Abstract
Recently, deubiquitinating enzymes (DUBs) are emerging as new regulators in cancer progression. However, understanding of the involvement of DUBs in non-small cell lung cancer (NSCLC) is just beginning. In this study, we investigated the expression and biological function of ubiquitin-specific peptidase 17 (USP17) in NSCLC progression in vitro and in vivo. We found that the expression of USP17 was higher than in a normal control. We further efficiently depleted USP17 expression in two different NSCLC cells, A549 and H1299. The anchorage-independent growth ability of these cells, estimated by soft agar colony formation assay, was significantly reduced after USP17 knockdown. Moreover, Matrigel–Transwell analysis showed that suppression of USP17 decreased cell migration and invasion capacity. Molecular mechanism studies found that USP17 silencing downregulated the expression of matrix metalloproteases (MMP3 and MMP9) in NSCLC cells. Furthermore, animal model results showed that USP17 suppression inhibited NSCLC tumorigenesis and growth. Altogether, this study illustrates the important functions of USP17 in NSCLC and suggests that USP17 might be an attractive target for NSCLC therapy.
Key words: Ubiquitin-specific peptidase 17 (USP17), Non-small cell lung cancer (NSCLC), Tumorigenesis, Invasion, Matrix metalloproteases (MMPs)
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide (1), and non-small cell lung cancer (NSCLC) accounts for approximately 80% of deaths (2). The 5-year survival rate of NSCLC remains poor for most patients (3). In NSCLC patients, regional lymph node, liver, contralateral lung, brain, and bone marrow are the most common metastatic sites causing death in NSCLC patients (4). Therefore, the major challenge in treating NSCLC is to find novel therapeutic targets that can complement current chemotherapy.
The ubiquitin proteasome system (UPS) is an essential metabolic constituent of cellular physiology that tightly regulates cellular protein levels with specificity and precision to optimize cellular function (5,6). In addition, several other mechanisms have emerged by which ubiquitination can regulate protein function, including regulation of subcellular localization, protein–protein interactions, and activity (7). The relationship between ubiquitin and cancer biology began early, and inhibition of the proteasome has proven very effective in the treatment of various forms of cancer (6). Deubiquitinating enzymes (DUBs) can digest ubiquitin chains and reverse the process of protein ubiquitination (8).
Ubiquitin-specific protease 17 (USP17), a novel DUB, has been implicated in the regulation of inflammation (9), cell motility (10), development of Th17 cells (11), and oncogenesis (12–14). It has been reported that USP17 was overexpressed in several tumor-derived cell lines (14). It regulates the Ras pathway by controlling the intracellular localization of Ras and other small guanosine triphosphatases (GTPases), which are crucial regulators of cell proliferation and migration (10). These functions demonstrate potential roles of USP17 in promoting tumor growth and metastasis. Interestingly, McFarlane et al. reported that USP17 was upregulated in squamous NSCLC patients. Patients with USP17-positive tumors had significantly reduced recurrence-free survival compared with those with USP17-negative tumors, and USP17 correlated with the recurrence of disease at distant sites (15). However, the biological function of USP17 that directly regulates NSCLC cancer progression and metastasis has not been discovered.
Here we showed that USP17 was highly expressed in NSCLC tumor tissues. In addition, we showed that USP17 knockdown significantly reduces the anchorage-independent growth and invasion abilities of NSCLC cells. Moreover, the in vivo animal models revealed that USP17 suppression reduced tumorigenesis and growth. In this study, we first identified that upregulated USP17 was involved in NSCLC tumorigenesis and invasion through targeting matrix metalloproteinases 3 and 9 (MMP3 and MMP9), indicating that USP17 might be an attractive target for NSCLC therapy.
MATERIALS AND METHODS
Patient Data
NSCLC cancer tissues and adjacent normal tissues were collected from patients and healthy volunteers at Zhongshan Hospital, Fudan University after obtaining the subjects’ informed consent and institutional review board approval.
Cell Lines
The human NSCLC cell lines A549 and H1299 were purchased from Stem Cell Bank, Chinese Academy of Sciences. A549 was maintained in Dulbecco’s modified Eagle’s medium (DMEM), and H1299 was maintained in 1640 medium. All the media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), penicillin (100 U/ml), and streptomycin (100 µg/ml) in a humidified atmosphere of 5% CO2 at 37°C. All the cells were confirmed to be free from mycoplasma contamination.
Plasmids and Reagents
The target sequence of USP17 knockdown was 5′-CTCTTGAGAATGTGCCGAT-3′. Antibodies used were the following: goat anti-human USP17 (AP5491b; Abgent), mouse anti-actin (3700P; Cell Signaling Technology), rabbit anti-human MMP2 (ab192082; Abcam), rabbit anti-human MMP3 (ab53015; Abcam), rabbit anti-human MMP9 (3852; Cell Signaling Technology). The lentivirus was packaged using psPAX2 and pMD2G, a three-plasmid system (Hanyin Biotech Co., Shanghai). To obtain stable cell lines, lentivirus supernatant was added to A549 and H1299 cells, followed by screening with 1 µg/ml puromycin for 2 weeks.
Cell Invasion Assay
After treatment, cells were detached and resuspended in a serum-free medium and seeded on the upper chamber of Matrigel-coated Transwell inserts with a pore size of 8 µm. Culture medium containing 10% FBS as a chemo-attractant was added to the lower chamber. After 24 h of incubation, the cells on the upper surface of the insert were gently removed with a cotton swab. Invading cells (lower surface of the insert) were fixed with 4% paraformaldehyde (Sigma-Aldrich), stained with crystal violet, and counted under a microscope. Five random microscopic fields were examined for each insert.
Soft Agar Colony Formation Assay
Soft agar assay was performed in six-well plates. An agar suspension (0.3% agar) containing 103 colony-forming cells was plated over an agar underlay (2.0% agar). The formed colonies were visible in about 14 days. The colonies were then counted using a dissecting microscope. The experiment was performed in triplicate and repeated three times.
Western Blot Analysis
Protein lysates (50 µg per lane) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking with 5% fat-free milk, the membranes were incubated with primary antibodies (1:500 dilution) at 4°C overnight, followed by horseradish peroxidase-conjugated secondary antibodies (1:3,000 dilution). Immune-reactive proteins were visualized by enhanced chemiluminescence (ECL).
Animal Models of NSCLC
To NSCLC models, cells with or without USP17 knockdown were injected into tail veins. After 6 weeks, all mice were euthanized using CO2, and lungs were removed and fixed in Bouin’s solution. Lung micrometastases larger than 0.5 mm in diameter in five lobes were counted using an anatomy microscope. Every group included eight mice. All animal studies were approved by the Institutional Animal Care and Use Committee of the Shanghai Institutes for Biological Sciences.
Statistics
Two-tailed Student’s t-tests were used to analyze differences between protein overexpression and control groups. One-way analysis of variance was initially performed to determine whether overall statistically significant change occurred before using two-tailed paired or unpaired Student’s t-tests. Multiple test-adjusted values of p < 0.05 were considered statistically significant.
RESULTS
Expression of USP17 in NSCLC Cells and USP17 Knockdown Strategy
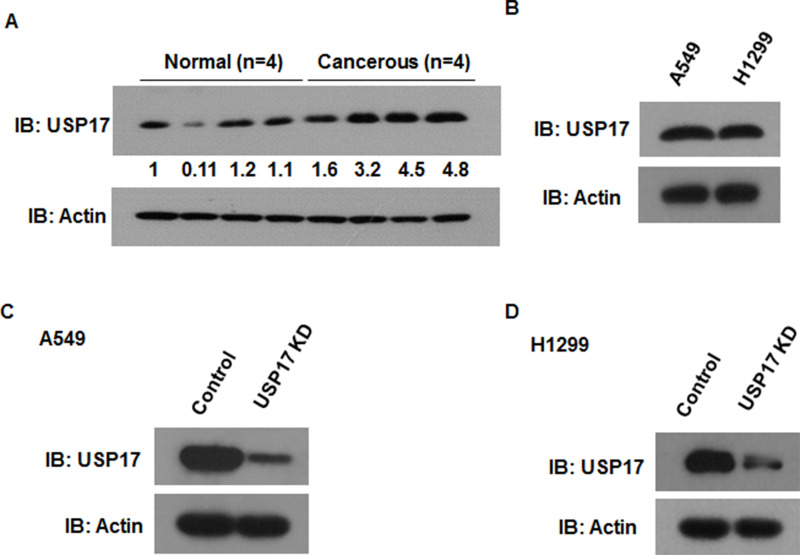
Some researchers have demonstrated that USP17 is overexpressed in both squamous and adenocarcinoma NSCLC tissues (15). We analyzed USP17 expression in NSCLC tumor tissues and adjacent normal tissues. Consistently, we found that USP17 was upregulated in NSCLC tumor tissues (Fig. 1A). We further confirmed USP17 expression in two different NSCLC cell lines, A549 and H1299. The protein expression level of USP17 was analyzed by Western blot. The Western blot analysis showed that USP17 was highly expressed in both NSCLC cell lines (Fig. 1B). The underlying mechanism accounting for the high expression of USP17 in NSCLC cells is yet unclear. To investigate the effects of USP17 on NSCLC progression, we used target-specific short hairpin RNA (shRNA) to knock down endogenous USP17 in two NSCLC cell lines, A549 and H1299. USP17 protein levels significantly decreased after stably transfecting recombinant shRNA in both cell lines (Fig. 1C and D).
Figure 1.
Expression of USP17 in NSCLC cells. (A) Western blot analysis of USP17 in NSCLC tumor tissues and adjacent normal tissues (n = 4). (B) Western blot analysis of USP17 in A549 and H1299 cells. (C) Western blot analysis of USP17 knockdown efficiency in A549 cells. (D) Western blot analysis of USP17 knockdown efficiency in H1299 cells.
USP17 Inhibition Reduces Cell Colony Formation Potential in NSCLC Cell Lines
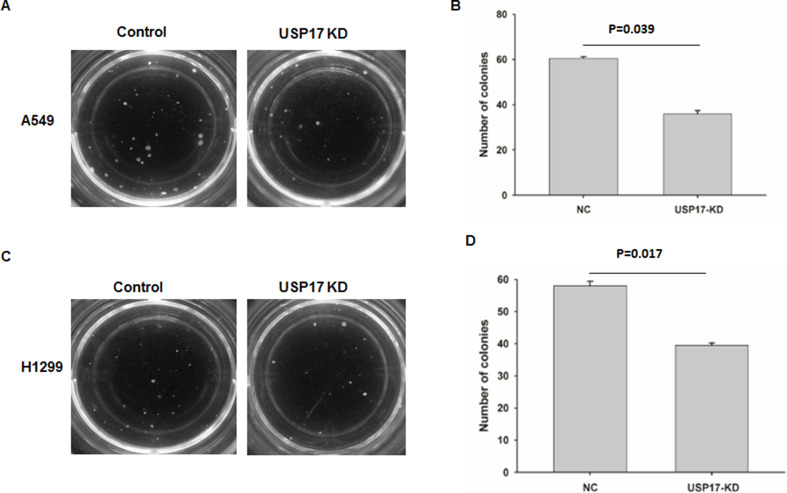
To investigate the biological functions of USP17 in the transformation of NSCLC cells, we conducted soft agar colony formation assay to determine the anchorage-independent growth and tumorigenesis abilities of the NSCLC cell lines A549 (Fig. 2A and B) and H1299 (Fig. 2C and D). The colony formation assay showed that the number of colonies decreased following USP17 downregulation in the two NSCLC cell lines (Fig. 2C).
Figure 2.
Knockdown (KD) of USP17 reduced the tumorigenesis ability of NSCLC cells. (A) Representative photo of the colony formation results of control or USP17 KD A549 cells. (B) Colony number of control or USP17 KD A549 cells. (C) Representative photo of the colony formation results of control or USP17 KD H1299 cells. (D) Colony number of control or USP17 KD H1299 cells.
USP17 Expression Is Required for the Migration and Invasion of NSCLC Cells
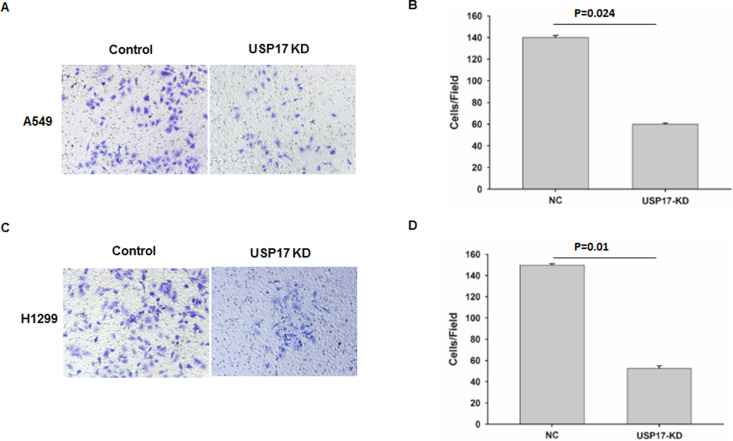
It has been reported that USP17 was more highly expressed in patients with recurrence of disease at distant sites, suggesting that USP17 levels may correlate with NSCLC distant metastasis (15). To figure out the function of USP17 on cell migration and invasion, we used A549 and H1299 cells to determine the effects of USP17 on NSCLC invasion. The effect of USP17 on invasion was assessed using a modified Boyden chamber assay. Knockdown of USP17 significantly decreased the invading capacity of both cells (Fig. 3A and C). In addition, depletion of USP17 in both cells showed more than 60% reduction of invasion capacity when compared with control cells (Fig. 3B and D). These results indicated that USP17 is indispensable for the regulation of NSCLC cell invasion.
Figure 3.
Suppression of USP17 reduced the invasion ability of NSCLC cells. (A, C) Matrigel-based Transwell assay in A549 (A) and H1299 (C) cells after USP17 knockdown. (B and D) The quantification of Transwell assay in A549 (B) and H1299 (D).
USP17 Knockdown Impairs Tumor Proliferation and Metastasis Through Targeting MMPs
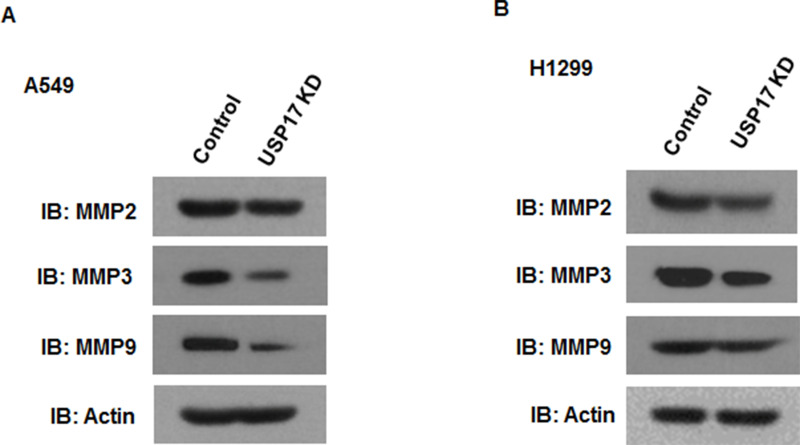
Because degradation of the basement membrane by MMPs is required for tumor cell migration and invasion (16,17), we sought to determine whether MMPs were responsible for USP17-dependent growth and invasion. Interestingly, our Western blot analysis found that depletion of USP17 dramatically reduced MMP3 and MMP9 but not MMP2 expression in both NSCLC cells (Fig. 4A and B).
Figure 4.
Inhibition of USP17 reduced the expression of MMPs in NSCLC cells. Western bolt analysis of MMP2, MMP3, and MMP9 in A549 (A) and H1299 (B) cells after USP17 knockdown.
Inhibition of USP17 Reduced Tumorigenesis and Progression of NSCLC In Vivo
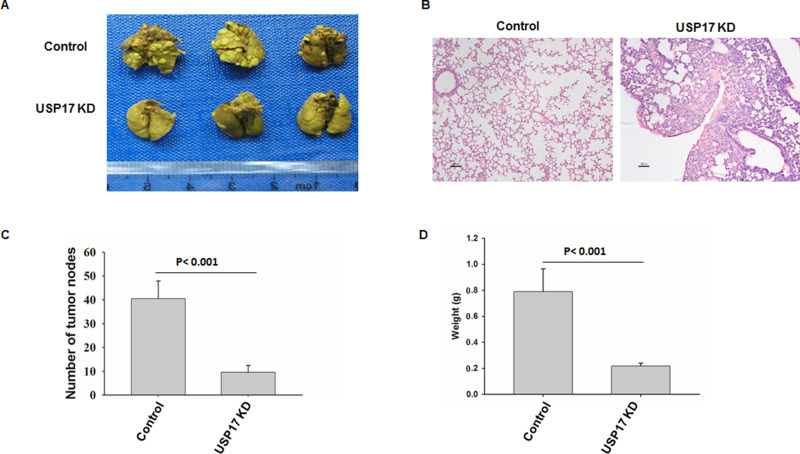
To further examine the role of USP17 in NSCLC progression in vivo, we injected H1299 cells with or without USP17 knockdown into the tail vein of nude mice, resulting in a substantial number of pulmonary tumor nodules (Fig. 5). However, knockdown of USP17 significantly reduced the number of pulmonary nodules (Fig. 5A–C) and the tumor weight (Fig. 5D).
Figure 5.
Suppression of USP17 reduced tumorigenesis and progression of NSCLC in vivo. (A) Tumor formation analysis of nude mice (n = 8/group) after 6-week inoculation of indicated H1299 cells with or without USP17 knockdown (1 × 106) through tail vein. Representative lungs are shown (n = 8/group). Data are representative of three independent experiments. (B) Representative hematoxylin and eosin (H&E) staining analysis of each group is shown. (C) The numbers of lung tumor nodules were counted under anatomy microscope. (D) The weight of the lung tumor nodules was analyzed. Data are representative of three independent experiments (mean and SEM).
Taken together, our study showed that USP17 promotes tumorigenesis and invasion through regulation of MMPs (MMP3 and MMP9) in NSCLC cells and provides a promising approach for NSCLC treatment and prevention.
DISSCUSSION
USP is one of the five subfamilies of DUB enzymes whose function is to deubiquitinate the polyubiquitin chains. USP17, a novel DUB, contains hyaluronan and RNA binding motifs and can be induced by cytokines such as interleukin-4 (IL-4) and IL-6 (18). Its function was initially identified as a regulator of cell viability through regulating the signaling pathway involved in cell death in cervical cancer cell (19). Subsequently, USP17 expression was reported to regulate Ras cellular localization and activation, thereby inhibiting phosphorylation of the downstream kinases MEK and ERK. This process was caused by the inactivation of Ras-converting enzyme 1 (RCE1) as a result of deubiquitination by USP17 (20). Another evidence of USP17 participation in cell progression was that USP17 depletion significantly impaired G1–S transition and blocked cell proliferation (14). The aberrant expression of USP17 was first identified in several tumor-derived cell lines and cervical tumor biopsies in vitro (14). Recently, USP17 expression was identified in NSCLC tissues, and it was found that USP17-positive patients had significantly reduced recurrence-free survival compared with those with USP17-negative tumors (15). However, there is still no direct experiment to prove the oncogenic role of USP17 in NSCLC.
In our work, we found that USP17 was expressed in the NSCLC cells A549 and H1299. USP17 knockdown significantly suppressed the tumorigenesis ability of both the NSCLC cell lines A549 and H1299. Moreover, USP17 participated in the invasion of NSCLC cells. These functions of USP17 are partially mediated by regulating the expression of MMP proteins MMP3 and MMP9. Our results confirmed the previous works on the clinical relevance of USP17 in NSCLC (15) and proved the hypothesis that USP17 could participate in NSCLC progression and metastasis. This is the first report of USP17 being required for NSCLC cell tumorigenesis and invasion.
In recent years, efforts have been made to discover agents targeting DUB activities. However, no efficient drugs were entered into clinical trials. Targeting USP17 might prove beneficial to prevent tumotigenesis and metastasis in NSCLC.
REFERENCES
- 1. Torre L. A.; Bray F.; Siegel R. L.; Ferlay J.; Lortet-Tieulent J.; Jemal A. Global cancer statistics. CA Cancer J. Clin. 65:87–108; 2012. [DOI] [PubMed] [Google Scholar]
- 2. Wood S. L.; Pernemalm M.; Crosbie P. A.; Whetton A. D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 40:558–566; 2014. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R.; Ma J.; Zou Z.; Jemal A. Cancer statistics. CA Cancer J. Clin. 64:9–29; 2014. [DOI] [PubMed] [Google Scholar]
- 4. Fidler I. J. Critical determinants of cancer metastasis: Rationale for therapy. Cancer Chemother. Pharmacol. 43 Suppl:S3–S10; 1999. [DOI] [PubMed] [Google Scholar]
- 5. D’Andrea A.; Pellman D. Deubiquitinating enzymes: A new class of biological regulators. Crit. Rev. Biochem. Mol. Biol. 33:337–352; 1998. [DOI] [PubMed] [Google Scholar]
- 6. Weathington N. M.; Mallampalli R. K. Emerging therapies targeting the ubiquitin proteasome system in cancer. J. Clin. Invest. 124:6–12; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welchman R. L.; Gordon C.; Mayer R. J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell. Biol. 6:599–609; 2005. [DOI] [PubMed] [Google Scholar]
- 8. Nijman S. M.; Luna-Vargas M. P.; Velds A.; Brummelkamp T. R.; Dirac A. M.; Sixma T. K.; Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Song H.; Tao L.; Chen C.; Pan L.; Hao J.; Ni Y.; Li D.; Li B.; Shi G. USP17-mediated deubiquitination and stabilization of HDAC2 in cigarette smoke extract-induced inflammation. Int. J. Clin. Exp. Pathol. 8:10707–10715; 2015. [PMC free article] [PubMed] [Google Scholar]
- 10. de la Vega M.; Kelvin A. A.; Dunican D. J.; McFarlane C.; Burrows J. F.; Jaworski J.; Stevenson N. J.; Dib K.; Rappoport J. Z.; Scott C. J.; Long A.; Johnston J. A. The deubiquitinating enzyme USP17 is essential for GTPase subcellular localization and cell motility. Nat. Commun. 2:259; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han L.; Yang J.; Wang X.; Wu Q.; Yin S.; Li Z.; Zhang J.; Xing Y.; Chen Z.; Tsun A.; Li D.; Piccioni M.; Zhang Y.; Guo Q.; Jiang L.; Bao L.; Lv L.; Li B. The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor gammat (RORgammat) in Th17 cells. J. Biol. Chem. 289:25546–25555; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borbely G.; Haldosen L. A.; Dahlman-Wright K.; Zhao C. Induction of USP17 by combining BET and HDAC inhibitors in breast cancer cells. Oncotarget 6:33623–33635; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu M.; Chen H.; Han C.; Lan J.; Xu Y.; Li C.; Xue Y.; Lou M. Expression and functional implications of USP17 in glioma. Neurosci. Lett. 616:125–131; 2016. [DOI] [PubMed] [Google Scholar]
- 14. McFarlane C.; Kelvin A. A.; de la Vega M.; Govender U.; Scott C. J.; Burrows J. F.; Johnston J. A. The deubiquitinating enzyme USP17 is highly expressed in tumor biopsies, is cell cycle regulated, and is required for G1-S progression. Cancer Res. 70:3329–3339; 2010. [DOI] [PubMed] [Google Scholar]
- 15. McFarlane C.; McFarlane S.; Paul I.; Arthur K.; Scheaff M.; Kerr K.; Stevenson M.; Fennell D. A.; Johnston J. A. The deubiquitinating enzyme USP17 is associated with non-small cell lung cancer (NSCLC) recurrence and metastasis. Oncotarget 4:1836–1843; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang M.; Dai C.; Zhu H.; Chen S.; Wu Y.; Li Q.; Zeng X.; Wang W.; Zuo J.; Zhou M.; Xia Z.; Ji G.; Saiyin H.; Qin L.; Yu L. Cyclophilin A promotes human hepatocellular carcinoma cell metastasis via regulation of MMP3 and MMP9. Mol. Cell. Biochem. 357:387–395; 2011. [DOI] [PubMed] [Google Scholar]
- 17. Kessenbrock K.; Plaks V.; Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 141:52–67; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baek K. H. Cytokine-regulated protein degradation by the ubiquitination system. Curr. Protein Pept. Sci. 7:171–177; 2006. [DOI] [PubMed] [Google Scholar]
- 19. Shin J. M.; Yoo K. J.; Kim M. S.; Kim D.; Baek K. H. Hyaluronan- and RNA-binding deubiquitinating enzymes of USP17 family members associated with cell viability. BMC Genomics 7:292; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burrows J. F.; Kelvin A. A.; McFarlane C.; Burden R. E.; McGrattan M. J.; De la Vega M.; Govender U.; Quinn D. J.; Dib K.; Gadina M.; Scott C. J.; Johnston J. A. USP17 regulates Ras activation and cell proliferation by blocking RCE1 activity. J. Biol. Chem. 284:9587–9595; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]