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 65:5–29; 2015. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis C. E.; Lin C. C.; Mariotto A. B.; Siegel R. L.; Stein K. D.; Kramer J. L.; Alteri R.; Robbins A. S.; Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 64:252–271; 2014. [DOI] [PubMed] [Google Scholar]
- 3. Nepple K. G.; Stephenson A. J.; Kallogjeri D.; Michalski J.; Grubb R. L.; Strope S. A.; Haslag-Minoff J.; Piccirillo J. F.; Ciezki J. P.; Klein E. A. Mortality after prostate cancer treatment with radical prostatectomy, external-beam radiation therapy, or brachytherapy in men without comorbidity. Eur. Urol. 64:372–378; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kroon J.; Metselaar J. M.; Storm G.; van der Pluijm G. Liposomal nanomedicines in the treatment of prostate cancer. Cancer Treat. Rev. 40:578–584; 2014. [DOI] [PubMed] [Google Scholar]
- 5. Tao J.; Wu D.; Xu B.; Qian W.; Li P.; Lu Q.; Yin C.; Zhang W. Microrna-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol. Rep. 27:1967–1975; 2012. [DOI] [PubMed] [Google Scholar]
- 6. Freundt E. C.; Bidere N.; Lenardo M. J. A different tipe of immune homeostasis. Cell 133:401–402; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmody R. J.; Hilliard B.; Maguschak K.; Chodosh L. A.; Chen Y. H. Genomic scale profiling of autoimmune inflammation in the central nervous system: The nervous response to inflammation. J. Neuroimmunol. 133:95–107; 2002. [DOI] [PubMed] [Google Scholar]
- 8. Sun H.; Gong S.; Carmody R. J.; Hilliard A.; Li L.; Sun J.; Kong L.; Xu L.; Hilliard B.; Hu S. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133:415–426; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z.; Qi H.; Hou S.; Jin X. TIPE2 mRNA overexpression correlates with TNM staging in renal cell carcinoma tissues. Oncol. Lett. 6:571–575; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gus-Brautbar Y.; Johnson D.; Zhang L.; Sun H.; Wang P.; Zhang S.; Zhang L.; Chen Y. H. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol. Cell 45:610–618; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao X.; Zhang L.; Shi Y.; Sun Y.; Dai S.; Guo C.; Zhu F.; Wang J.; Wang X.; Chen Y. H. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer. 12:149; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y.; Li X.; Liu G.; Sun R.; Wang L.; Wang J.; Wang H. Downregulated TIPE2 is associated with poor prognosis and promotes cell proliferation in non-small cell lung cancer. Biochem. Bioph. Res. Co. 457:43–49; 2015. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y. H.; Yan H. Q.; Wang F.; Wang Y. Y.; Jiang Y. N.; Wang Y. N.; Gao F. G. TIPE2 inhibits TNF-α-induced hepatocellular carcinoma cell metastasis via Erk1/2 downregulation and NF-κB activation. Int. J. Oncol. 46:254–264; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Osaki M.; Oshimura M. A.; Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 9:667–676; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Vara J. Á. F.; Casado E.; de Castro J.; Cejas P.; Belda-Iniesta C.; González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 30:193–204; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Liu Q.-Q.; Zhang F. F.; Wang F.; Qiu J. H.; Luo C. H.; Zhu G. Y.; Liu Y. F. TIPE2 inhibits lung cancer growth attributing to promotion of apoptosis by regulating some apoptotic molecules expression. PLoS One 10:e0126176; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huber M. A.; Kraut N.; Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558; 2005. [DOI] [PubMed] [Google Scholar]
- 18. Yilmaz M.; Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Met. Rev. 28:15–33; 2009. [DOI] [PubMed] [Google Scholar]
- 19. Wu J.; Zhang H.; Xu C.; Xu H.; Zhou X.; Xie Y.; Tao M. TIPE2 functions as a metastasis suppressor via negatively regulating β-catenin through activating GSK3β in gastric cancer. Int. J. Oncol. 48:199–206; 2016. [DOI] [PubMed] [Google Scholar]
- 20. Bitting R. L.; Armstrong A. J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 20:R83–R99; 2013. [DOI] [PubMed] [Google Scholar]
- 21. Gan Y.; Shi C.; Inge L.; Hibner M.; Balducci J.; Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29:4947–4958; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Chang L.; Graham P.; Hao J.; Ni J.; Bucci J.; Cozzi P.; Kearsley J.; Li Y. Acquisition of epithelial–mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 4:e875; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barber A. G.; Castillo-Martin M.; Bonal D. M.; Jia A. J.; Rybicki B. A.; Christiano A. M.; Cordon-Cardo C. PI3K/AKT pathway regulates E-cadherin and desmoglein 2 in aggressive prostate cancer. Cancer Med. 4:1258–1271; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henderson V.; Smith B.; Burton L. J.; Randle D.; Morris M.; Odero-Marah V. A. Snail promotes cell migration through PI3K/AKT-dependent Rac1 activation as well as PI3K/AKT-independent pathways during prostate cancer progression. Cell Adhes. Migr 9:255–264; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Y.; Tao M.; Wu J.; Meng Y.; Xu C.; Tian Y.; Zhou X.; Xiang J.; Zhang H.; Xie Y. Adenovirus-directed expression of TIPE2 suppresses gastric cancer growth via induction of apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther. 23(4):98–106; 2016. [DOI] [PubMed] [Google Scholar]