Abstract
Sparc/osteonectin, cwcv, and kazal-like domains proteoglycan (testican) 1 (SPOCK1), known as testican-1, were found to be involved in the development and progression of tumors. However, in colorectal cancer (CRC), the expression pattern of SPOCK1 and its functional role remain poorly investigated. In the present study, we explored the role of SPOCK1 in CRC. Our results demonstrated that SPOCK1 is overexpressed in CRC cell lines. SPOCK1 silencing significantly inhibited the proliferation in vitro and the tumor growth in vivo. Furthermore, SPOCK1 silencing significantly attenuated the migration/invasion by reversing the EMT process in CRC cells. Finally, knockdown of SPOCK1 obviously decreased the protein expression levels of p-PI3K and p-Akt in HCT116 cells. In total, our study demonstrated for the first time that knockdown of SPOCK1 inhibits the proliferation and invasion in CRC cells, possibly through the PI3K/Akt signaling pathway. Therefore, SPOCK1 may be a potential therapeutic target for the treatment of CRC.
Key words: Sparc/osteonectin, cwcv, and kazal-like domains proteoglycan (testican) 1 (SPOCK1); Colorectal cancer (CRC); Invasion; PI3K/Akt pathway
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies and is the second leading cause of cancer death in humans (1). Despite very aggressive treatments, including surgery and combined radiation treatment and chemotherapy, most patients are initially diagnosed at the advanced stages, and the 5-year survival rate is very low (2–4). Therefore, efforts are urgently needed to explore the molecular mechanisms of CRC for cancer-specific cellular targets for novel therapeutic approaches.
Sparc/osteonectin, cwcv, and kazal-like domains proteoglycan 1 (SPOCK1) is a proteoglycan that was first isolated in human testes and encodes a matricellular glycoprotein belonging to a novel Ca2+-binding proteoglycan family (5). It is dysregulated in many organs and tissues including the brain, cartilage, vascular endothelium, lymphocytes, and neuromuscular junctions (6–8). A growing body of evidence indicates that SPOCK1 plays a critical role in cell cycle control, apoptosis, DNA repair, and metastasis (9–11). Recently, several studies showed that SPOCK1 is highly expressed in different human cancers (12–15). One study showed that SPOCK1 expression was significantly increased in human gallbladder cancer tissues, and downregulation of SPOCK1 expression significantly inhibited the proliferation, colony formation, and invasion, as well as inhibited the tumor growth in vivo (16). However, in CRC, the expression pattern of SPOCK1 and its functional role remain poorly investigated. In the present study, we explored the role of SPOCK1 in CRC. We found that knockdown of SPOCK1 inhibits the proliferation and invasion in CRC cells by suppressing the PI3K/Akt signaling pathway.
MATERIALS AND METHODS
Cell Culture
Four human CRC cell lines (HCT116, HT29, SW480, and Lovo) and normal colonic mucosa epithelial cell line (NCM460) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) with 10% (v/v) fetal bovine serum (FBS; Gibco) and 100 U/ml streptomycin and penicillin (Gibco). All cells were incubated in a 5% CO2 humidified atmosphere at 37°C.
Short Hairpin RNA-Mediated Knockdown of SPOCK1 and Cell Transfection
A specific SPOCK1 short hairpin RNA (shRNA) (shSPOCK1; 5′-GUAAUGAGGAGGGCUAUUA-3′) and the negative control shRNA (shNC; 5′-TTCTCCGAACGTGTCACGT-3′) were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, P.R. China). For in vitro transfection, CRC cells were transfected with shSPOCK1 or shNC using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The transfection efficiency was confirmed by RT-PCR and Western blot.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from CRC cells using TRIzol reagent (Abcam, Cambridge, UK). Complementary DNA was prepared with the use of a First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany). RT-qPCR reactions were performed on the Bio-Rad iQ5 real-time thermal cyclers using SYBRH Premix Ex Taq™ II kit (Takara, Dalian, P.R. China). The following primers were used for qPCR: SPOCK1, 5′-CAACTGCTTGTTCCCAGAGG-3′ (sense) and 5′-GCCAATGACTTCCCTATCCA-3′ (antisense); and β-actin, 5′-TTAGTTGCGTTACACCCTTTC-3′ (sense) and 5′-ACCTTCACCGTTCCAGTTT-3′ (antisense). All reactions were run in triplicate, and the relative gene expression was calculated using the comparative threshold cycle (Ct) method [relative gene expression = 2−(ΔCt sample − ΔCt control)].
Western Blot
The cells were lysed in lysis buffer containing 1% NP40, 1 mM EDTA, 50 mM Tris–HCl (pH 7.5), and 150 mM NaCl, supplemented with complete protease inhibitors mixture (Roche, Monza, Italy). The protein concentration was then determined using the Pierce® BCA Protein Assay Kit (23225; Pierce). Then 30 μg of protein per lane was separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). After blocking with 2% nonfat dry milk in Tris-buffered saline (TBS) for 1 h at room temperature, the membrane was incubated with primary antibodies (SPOCK1, E-cadherin, N-cadherin, PI3K, p-PI3K, Akt, and p-Akt antibodies; all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.). Protein bands were visualized by enhanced chemiluminescence (Thermo Fisher Scientific, Rockford, IL, USA).
Cell Proliferation Assay
Cell proliferation was evaluated by MTT assay. Briefly, infected cells were seeded in 96-well plates and cultured until 80% confluence was reached. They were then cultured for 24, 48, 72, or 96 h. At each time point, cells were stained with 100 μl of MTT dye (0.5 mg/ml; Sigma-Aldrich) for 4 h at 37°C, followed by removal of the culture medium and addition of 100 μl of dimethyl sulfoxide (Sigma-Aldrich). Absorbance was determined at 490 nm.
Transwell Migration and Invasion Assays
Cell migration and invasion assays were performed in a 24-well Boyden chamber with an 8-μm pore size polycarbonate membrane (Millipore). For the migration assay, 5 × 104 cells transfected with shRNA-SPOCK1 were suspended in serum-free medium and plated on chambers (Corning Costar, NY, USA), and 500 μl of DMEM with 10% FBS was added into the lower compartment. After incubating for 24 h at 37°C in the incubator supplemented with 5% CO2, the tumor cells remaining inside the upper chamber were removed with cotton swabs. The cells on the lower surface of the membrane were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and then counted in five random fields per well under a light microscope (magnification: 100×). The invasion assay was done by the same procedure, except that the membrane was coated with Matrigel to form a matrix barrier.
Xenografted Tumor Model
Female Balb/c nude mice (4–5 weeks of age, 18–20 g) were purchased from the Laboratory Animal Center of Xi’an Jiaotong University Health Science Center and were housed in barrier facilities on a 12-h light/dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. HCT116 cells transfected with shSPOCK1 and the corresponding control cells (5 × 106) were suspended in 200 μl of PBS and then injected subcutaneously into the left axilla of the mice (five mice/group). Tumors were examined every week; length, width, and thickness measurements were obtained with calipers, and tumor volumes were calculated. On day 29, animals were euthanized and tumors were excised and weighed.
Statistical Analysis
All statistical analyses were conducted using the SPSS version 13.0 software, and the data are reported as means ± SD. Statistical analysis involved using the Student’s t-test for comparison of two groups or one-way ANOVA for multiple comparisons. Differences with values of p < 0.05 were considered significant.
RESULTS
SPOCK1 Is Highly Expressed in CRC Cell Lines
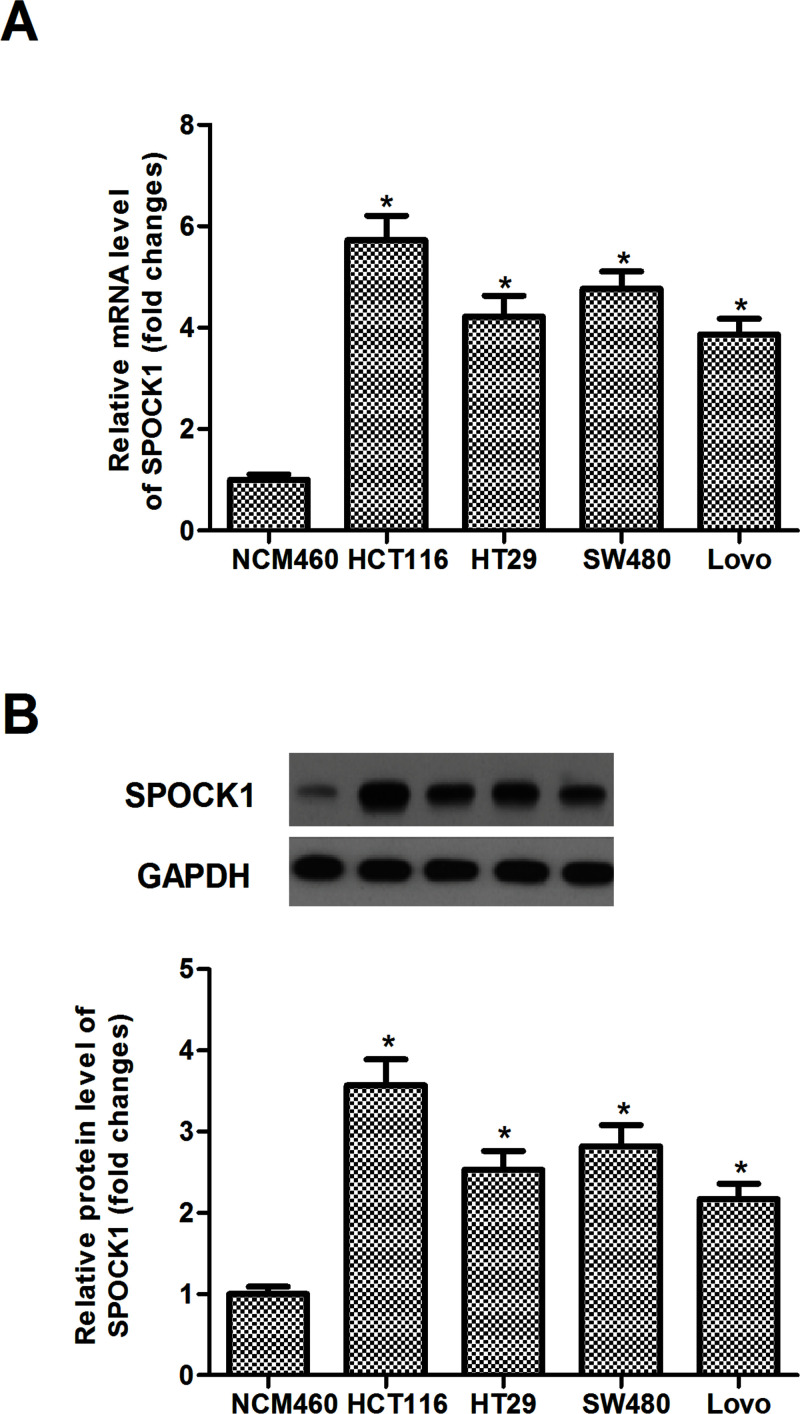
SPOCK1 expression was analyzed in four human CRC cell lines (HCT116, HT29, SW480, and Lovo) and normal colonic mucosa epithelial cell line (NCM460) by RT-qPCR and Western blot. Our results demonstrated that the expression levels of SPOCK1 of both mRNA and protein were significantly increased in human CRC cell lines, compared with the NCM460 cells (Fig. 1A and B). HCT116 displayed a higher expression level of SPOCK1. Therefore, we used HCT116 cells as a model to investigate the effect of SPOCK1 on CRC cell proliferation and invasion.
Figure 1.
SPOCK1 is highly expressed in CRC cell lines. (A) RT-qPCR analysis of SPOCK1 mRNA in four human CRC cell lines (HCT116, HT29, SW480, and Lovo) and normal colonic mucosa epithelial cell line (NCM460). (B) Western blot analysis of SPOCK1 protein in four human CRC cell lines (HCT116, HT29, SW480, and Lovo) and normal colonic mucosa epithelial cell line (NCM460). Data are expressed as mean ± SD. *p < 0.05 compared with the NCM460 group.
Knockdown of SPOCK1 Inhibits CRC Cell Proliferation In Vitro
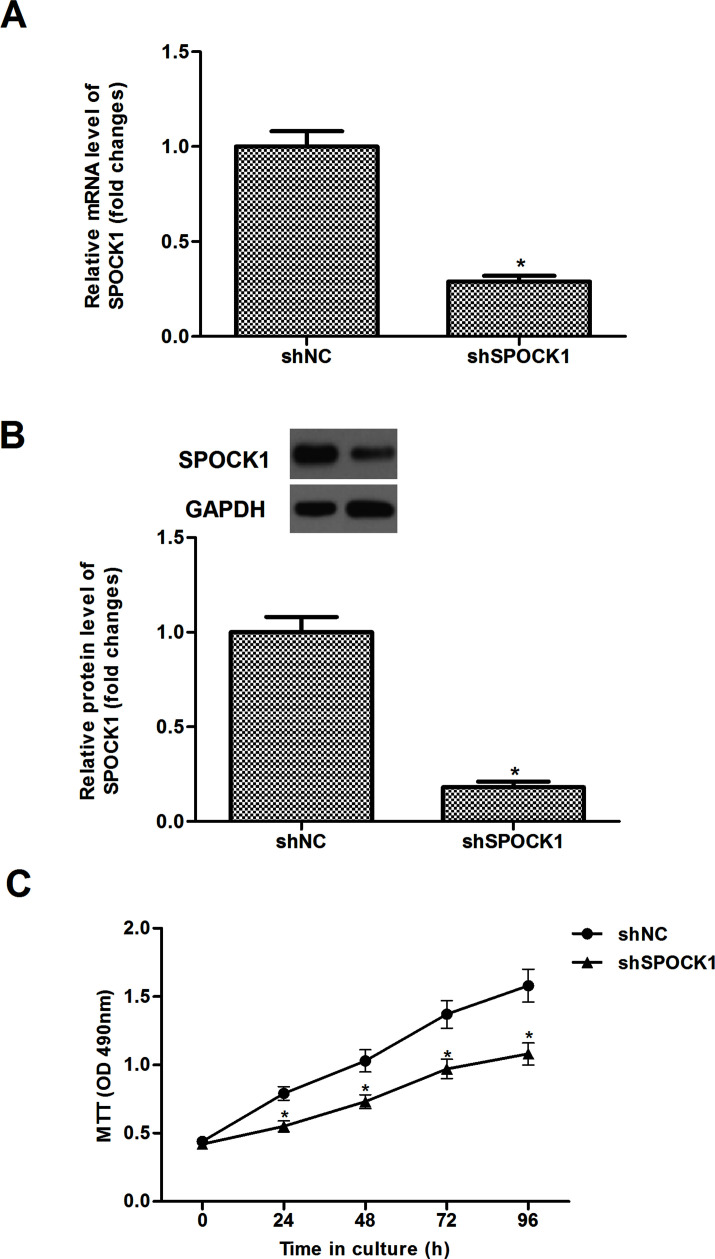
To further investigate the effect of SPOCK1 on proliferation, HCT116 cells were transfected with shSPOCK1 or shNC for 48 h. The results of the RT-qPCR demonstrated that the mRNA expression of SPOCK1 was obviously reduced in HCT116 cells transfected with shSPOCK1, compared to the shNC group (Fig. 2A). Similarly, Western blot analysis showed that shSPOCK1 significantly decreased the protein expression of SPOCK1 in HCT116 cells (Fig. 2B). We used the MTT assay to investigate the role of SPOCK1 in the proliferation of CRC cells. As shown in Figure 2C, knockdown of SPOCK1 significantly inhibited the proliferation of HCT116 cells.
Figure 2.
Knockdown of SPOCK1 inhibits CRC cell proliferation in vitro. HCT116 cells were transfected with shSPOCK1 or shNC for 48 h. The transfection efficiency was confirmed by RT-qPCR (A) and Western blot (B). Proliferation of HCT116 was detected by the MTT assay (C). Data are expressed as mean ± SD. *p < 0.05 compared with the shNC group.
Knockdown of SPOCK1 Inhibits CRC Cell Migration and Invasion by Reversing EMT Process
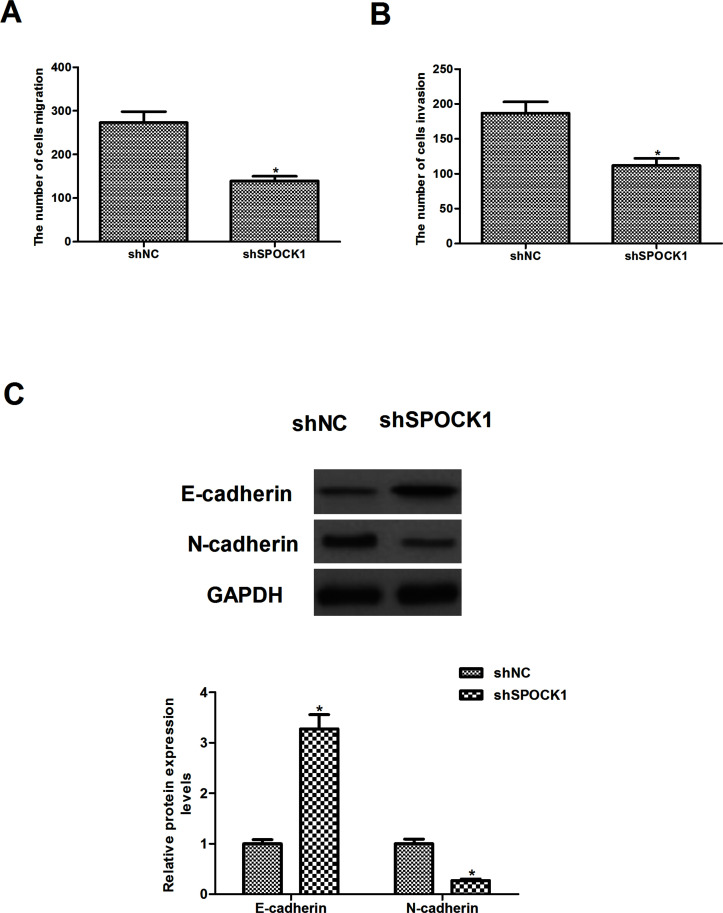
To investigate the effects of SPOCK1 on CRC cell migration and invasion, we performed in vitro Transwell migration assays. As shown in Figure 3A, compared with the shNC group, knockdown of SPOCK1 obviously inhibited the migration of HCT116 cells. Consistent with results of the Transwell assay, knockdown of SPOCK1 also greatly inhibited HCT116 cell invasion (Fig. 3B). To determine whether SPOCK1 regulates the migration/invasion of CRC cells through the EMT processes, we detected the expression levels of EMT-related markers by Western blot. The results showed that knockdown of SPOCK1 significantly increased the protein expression level of E-cadherin, but reduced the protein expression level of N-cadherin in HCT116 cells, compared to the shNC group (Fig. 3C).
Figure 3.
Knockdown of SPOCK1 inhibits CRC cell migration and invasion by reversing the EMT process. HCT116 cells were transfected with shSPOCK1 or shNC for 48 h. (A) The migration of HCT116 cells was evaluated by a Transwell assay. (B) The invasiveness of HCT116 cells was evaluated by a Matrigel-coated Transwell assay. (C) The expression levels of EMT markers were measured using Western blot analysis. Data are expressed as mean ± SD. *p < 0.05 compared with the shNC group.
Knockdown of SPOCK1 Inhibits the Growth of CRC In Vivo
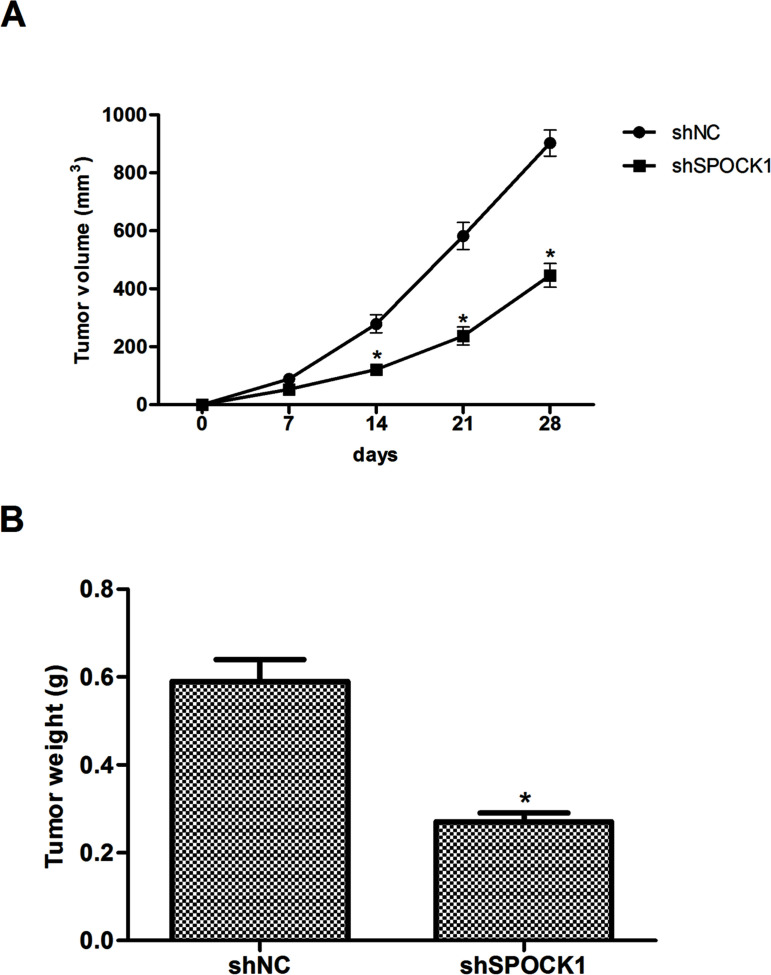
To investigate the effects of SPOCK1 on CRC growth in vivo, SPOCK1-depleted or control HCT116 cells were injected into the left axilla of nude mice, and then the tumor growth was monitored. The results indicated that the tumors formed by SPOCK1-silenced cells were smaller, in both size (Fig. 4A) and weight (Fig. 4B), than the tumors formed from shRNA vector control cells.
Figure 4.
Knockdown of SPOCK1 inhibits the growth of CRC in vivo. SPOCK1-depleted or control HCT116 cells were injected into the left axilla of nude mice, and then the tumor growth was monitored. (A) Tumor weights of the two groups. (B) Growth curves of tumor size. Data are expressed as mean ± SD. *p < 0.05 compared with the shNC group.
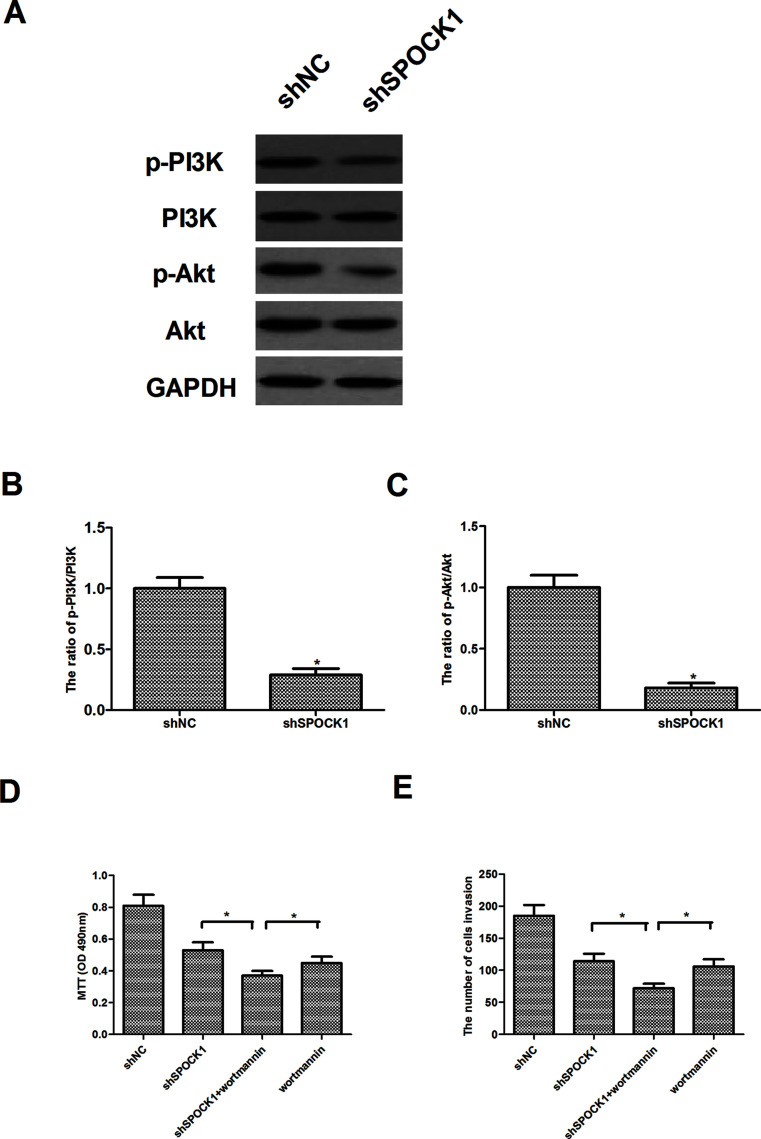
Knockdown of SPOCK1 Inhibits the Proliferation and Invasion Through Inactivation of the PI3K/Akt Signaling Pathway
To further investigate the mechanism of SPOCK1-mediated CRC cell proliferation and invasion, we investigated the effect of SPOCK1 on the expression of certain molecules involved in the PI3K/Akt signaling pathway in HCT116 cells. Western blot analysis showed that knockdown of SPOCK1 obviously decreased levels of PI3K and Akt phosphorylation in HCT116 cells, compared to the shNC group (Fig. 5A). Furthermore, we examined the effects of an Akt inhibitor (wortmannin) on SPOCK1-mediated CRC cell proliferation and invasion. The results showed that wortmannin enhanced the effects shSPOCK1 on CRC cell proliferation (Fig. 5B) and invasion (Fig. 5C).
Figure 5.
Knockdown of SPOCK1 inhibits the proliferation and invasion through inactivation of the PI3K/Akt signaling pathway. HCT116 cells were transfected with shSPOCK1 or shNC for 30 min. (A) The expression levels of PI3K, p-PI3K, Akt, and p-Akt were determined by Western blot. (B, C) Quantification analysis of p-PI3K/PI3K and p-Akt/Akt and was performed using Gel-Pro Analyzer version 4.0 software. (D) HCT116 cells were transfected with shSPOCK1 or shNC in the presence or absence of wortmannin (100 nM) for 24 h. Cell proliferation was detected by the MTT assay. (E) Cell invasion was evaluated by the Matrigel-coated Transwell assay. Data are expressed as mean ± SD. *p < 0.05 compared with the shNC group.
DISCUSSION
In the present study, we focused on the role of SPOCK1 in CRC. Our results demonstrated that SPOCK1 is overexpressed in CRC cell lines. SPOCK1 silencing significantly inhibited the proliferation in vitro and the tumor growth in vivo. Furthermore, SPOCK1 silencing significantly attenuated the migration/invasion by reversing the EMT process in CRC cells. Finally, knockdown of SPOCK1 obviously decreased the protein expression levels of p-PI3K and p-Akt in HCT116 cells.
Previous studies reported that SPOCK1 is implicated in the development and progression of tumors. A recent study showed that the expression of SPOCK1 dramatically upregulated in human glioma tissues and cell lines, and overexpression of SPOCK1 promoted the proliferation and inhibited apoptosis in glioma cells (15). Another study reported that both ovarian cancer tissues and the cell lines exhibited a higher level of SPOCK1, and knockdown of SPOCK1 by specific shRNA significantly inhibited ovarian cancer cell proliferation and colony formation in vitro and suppressed xenografted tumor growth in vivo (17). In line with the results, we found that downregulation of SPOCK1 expression greatly inhibited the proliferation in vitro and tumor growth in vivo. Collectively, these results obtained from both in vivo and in vitro experiments strongly suggest that SPOCK1 may function as an oncogene and plays an important role in the development and progression of CRC.
EMT has been reported to play an important role in the migration and invasion of tumor cells (18). Emerging studies have indicated that reduction or a loss of E-cadherin expression is also one of the well-established hallmarks of EMT and promotes the migration and invasion of tumor cells (19–21). Recently, Song et al. confirmed that upregulation of SPOCK1 induces EMT and promotes migration and invasion in esophageal squamous cell carcinoma (13). Ectopic expression of SPOCK1 was also found to induce EMT with increased expression of the mesenchymal marker vimentin and decreased expression of epithelial marker E-cadherin in epithelial lung cancer cells (14). Consistent with the results of previous studies, in the present study, we observed that knockdown of SPOCK1 obviously inhibited the migration and invasion in CRC cells. Moreover, knockdown of SPOCK1 significantly increased the protein expression level of E-cadherin, but decreased the protein expression level of N-cadherin in HCT116 cells. These results suggested that knockdown of SPOCK1 inhibits CRC cell migration and invasion by reversing the EMT process.
PI3K/Akt signaling pathway plays an important role in regulating cancer cell proliferation, differentiation, invasion, and apoptosis (22). PI3K/Akt signaling is closely related with CRC development and progression. Both PI3K and Akt are frequently activated in CRC and are critical for CRC cell growth (23–26). It was reported that activated Akt can drive epithelial mesenchymal transition as cells acquire fibroblast-like characteristics and decreases the transcription of E-cadherin, which would reduce cell adhesion and increase cell motility and invasion (27). In this study, we examined the effect of SPOCK1 on the expression of PI3K and Akt phosphorylation in HCT116 cells and found that knockdown of SPOCK1 obviously decreased levels of PI3K and Akt phosphorylation in HCT116 cells. An Akt inhibitor (wortmannin) could enhance the effects of shSPOCK1 on CRC cell proliferation and invasion. These data suggest that SPOCK1 silencing inhibits the proliferation and invasion by suppressing the PI3K/Akt signaling pathway.
In total, our study demonstrated for the first time that knockdown of SPOCK1 inhibits the proliferation and invasion in CRC cells, possibly through the PI3K/Akt signaling pathway. Therefore, SPOCK1 may be a potential therapeutic target for the treatment of CRC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A.; Bray F.; Center M. M.; Ferlay J.; Ward E.; Forman D. Global cancer statistics. CA Cancer J. Clin. 61:69–90; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Ducreux M.; Adenis A.; Pignon J.-P.; François E.; Chauffert B.; Ichanté J.; Boucher E.; Ychou M.; Pierga J.-Y.; Montoto-Grillot C. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: Final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur. J. Cancer 49:1236–1245; 2013. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E.; Köhne C.-H.; Láng I.; Folprecht G.; Nowacki M. P.; Cascinu S.; Shchepotin I.; Maurel J.; Cunningham D.; Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 29:2011–2019; 2011. [DOI] [PubMed] [Google Scholar]
- 4. Benedix F.; Kube R.; Meyer F.; Schmidt U.; Gastinger I.; Lippert H.; Group, C. R. C.S. Comparison of 17,641 patients with right-and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis. Colon Rectum 53:57–64; 2010. [DOI] [PubMed] [Google Scholar]
- 5. Bradshaw A. D. Diverse biological functions of the SPARC family of proteins. Int. J. Biochem. Cell B 44:480–488; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartmann U.; Hülsmann H.; Seul J.; Röll S.; Midani H.; Breloy I.; Hechler D.; Müller R.; Paulsson M. Testican-3: A brain-specific proteoglycan member of the BM-40/SPARC/osteonectin family. J. Neurochem. 125:399–409; 2013. [DOI] [PubMed] [Google Scholar]
- 7. Hausser H.-J.; Decking R.; Brenner R. E. Testican-1, an inhibitor of pro-MMP-2 activation, is expressed in cartilage. Osteoarthr Cartilage 12:870–877; 2004. [DOI] [PubMed] [Google Scholar]
- 8. Cifuentes-Diaz C.; Alliel P. M.; Charbonnier F.; De La Porte S.; Molgó J.; Goudou D.; Rieger F.; Périn J.-P. Regulated expression of the proteoglycan SPOCK in the neuromuscular system. Mech. Dev. 94:277–282; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Li Y.; Chen L.; Chan T. H. M.; Liu M.; Kong K. L.; Qiu J. L.; Yuan Y. F.; Guan X. Y. SPOCK1 is regulated by CHD1l and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology 144:179–191; 2013. [DOI] [PubMed] [Google Scholar]
- 10. Röll S.; Seul J.; Paulsson M.; Hartmann U. Testican-1 is dispensable for mouse development. Matrix Biol. 25:373–381; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Szliter E.; McClellan S.; Barrett R.; Hazlett L. Testican-1/SPOCK1 promotes resistance against P. aeruginosa-induced keratitis via modulation of MMP-driven wound healing and ECM restoration. Invest. Ophthalmol. Vis. Sci. 50:1199–1199; 2009. [Google Scholar]
- 12. Guan X.-Y.; Li Y.; Chen L.; Yuan Y.-F. Characterization of a novel oncogene SPOCK1 in humanhepatocellular carcinoma. Cancer Res. 73:5309–5309; 2013.23970479 [Google Scholar]
- 13. Song X.; Han P.; Liu J.; Wang Y.; Li D.; He J.; Gong J.; Li M.; Tu W.; Yan W. Up-regulation of SPOCK1 induces epithelial–mesenchymal transition and promotes migration and invasion in esophageal squamous cell carcinoma. J. Mol. Histol. 46:347–356; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Miao L.; Wang Y.; Xia H.; Yao C.; Cai H.; Song Y. SPOCK1 is a novel transforming growth factor-β target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem. Bioph. Res. Commun. 440:792–797; 2013. [DOI] [PubMed] [Google Scholar]
- 15. Yang J.; Yang Q.; Yu J.; Li X.; Yu S.; Zhang X. SPOCK1 promotes the proliferation, migration and invasion of glioma cells through Pi3k/Akt and Wnt/β-catenin signaling pathways. Oncol. Rep. 35:3566–3576; 2016. [DOI] [PubMed] [Google Scholar]
- 16. Shu Y.-J.; Weng H.; Ye Y.-Y.; Hu Y.-P.; Bao R.-F.; Cao Y.; Wang X.-A.; Zhang F.; Xiang S.-S.; Li H.-F. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol. Cancer 14:1; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L.; Wang Y.; Zhang L. Effects of shRNA-mediated knockdown of SPOCK1 on ovarian cancer growth and metastasis. Cell. Mol. Biol. 61:102–109; 2014. [PubMed] [Google Scholar]
- 18. Yilmaz M.; Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 28:15–33; 2009. [DOI] [PubMed] [Google Scholar]
- 19. De Craene B.; Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13:97–110; 2013. [DOI] [PubMed] [Google Scholar]
- 20. Bates R. C.; Mercurio A. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol. Ther. 4:371–376; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Hur K.; Toiyama Y.; Takahashi M.; Balaguer F.; Nagasaka T.; Koike J.; Hemmi H.; Koi M.; Boland C. R.; Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 62:1315–1326; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martini M.; De Santis M. C.; Braccini L.; Gulluni F.; Hirsch E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 46:372–383; 2014. [DOI] [PubMed] [Google Scholar]
- 23. Pandurangan A. K. Potential targets for prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac. J. Cancer Prev. 14:2201–2205; 2013. [DOI] [PubMed] [Google Scholar]
- 24. Ye Q.; Cai W.; Zheng Y.; Evers B. M.; She Q.-B. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene 33:1828–1839; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson S. M.; Gulhati P.; Rampy B. A.; Han Y.; Rychahou P. G.; Doan H. Q.; Weiss H. L., Evers B. M. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J. Am. Coll. Surg. 210:767–776; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H.-Y.; Zhang Y.; Cai J.-H.; Bian H.-L. Microrna-451 inhibits growth of human colorectal carcinoma cells via downregulation of PI3K/AKT pathway. Asian Pac. J. Cancer Prev. 14:3631–3634; 2013. [DOI] [PubMed] [Google Scholar]
- 27. Grille S. J.; Bellacosa A.; Upson J.; Klein-Szanto A. J.; Van Roy F.; Lee-Kwon W.; Donowitz M.; Tsichlis P. N.; Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172–2178; 2003. [PubMed] [Google Scholar]