Abstract
Triple-negative breast cancer (TNBC) is associated with high recurrence rates of metastasis and death. miR-509 has been reported to be a tumor suppressor in many cancers, but its effect in TNBC has not yet been identified. In this article, we explored the effects of miR-509 on the malignant phenotype of TNBC cells, including proliferation, apoptosis, migration, and invasion. We transiently transfected TNBC cells, Hs578T, with miR-509 mimic. Upon transfection, the expression of miR-509 was upregulated about 50-fold compared with cells transfected with scramble mimic. Overexpression of miR-509 inhibited cell proliferation, induced cell apoptosis, and suppressed cell invasion of Hs578T cells. Moreover, tumor necrosis factor-α (TNF-α) was involved in miR-509-mediated suppressive effects of TNBC cells, as being treated with TNF-α could partially abolish the suppressive effects of miR-509. Collectively, these data suggest that miR-509 could reverse the malignant phenotype of TNBC cells, probably by suppressing TNF-α.
Key words: miR-509, Tumor necrosis factor-α (TNF-α), Breast cancer (BC), Triple-negative breast cancer (TNBC)
INTRODUCTION
Breast cancer (BC) is the most frequent type of malignancy in women in both the developed and the developing world (1). This malignancy represents a group of heterogeneous tumors with various character molecular features, prognosis, and responses to available therapy (2). Currently, according to different gene expression profiles, BC has been classified into four subtypes, which are triple-negative breast cancer (TNBC), luminal A, luminal B, as well as human epidermal growth factor receptor 2 (HER2)-enriched BCs. The genomic complexity, genetic alteration, and clinical prognosis among these subtypes are definitely different (3).
Because of the intense effort in public education and mammogram screening followed by early treatments, BC is becoming a curable disease. However, the overall survival rate of TNBC is still poor. TNBC is frequently observed in young patients, especially ones with larger and higher grade tumors (4). It is also associated with higher recurrence rates of metastasis and death, especially in ones within 3 years after diagnosis (5). Thus, identifying the mechanisms involved in the tumorigenesis of TNBC is crucial to the treatment of BC.
MicroRNAs (miRNAs) are noncoding RNAs that regulate gene expression by targeting the sequences on their untranslated region. During the past decades, more than 1,000 human miRNAs have been identified, and many of them are found to be involved in the carcinogensis of BC (6). Among these miRNAs, miR-509 is an interesting member. Upon targeting a series of oncogenes, miR-509 has been reported to function as a tumor suppressor in a quantity of cancers, such as cervical cancer and renal cell cancer (7,8). For BC, the expression of miR-509 was reported to be attenuated in brain metastatic lesions, and overexpression of miR-509 suppressed transendothelial cell migration by blocking RhoC (9). Since TNBC is a highly aggressive subtype, we speculate that miR-509 might participate in the dysregulated molecular signaling pathways involved in the aggressive behavior of TNBC.
In this study, we analyzed the expression of miR-509 in a cohort of 25 human TNBCs and adjacent normal tissues. Next, we explored a series of cellular functional assays to identify the effects of miR-509 on the malignant phenotypes of a TNBC cell line, Hs578T. Furthermore, our subsequent experiments found that tumor necrosis factor-α (TNF-α) might be involved in the suppressive effects of miR-509 on the TNBC cells.
MATERIALS AND METHODS
Cell Culture and Cell Treatment
The human TNBC cell line Hs578T was obtained from the Cell Bank of the Chinese Academy of Science. Cells were maintained in the Dulbecco’s modified Eagle’s medium (DMEM) with a high glucose concentration, supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), gluatamine 2 mM, penicillin 100 UI/ml, and insulin. miR-509 mimic and scramble mimic were obtained from Shanghai GenePharma Company (Shanghai, China) and were transfected using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s protocol at a final concentration of 50 nM. Cells were cultured for 48 h and harvested for the next experiments.
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was exacted from cells using TRIzol (Invitrogen). The RNA was quantified by assessing its absorbance at 260 nm. Stem-loop reverse transcription polymerase chain reaction (RT-PCR) primers were used for the reverse transcription of miR-509. Quantitative RT-PCR was conducted using ABI PRISM 7500 real-time PCR system. For the PCR amplification, the SYBR Premix Ex Taq Kit (Takara, Dalian, China) was utilized according to the manufacturer’s instructions. U6 snRNA was adopted as the endogenous control for miR-509 [the sequence of the primers used for reverse transcription: miR-509, 5′-GTCGTATCCAG TGCA GGGTCCGAGGTATTCGCACTGGTGGGTAG-3′; the sequences of the primers used for quantitative PCR: miR-509, 5′-GCGCTGATTGGTACG TCTG-3′ (forward) and 5′-CAGTGCAG GGTCCGAGGT-3′ (reverse)]. The comparative Ct method was used to quantify target gene relative to their endogenous control. For each individual analysis, one of the samples was designated as the calibrator and given a relative value of 1.0; all of the quantities were then expressed as n-fold relative to the calibrator.
CCK-8 Assays
To explore the effects of miR-509 on cell proliferation, Hs578T cells were incubated in 10% CCK-8 reagent (Dojindo, Kumamoto, Japan) diluted in normal culture medium at 37°C until visual color conversion occurred. Proliferation rates were determined at 0, 24, 48, and 72 h after transfection. The absorbance of each well was measured with a microplate reader set at 450 and 650 nm.
FACS Assays
The fluorescence-activated cell sorting (FACS) assays were adopted to identify the effects of miR-509 on cell apoptosis. The apoptosis assays was performed after 72 h of transfection using the PE Annexin-V Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA, USA) and then analyzed by FACS. Hs578T cells were transfected with miR-509 and scramble mimic. Cells were harvested, washed twice with cold PBS, incubated with PE Annexin-V and 7-AAD according to the manufacturer’s instruction, and then analyzed by FACS. Each sample was run in triplicate.
Cell Invasion Assays
For the invasion assays, after 24-h transfection, 2 × 105 cells in serum-free media were seeded onto the Transwell migration chambers (8-µm pore size; BD Pharmingen) coated with Matrigel (BD Pharmingen) on the upper chamber. Medium containing 20% FBS was added to the lower chamber. After 24 h, the noninvading cells were removed by a cotton wool, and the invasive cells located on the lower surface of the chamber were stained with crystal violet stain and counted with a microscope. Experiments were repeated three times.
Western Blot
At the indicated times, Hs578T cells were harvested in ice-cold PBS and lysed on ice in a cold preparation of modified radioimmunoprecipitation buffer supplemented with protease inhibitors. Protein concentration was determined using the BCA Protein Assay Kit (Vigorous Biotechonology, Beijing, China), and equal amounts of proteins were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide). Gels were electroblotted onto nitrocellulose membranes (BD Pharmingen). For immunoblot experiments, membranes were blocked for 2 h with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, and incubated at 4°C overnight with primary antibody. Detection was performed by peroxidase-conjugated secondary antibodies using the enhanced chemiluminescence system. Primary antibodies used were GAPDH (Zhong-shan JinQiao, Beijing, China) and TNF-α (Cell Signaling Technology, Danvers, MA, USA).
Statistical Analysis
A two-tailed Student’s t-test was performed to analyze the data. Values of p < 0.05 were considered to be significant.
RESULTS
Overexpression of miR-509 Suppresses the Cell Proliferation of TNBC Cell Line Hs578T
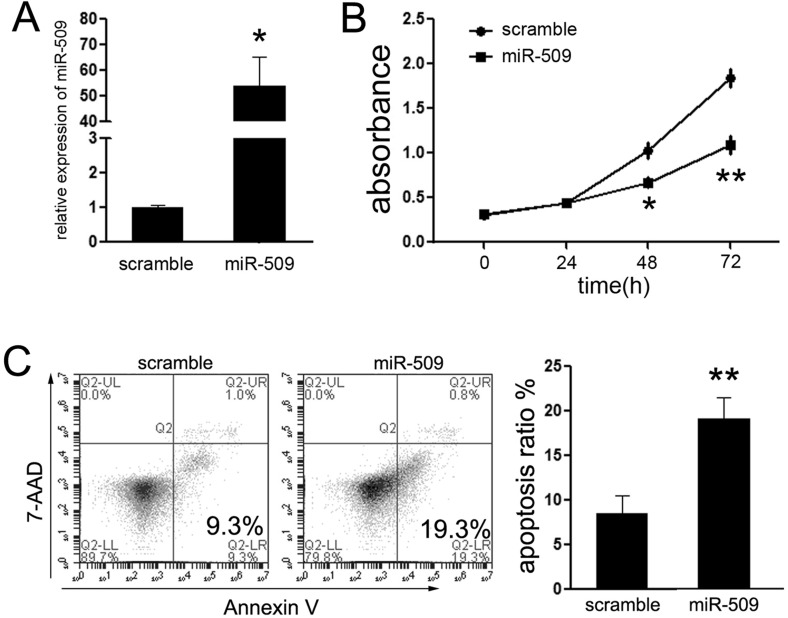
Although a previous work reported that miR-509 was suppressed in BC tissues, the relationship between miR-509 and TNBC remained unclear. Thus, we explored the expression of miR-509 in TNBC tissues. The results showed that the expression of miR-509 was suppressed in TNBC tissues compared with the adjacent nontumor tissues (data not shown). The prominent reduction of miR-509 in TNBC tissues prompted us to evaluate the potential biological significance between miR-509 and TNBC cells. Accordingly, TNBC cell line Hs578T was picked up for further experiments. First, we restored the expression of miR-509 in Hs578T cells upon exogenous transfection with miR-509 mimic. Upon transfection, the expression of miR-509 was significantly increased by more than 50-fold in transfected Hs578T cells (Fig. 1A).
Figure 1.
miR-509 suppressed cell proliferation of TNBC cells in vitro. (A) Quantitative polymerase chain reaction (qPCR) analysis of miR-509 in Hs578T cells after transfection of miR-509 mimic for 48 h. The expression of miR-509 in Hs578T cells transfected with miR-509 mimic was upregulated. (B) Cellular viability assay was detected by CCK-8 assay. (C) Analysis of apoptosis was performed by fluorescence-activated cell sorting (FACS) after transfection at 48 h, and showed that miR-509 induced early apoptosis of Hs578T cells. *p < 0.05; **p < 0.01.
Next, the CCK-8 proliferation assays showed that the growth rate was reduced in Hs578T cells transfected with miR-509 mimic compared with cells transfected with scramble mimic (Fig. 1B). To determine whether miR-509-induced inhibition of proliferation might be due to induction of cell death, the number of early apoptotic Hs578T cells following transfection was examined. As expected, few early apoptotic cells (9.3%) were detected in the scramble mimic-treated cells, whereas treatment with miR-509 mimic increased the percentage of early apoptotic cells (19.3%) as judged by PE Annexin-V staining (Fig. 1C). Taken together, the results demonstrate that the restoration of miR-509 activity in TNBC cells markedly inhibited cell proliferation.
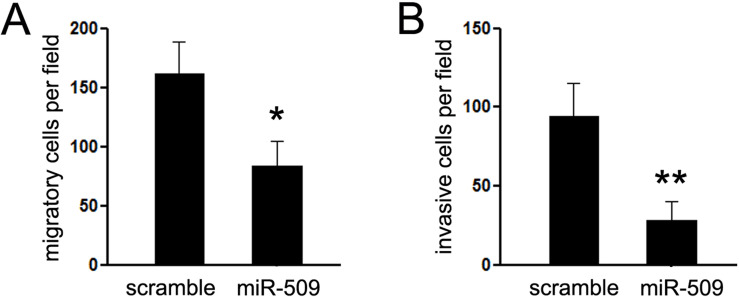
miR-509 Suppresses the Migration and Invasion of Hs578T In Vitro
Given that Hs578T is a highly metastatic cell line, we then investigated the effects of miR-509 on the migratory and invasive capacity of Hs578T cells. A Matrigel migration assay was performed to analyze the effect of miR-509 expression on the migratory capacity of Hs578T cells. Hs578T cells were maintained in serum-free medium during the course of the assays to avoid any augmented invasive behavior that could be affected by altered cell proliferation. We found that ectopic expression of miR-509 in Hs578T cells resulted in a significant reduction of cell migratory capacity compared with the control groups (Fig. 2A). Then the invasive capacity of Hs578T cells transfected with miR-509 or scramble mimic was evaluated by Matrigel invasion chamber assays. The chambers were coated with Matrigel to mimic the membrane of the cell. As shown in Figure 2B, ectopic expression of miR-509 in Hs578T cells clearly suppressed cells passing through the chamber. The results indicated that miR-509 could efficiently repress cell motility and invasiveness of TNBC cells in vitro.
Figure 2.
miR-509 suppressed cell migration and invasion of TNBC cells in vitro. (A) Cell migration assay was detected by Matrigel migration chamber assays. Restoring the expression of miR-509 reduced cell invasion through the chamber. (B) Cell invasion assay was performed using Matrigel invasion chamber assay. *p < 0.05; **p < 0.01.
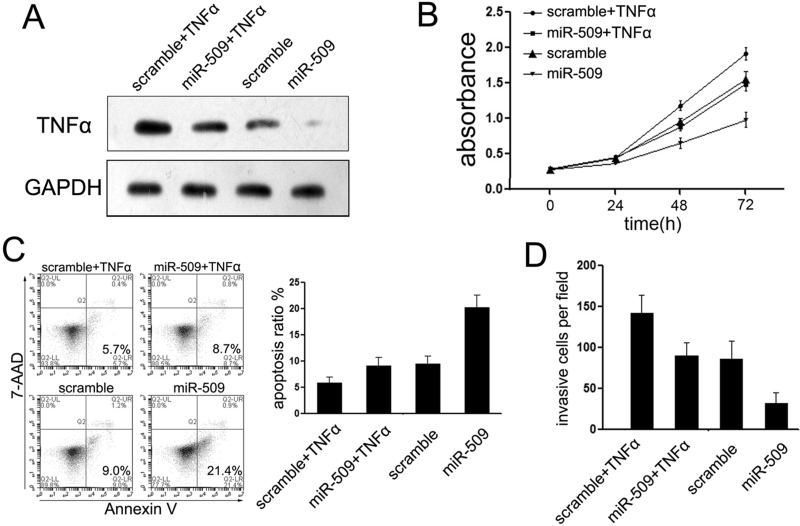
TNF-α Was Involved in miR-509-Mediated Suppression of TNBC Cells
Since a previous work reported that TNF-α is highly expressed in the brain metastatic tumors of BC patients and it is involved in miR-509-meidated suppression of brain metastasis, we further identified whether it is involved in miR-509-mediated suppression of TNBC cells. Upon transfection with miR-509, the expression of TNF-α was suppressed in Hs578T cells (Fig. 3A). The Hs578T cells were transfected with miR-509 or scramble mimic for 24 h and then treated with 10 ng/ml TNF-α for 48 h to identify the effects of TNF-α in the suppressive role of miR-509. As expected, the expression of TNF-α was partially restored upon treatment with TNF-α (Fig. 3A). To determine whether TNF-α was involved in miR-509 effects, we measured the effect of TNF-α on Hs578T cell proliferation and apoptosis upon overexpression of miR-509. Strikingly, compared with cells treated with miR-509 mimic only, cotreatment with TNF-α cytokine decreased the proliferation rate of Hs578T cells (Fig. 3B). In accordance with cell proliferation, cotransfection with TNF-α cytokine partly blocked miR-509-mediated apoptosis induction (Fig. 3C), although much less effectively than restored miR-509 expression. The same results were also observed in cell Matrigel assays, which suggested that TNF-α was involved in miR-509-mediated suppression on Hs578T cell invasion (Fig. 3D).
Figure 3.
TNF-α is involved in miR-509-mediated suppressive effects on TNBC cells. (A) Western blotting was performed to examine the expression of TNF-α upon transfection with miR-509 in Hs578T cells. (B) Treatment with TNF-α partially abolished the suppressive effects of miR-509 on cell proliferation. (C) Cell apoptosis of Hs578T cells as described after treatment with TNF; TNF induced TNBC cell apoptosis. (D) Transwell assays were conducted to detect the effects on cell invasion of Hs578T cells treated with TNF. TNF increased the cell invasive ability of Hs578T cells.
DISCUSSION
Accumulating evidence has identified that unrevealed molecular factors, particularly noncoding RNAs, play an important role in tumorigenesis or tumor progression through targeting various genes that were involved in the biological progression. In this article, we explored the biological role and putative regulatory mechanisms of miR-509 on the malignant phenotype of TNBC cells.
The name of TNBC comes from the lack of cell membrane receptors for progesterone, estrogen, as well as HER2 (10). It is frequently observed in young patients (<35 years) and in patients with larger and higher grade tumors (11). Thus, understanding the mechanisms involved in the tumorigenesis and progression of the TNBC subtype of BC is particularly important for targeted therapy in the future.
We have detected the expression of miR-509 in the TNBC subtypes of BC patients (data not shown). The results showed that miR-509 was suppressed in TNBC subtypes of BC patients, suggesting that miR-509 might function as a tumor suppressor in TNBCs. These results were in partial agreement those of with Xing et al., who reported that miR-509 was suppressed in BC patients (9), while they did not analyze the expression of miR-509 in the subtypes of TNBC. They also suggested that miR-509 was significantly enriched in patients with brain metastasis, and suppressing miR-509 increases the blood–brain barrier (BBB) permeability and induces transendothelial cell migration (9). To further identify the putative role of miR-509 in TNBC cells, we analyzed the effects of miR-509 overexpression on the proliferation, cell cycle distribution, apoptosis, as well as invasion assays. Overexpression of miR-509 in the TNBC cell line, Hs578T cells, induced a complex phenotype, namely, an inhibition of cell proliferation, block of G1/S phase transition, induction of cell apoptosis, and suppression of cell invasion, indicating that miR-509 functions as a tumor suppressor in TNBC BCs.
What is the mechanism that might be involved in the miR-509-mediated effects on the malignant phenotype of TNBC cells? TNF-α attracted our attention most, as a previous work reported that TNF-α was involved in miR-509-mediated BBB permeability and the penetration of BC cells into the brain (9). Thus, we further identified whether it was involved in miR-509-mediated suppression on TNBC. We found that overexpression of miR-509 significantly suppressed the secretion of TNF-α by Hs578T cells. TNF-α is a proinflammatory cytokine involved in the promotion and progression of cancer (12). Several studies have suggested that TNF-α plays a critical role in the molecular events that mediate inflammation toward development and evolution of BC, especially the TNBC subtypes (13). TNF-α-308G > A polymorphism is positively associated with metastasis of TNBC patients (14). Moreover, knockdown of the TNF-α gene through blockage of the NF-κB pathway inhibited cell proliferation and induced apoptosis of TNBC cells (13). Herein, we found that TNF-α is involved in miR-509-mediated suppression of TNBC cells, Hs578T, since treatment of TNF-α could partially abolish miR-509-mediated suppression on cell proliferation and migration, and induction on cell apoptosis. However, more experiments are warranted to identify whether other genes were involved in the suppression effects.
Our findings strongly demonstrate that miR-509 functions as tumor suppressor in TNBC BC cells through, at least partially, suppressing the secretion of TNF-α. To the best of our knowledge, this is the first comprehensive study to explore the role of miR-509 in TNBC. The combined miRNA-based and epigenetic treatment may be a novel potential therapeutic target for BCs, especially the ones with TNBC subtypes.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Development Plan of Binzhou (No. 2013ZC1708); the Medical and Health Science and Technology Development Plan of Shandong (No. 2015WS0484); and the National Natural Science Foundation of China (Nos. 81173601 and 30973932).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. DeSantis C. E.; Siegel R. L.; Sauer A. G.; Miller K. D.; Fedewa S. A.; Alcaraz K. I.; Jemal A. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 66(4):290–308; 2016. [DOI] [PubMed] [Google Scholar]
- 2. Gatza M. L.; Lucas J. E.; Barry W. T.; Kim J. W.; Wang Q.; Crawford M. D.; Datto M. B.; Kelley M.; Mathey-Prevot B.; Potti A.; Nevins J. R. A pathway-based classification of human breast cancer. Proc. Natl. Acad. Sci. USA 107:6994–6999; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianchini G.; Balko J. M.; Mayer I. A.; Sanders M. E.; Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol.; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer K. R.; Brown M.; Cress R. D.; Parise C. A.; Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 109:1721–1728; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Dent R.; Trudeau M.; Pritchard K. I.; Hanna W. M.; Kahn H. K.; Sawka C. A.; Lickley L. A.; Rawlinson E.; Sun P.; Narod S. A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 13:4429–4434; 2007. [DOI] [PubMed] [Google Scholar]
- 6. O’Day E.; Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 12:201; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhai Q.; Zhou L.; Zhao C.; Wan J.; Yu Z.; Guo X.; Qin J.; Chen J.; Lu R. Identification of miR-508-3p and miR-509-3p that are associated with cell invasion and migration and involved in the apoptosis of renal cell carcinoma. Biochem. Biophys. Res. Commun. 419:621–626; 2012. [DOI] [PubMed] [Google Scholar]
- 8. Ren Z. J.; Nong X. Y.; Lv Y. R.; Sun H. H.; An P. P.; Wang F.; Li X.; Liu M.; Tang H. Mir-509-5p joins the Mdm2/p53 feedback loop and regulates cancer cell growth. Cell Death Dis. 5:e1387; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xing F.; Sharma S.; Liu Y.; Mo Y. Y.; Wu K.; Zhang Y. Y.; Pochampally R.; Martinez L. A.; Lo H. W.; Watabe K. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-alpha. Oncogene 34:4890–4900; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffiths C. L.; Olin J. L. Triple negative breast cancer: A brief review of its characteristics and treatment options. J. Pharm. Pract. 25:319–323; 2012. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L.; Hao C.; Dong G.; Tong Z. Analysis of clinical features and outcome of 356 triple-negative breast cancer patients in China. Breast Care (Basel) 7:13–17; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown E. R.; Charles K. A.; Hoare S. A.; Rye R. L.; Jodrell D. I.; Aird R. E.; Vora R.; Prabhakar U.; Nakada M.; Corringham R. E.; Dewitte M.; Sturgeon C.; Propper D.; Balkwill F. R.; Smyth J. F. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann. Oncol. 19:1340–1346; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Pileczki V.; Braicu C.; Gherman C. D.; Berindan-Neagoe I. TNF-alpha gene knockout in triple negative breast cancer cell line induces apoptosis. Int. J. Mol. Sci. 14:411–420; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H. H.; Zhu H.; Liu L. S.; Huang Y.; Guo J.; Li J.; Sun X. P.; Chang C. X.; Wang Z. H.; Zhai K. Tumour necrosis factor-alpha gene polymorphism is associated with metastasis in patients with triple negative breast cancer. Sci. Rep. 5:10244; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]