Abstract
Triple negative breast cancer (TNBC) is a phenotype of breast cancer with aggressive clinical behavior. Because of the absence of optimal treatment, the prognosis of this disease is poor. The main purpose of this study was to detect the response to neoadjuvant chemotherapy (NACT) in a TNBC cohort and compare the long-term survival between patients with and without pathological complete response (pCR). A total of 53 patients diagnosed with TNBC from 2005 to 2013 who received NACT at the University Hospital Birmingham were enrolled in this study. Overall survival (OS) and progression-free survival (PFS) were compared between the pCR group and non-pCR group. Demographic information and clinical or pathologic parameters were also analyzed to explore potential predictive and prognostic factors. Fourteen patients (26.4%) achieved pCR to NACT. In univariate analysis, patients with pCR had longer PFS time (p = 0.013) and OS time (p = 0.054) compared with their counterparts without pCR. In multivariate analysis, the existence of lymphovascular invasion (LVI) significantly reduced OS (HR = 17.404, 95% CI = 2.923–103.644) and PFS (HR = 7.776, 95% CI = 1.645–36.753). The achievement of pCR to NACT can significantly postpone the incidence of disease progression in patients with TNBC. There is not enough evidence showing its influence on ultimate survival. LVI may be a more potent prognostic factor than pCR in the TNBC cohort.
Key words: Breast cancer, Triple negative, Neoadjuvant chemotherapy (NACT), Pathological complete response (pCR)
INTRODUCTION
Triple negative breast cancer (TNBC) is an aggressive clinical phenotype of breast cancer with negative expression of estrogen receptor (ER) and progesterone receptor (PgR) and absence of human epidermal growth factor receptor 2 (HER-2) amplification (1,2). TNBC accounts for 10–20% of all breast cancers, and the incidence of TNBC is epidemiologically associated with younger women, younger age at menarche, short duration of breastfeeding, obesity of premenopausal women, BRCA1 mutations, and African-American or non-Hispanic black race (3–9). Although the incidence of TNBC is relatively lower compared with other subtypes (e.g., HER-2 or ER/PgR overexpressing subtypes), TNBC is still more likely to have aggressive clinical behavior, such as larger tumor size, high histological grades, and involvement of regional lymph nodes (1,3).
Unlike hormone receptor or HER-2-positive tumors, which are eligible for endocrine treatment or HER-2-targeted therapy with agents such as trastuzumab (10,11), systematic treatment of TNBC is limited to cytotoxic chemotherapy due to the lack of molecular targets, and there is no specific guideline for its clinical management (5,12–16). Therefore, TNBC is related to a worse clinical outcome and higher risk of early metastatic diseases compared with other breast cancer phenotypes (1,8,9,13,17). Previous studies reported that the prognosis of TNBC was the poorest with an overall survival (OS) rate of 76.2% and a hazard ratio (HR) of 1.8 using the subtype with positive ER/PgR and negative HER-2 as a reference (8,17).
To improve the clinical outcome of TNBC, previous studies focused on evaluating existing chemotherapy regimens. Cumulative evidence shows that TNBC compared with non-TNBC has higher chemosensitivity, especially in a neoadjuvant setting with a higher percentage of pathological complete response (pCR) (18,19). Because optimal chemotherapy regimen has not yet been established, standard anthracycline- and/or taxane-based chemotherapy is still widely used (20). TNBC is sensitive to neoadjuvant anthracycline-based regimens with higher pCR rates around 17–58% compared with luminal breast cancer (14,21). Furthermore, neoadjuvant chemotherapy (NACT) is considered to be advantageous to aggressive tumors like TNBC because it can reduce tumor size and makes surgery feasible for initially unresectable tumors or makes breast-conserving surgery eligible for resectable tumors (18).
Nevertheless, whether the short-term benefit of NACT can be converted into the improvement of long-term survival in a TNBC cohort is still controversial (5,18,19). On the one hand, a few studies (15,16,21) proposed that patients with pCR to NACT had a better prognosis across different subtypes of breast cancer. However, for TNBC patients in the non-pCR group, the survival rate was lower than that with adjuvant chemotherapy (ACT), which may be explained as an elevated risk of disease relapse if pCR was not achieved after NACT (21). On the other hand, a recent neoadjuvant study (22) compared the survival difference between ACT and NACT in patients with TNBC and demonstrated that chemotherapy delivered in a postsurgery setting significantly reduced the risk of overall death compared with presurgery or none/unknown chemotherapy (HR = 0.476, 95% CI 0.295–0.770). Their subsequent study (23) further illustrated that patients with pCR to NACT had better OS than patients who received ACT, but the result was not statistically significant (HR = 0.19, p = 0.10). Therefore, further neoadjuvant studies are required to examine the relationship between short-term and long-term benefits of NACT to TNBC patients. This study evaluated the prognostic value of pCR to NACT in patients with TNBC.
MATERIALS AND METHODS
This study had four key objectives: (a) to investigate the short-term response to NACT, (b) to establish the association between pCR and OS on progression-free survival (PFS), (c) to identify prognostic factors other than pCR, and (d) to identify potential factors that influence the rates of pCR.
Study Population
Women eligible for the study were patients newly diagnosed with breast cancer between January 2005 and November 2013 who met the following criteria: (a) patients diagnosed with breast cancer in University Hospital Birmingham (UHB) or patients referred from other hospitals but treated in UHB, (b) quick scores of ER/PgR were ≤2, and immunohistochemistry scores of HER-2 were ≤1+ or 2+ without amplification in fluorescence in situ hybridization analysis, (c) confirmation of hormone receptors and HER-2 status was based on core needle biopsy at diagnosis or excisional biopsy after surgery, (d) primary breast tumors were treated with NACT followed by surgery (mastectomy or breast-conserving surgery) in UHB.
Exclusion criteria are shown as follows: (a) NACT for recurrent breast cancer; (b) no surgery after NACT; (c) insufficient data for analysis.
Data Collection
Data including demographic information, tumor features, and treatment records were collected from the clinical portal system and medical correspondence in UHB. In addition, expression of ER, PgR, and HER-2 was confirmed by checking pathological reports from the Department of Histopathology in UHB.
All measurements of tumor size were based on radiological, clinical, or histopathological examinations. In particular, pretreatment pathological diagnosis was based on core needle biopsy of primary breast tumor at diagnosis, while the posttreatment part was based on surgery specimens of breast tumor and axillary lymph nodes. Histological grade was subjected to excisional material. Alternatively, histological grade was cited from pretreatment biopsy if no residual invasive tumor was seen or no record was available in surgery specimens.
Data Evaluation
Short-term responses to NACT were evaluated by use of clinical response rates and pCR rates. pCR was investigated with surgery specimens and defined as disappearance of all invasive tumors plus the involved lymph nodes (ypT0/is pN0) (15).
Clinical response rates were evaluated on the basis of Response Evaluation Criteria in Solid Tumours (RECIST) Guideline (version 1.1) but modulated to measure primary tumors in breast only (24). Therefore, complete response (CR) was defined as disappearance of all primary tumors detected radiologically or clinically; partial response (PR) was defined as at least 30% reduction in the sum of diameters of breast tumors; progressive disease (PD) was defined as at least 20% increase in the sum of diameters of breast tumors compared with the smallest sum during clinical detection; and stable disease (SD) described the change of tumor sizes between PR and PD. Among them, objective response equaled PR plus CR. For patients who attained fluctuating response to NACT, the best response across all time points was recorded for analysis.
OS and PFS were applied to evaluated long-term prognosis. OS was calculated from the date of diagnosis to the date of death or censored if the patient was known to be alive on the last day of data collection (i.e., July 31, 2014). PFS was calculated from date of diagnosis to the earliest date of disease progression or death or censored in the same situation as OS.
Statistical Analysis
Data were analyzed via SPSS (version 2.2; IBM Corporation, Armonk, NY, USA). Statistical significance was confirmed with a significance level of 5%. The 95% CI was indicated if necessary. Univariate analysis of pCR rates was conducted with personal chi-square or Fisher’s exact probability tests for categorical data, and with a logistic regression model for continuous data, while multivariate analysis of pCR rates was not implemented since only one significant factor was identified in univariate analysis.
Survival rates influenced by each factor were analyzed with Kaplan–Meier curves with log-rank test for calculation of p value. Cox regression was operated in the following situations: (a) multivariate survival analysis of continuous variates [e.g., body mass index (BMI) tumor size, and Nottingham prognostic index (NPI)], (b) output of HR for each significant factor in univariate analysis, and (c) multivariate survival analysis.
RESULTS
Among about 6,000 medical records, a total of 53 valid cases of TNBC were diagnosed between 2005 and 2013 and received chemotherapy before definitive surgery. This study period was selected because the expression of ER, PgR, and HER-2 was routinely tested upon diagnosis. The status of hormone receptors or HER-2 changed in seven cases: Five cases transformed from negative ER and/or PgR (quick scores ≤2) to positive expression (quick score ≥3), one from positive to negative, and one initial TNBC presented with HER-2 amplification in surgery specimen. These cases were not excluded but recorded.
Clinical and Pathological Response
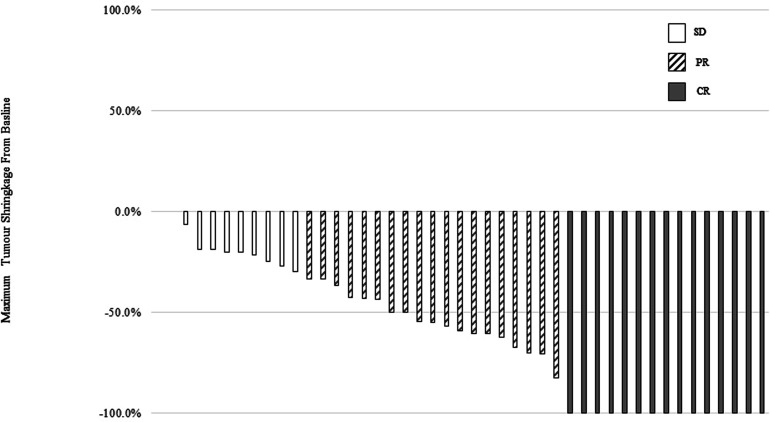
In clinical practice, CR to NACT can be evaluated by imaging studies and clinical examinations at the end of NACT. Table 1 summarizes different CR and PR after NACT. The data for CR were available in 45 patients. Figure 1 shows a waterfall graph of the maximum tumor shrinkage from baseline. Approximately 80.0% of patients responded objectively, including 15 (33.3%) with CR and 21 (46.7%) with PR. Meanwhile, nine patients (17.0%) did not respond significantly to NACT (i.e., SD). No disease progression happened before surgery.
Table 1.
Summary of Clinical and Pathological Response After Neoadjuvant Chemotherapy
| Clinical Response | Pathological Response | ||||
|---|---|---|---|---|---|
| No. of Patients | Valid Percentage (n = 45) | No. of Patients | Valid Percentage (n = 50) | ||
| CR | 15 | 33.3% | pCR | 14 | 28.0% |
| PR | 21 | 46.7% | Non-pCR | 36 | 72.0% |
| SD | 9 | 17.0% | Missing | 3 | |
| Missing | 8 | ||||
CR, complete response; PR, partial response; SD, stable disease; pCR, pathological complete response; Non-pCR, no pathological complete response.
Figure 1.
Waterfall plots of best percent change from baseline in measurable tumor during neoadjuvant chemotherapy courses. Different responses were in different patterned bars. Among them, 33.3% of patients receive clinical complete response (CR) from baseline, 46.7% of patients received clinical partial response (PR), and 17.0% of patients received stable disease (SD).
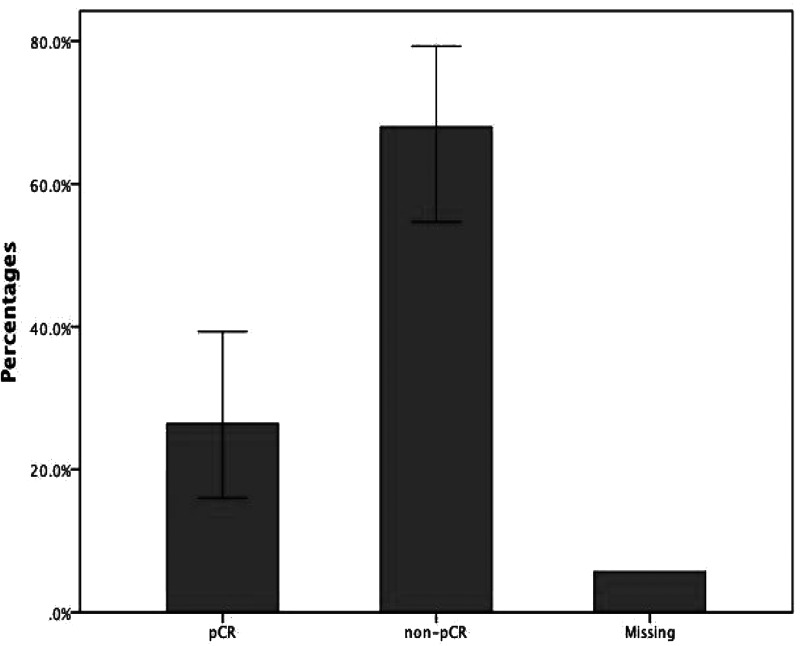
After definitive surgery, the response was also confirmed pathologically using excision samples (Fig. 2). The 53 cases involved 14 patients with pCR, 36 patients with residual tumors in breast or regional lymph nodes after NACT (i.e., non-pCR), and three patients with unidentified response due to incomplete pathological report (not stated). Hence, the valid percentage of patients who received pCR was 28.0% (26/50).
Figure 2.
Percentages of patients who achieved pathological complete response (pCR) and patients who did not receive pCR. A total of 14 patients received pCR after neoadjuvant chemotherapy, while 36 patients still had residual tumor in breast or axillary lymph nodes. Three patients had missing values.
Univariate Analysis of pCR
Univariate analysis of pCR involved 11 factors related to patients, tumor, or anticancer treatment (Table 2). Among them, BMI was the only risk factor that significantly influences pCR rates [odds ratio (OR) = 0.803, 95% CI = 0.658–0.979]. In total, 27 patients received both anthracycline and a taxane for their NACT, while 24 patients received anthracycline-based regimens and one received a taxane-based regimen. Table 3 summarized the regimens used in our study and pCR rates for each group. Three patients without pCR record were not listed in the table, including one in the E-CMF (epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil) group and two in the ET (epirubicin and docetaxel) group. Chi-square and Fisher’s exact tests were conducted among E-CMF, ET, and T-FEC/FEC-T (fluorouracil, epirubicin, and cyclophosphamide followed/following docetaxel) containing regimens to compare their pCR rates but failed to conclude significant difference (p = 0.064).
Table 2.
Univariate Analysis of pCR
| OR | 95% CI | p Value | |
|---|---|---|---|
| Age | 0.983 | 0.935–1.036 | 0.553 |
| Body mass index | 0.803 | 0.658–0.979 | 0.030 |
| Tumor size at presentation | 0.986 | 0.962–1.013 | 0.312 |
| Sides of tumor | |||
| Right | 1 | ||
| Left | 0.500 | 0.142–1.756 | 0.276 |
| Nodes at presentation | |||
| Negative | 1 | ||
| Positive | 1.185 | 0.338–4.149 | 0.791 |
| Smoking | |||
| No | 1 | ||
| Yes | 0.417 | 0.092–1.888 | 0.305 |
| Family history | |||
| No | 1 | ||
| Yes | 1.401 | 0.345–5.682 | 0.718 |
| Comorbidity | |||
| No | 1 | ||
| Yes | 0.429 | 0.101–1.812 | 0.203 |
| Regimens of NACT | |||
| Anthracycline/taxanes | 1 | ||
| Anthracycline and taxanes | 1.143 | 0.318–4.109 | 0.838 |
| Severe side effects | |||
| No | 1 | ||
| Yes | 1.499 | 0.423–5.319 | 0.529 |
| Type of surgery | |||
| Mastectomy | 1 | ||
| Breast conserving | 2.012 | 0.563–7.193 | 0.278 |
NACT, neoadjuvant chemotherapy; OR, odds ratio; CI, confidence interval.
Table 3.
pCR Rates Among Different NACT Regimens
| Regimens | pCR (%) | Total | |
|---|---|---|---|
| Non-pCR | pCR | ||
| E-CMF | 13 (72.2%) | 5 (27.8%) | 18 |
| T-FEC/FEC-T | 9 (56.3%) | 7 (43.8%) | 16 |
| ET | 9 (100.0%) | 0 (0.0%) | 9 |
| Others | 4 (77.8%) | 2 (22.2%) | 6 |
| Not stated | 1 (100.0%) | 0 (0.0%) | 1 |
| Total | 38 (73.6%) | 14 (26.4%) | 50 |
E-CMF, epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil; T-FEC/FEC-T, fluorouracil, epirubicin, and cyclophosphamide followed/following docetaxel; ET, epirubicin and docetaxel.
Overall Survival and Progression-Free Survival
Information on OS was available from 52 patients, with a median follow-up duration of 54.5 (9–100) months and a median OS time of 53 months. The median survival time of the pCR group could not be computed because more than 50% of patients were still alive at the end of the study, while the median survival time of the non-pCR group was 45 (95% CI = 20–70) months. The 5-year survival rates of the pCR and non-pCR groups were 71% (95% CI = 62–80%) and 42% (95% CI = 39–45%), respectively (Table 4).
Table 4.
Long-Term Survival in pCR or Non-pCR Patients
| Median PFS (Months) | 95% CI (Months) | Median OS (Months) | 95% CI (Months) | 5-Year OS | 95% CI | |
|---|---|---|---|---|---|---|
| pCR | Not researched | – | Not researched | – | 71% | 62–80% |
| Non-pCR | 34 | 10–58 | 45 | 20–70 | 45% | 39–45% |
PFS, progression-free survival; CI, confidence interval; OS, overall survival; pCR, pathological complete response; non-pCR, no pathological complete response.
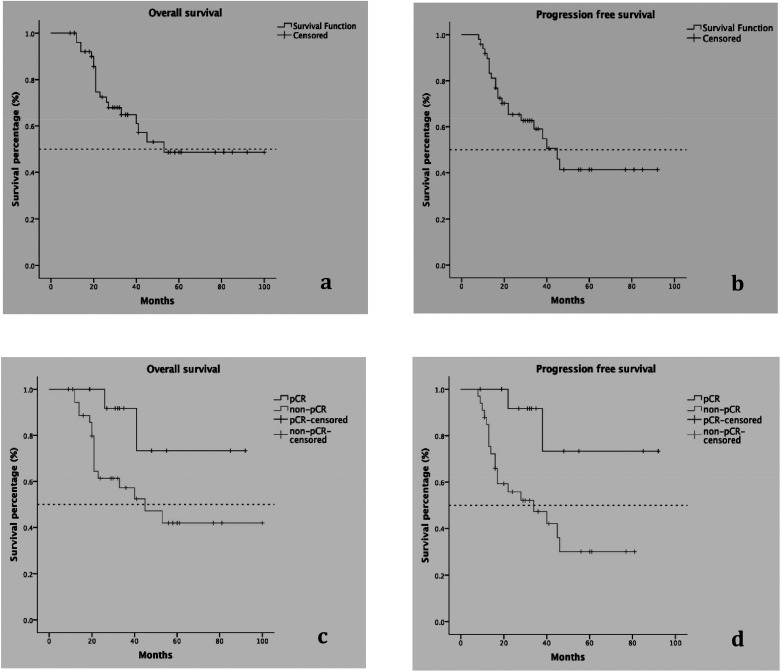
Information on PFS was available in 49 patients, with 22 cases of progression or death and 27 cases of survival at the end of data collection. The median survival time was 45 (95% CI = 33–57) months. The median survival time in the pCR group still could not be computed, but it was 34 (95% CI = 10–58) months in the non-pCR group (Table 4) because there were only two patients getting disease progression in pCR group compared with eight in the non-pCR group. Furthermore, the only two cases of progression in the pCR group occurred at 1.8 and 3.2 years after diagnosis. In contrast, the earliest progression in the non-pCR group happened 8 months after diagnosis. These results indicated that disease progression was delayed in the pCR group (HR = 0.193, 95% CI = 0.045–0.832). Figure 3 shows the Kaplan–Meier curves of OS and PFS in all patients, and the different survival curves between patients with pCR and without pCR.
Figure 3.
Long-term survival in all TNBC patients and the comparison between patients with pCR and those with no pCR. (a) OS in all TNBC patients, (b) PFS in all TNBC patients, (c) comparison of OS in patients with pCR (upper line) and those without pCR (lower line), (d) comparison of PFS in patients with pCR (upper line) and those without pCR (lower line). Dashed line refers to the level of median survival rates. Patients with pCR had improved OS and PFS compared with their non-pCR counterparts.
Univariate Analysis of Long-Term Survival
A total of 22 factors related to patients, tumors, or treatment were analyzed separately in univariate survival analysis (Table 5). Among them, OS rates between the pCR group and non-pCR group were borderline different (p = 0.054), while the variates that significantly influence OS were tumor size at presentation (p = 0.029), residual tumor size after NACT (p = 0.034), number of residual positive lymph nodes (p = 0.000), existence of lymphovascular invasion (LVI) (p = 0.000), type of surgery (p = 0.028), and administration of ACT (p = 0.005). In addition to pCR, there were another four factors with borderline significant influence, which were achievement of clinical CR (p = 0.056), age (p = 0.046), smoking history (p = 0.048), and existence of distant metastases at presentation (p = 0.041).
Table 5.
Univariate Analysis of Overall Survival (OS) and Progression-Free Survival (PFS)
| p Value | ||
|---|---|---|
| OS | PFS | |
| pCR or not | 0.054 | 0.013 |
| cCR or not | 0.056 | 0.062 |
| Age | 0.046 | 0.438 |
| Race | 0.094 | 0.211 |
| Body mass index | 0.840 | 0.477 |
| Smoking or not | 0.074 | 0.016 |
| Family history of cancer | 0.819 | 0.660 |
| Comorbidity or not | 0.433 | 0.397 |
| Tumor size at diagnosis | 0.029 | 0.080 |
| Tumor after NACT | 0.034 | 0.000 |
| Nodes at diagnosis | 0.138 | 0.082 |
| Nodes after NACT | 0.000 | 0.000 |
| LVI | 0.000 | 0.000 |
| Tumor grade | 0.911 | 0.344 |
| Tumor type | 0.200 | 0.123 |
| Change of receptors | 0.791 | 0.832 |
| Tumor sides | 0.559 | 0.755 |
| Regimens of NACT | 0.230 | 0.689 |
| Side effects | 0.268 | 0.185 |
| Type of surgery | 0.028 | 0.039 |
| ACT | 0.005 | 0.078 |
| ART | 0.102 | 0.163 |
pCR, pathological complete response; NACT, neoadjuvant chemotherapy; LVI, lymph vascular invasion; ACT, adjuvant chemotherapy; ART, adjuvant radiotherapy.
PFS rates were statistically different between the pCR group (n = 14) and non-pCR group (n = 33) (p = 0.013). Other variates that significantly influence PFS were smoking history (p = 0.031), existence of distant metastases at presentation (p = 0.000), residual tumor size after NACT (p = 0.000), existence of LVI (p = 0.000), number of residual positive lymph nodes (p = 0.000), and type of surgery (p = 0.039). Only one factor was found with borderline significance, which was achievement of clinical CR (p = 0.062).
Multivariate Analysis of Long-Term Survival
In terms of multivariate analysis, five variates for OS (Table 6) and five variates for PFS (Table 7) were analyzed via Cox regression model. The results showed that the achievement of pCR failed to prolong long-term survival (OS, p = 0.309; PFS, p = 0.247), but the existence of LVI is an independent variate that may harm OS (HR = 17.404, 95% CI = 2.923–103.644) and PFS (HR = 7.776, 95% CI = 1.645–36.753).
Table 6.
Multivariate Analysis of Overall Survival
| p Value | HR | 95.0% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| LVI | 0.002 | 17.404 | 2.923 | 103.644 |
| Type of surgery | 0.943 | 1.058 | 0.221 | 5.075 |
| pCR | 0.309 | 0.266 | 0.021 | 3.414 |
| ACT | 0.267 | 2.272 | 0.534 | 9.674 |
| Tumor size | 0.165 | 1.023 | 0.991 | 1.057 |
LVI, lymph vascular invasion; pCR, pathological complete response; ACT, adjuvant chemotherapy; HR, hazard ratio; CI, confidence interval.
Table 7.
Multivariate Analysis of Progression-Free Survival
| p Value | HR | 95.0% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| LVI | 0.010 | 7.776 | 1.645 | 36.753 |
| Type of surgery | 0.794 | 1.225 | 0.267 | 5.616 |
| pCR | 0.247 | 0.221 | 0.017 | 2.842 |
| Tumor size | 0.599 | 1.009 | 0.975 | 1.045 |
| Smoking | 0.140 | 2.513 | 0.740 | 8.539 |
LVI, lymph vascular invasion; pCR, pathological complete response; HR, hazard ratio; CI, confidence interval.
DISCUSSION
TNBC is an aggressive phenotype of breast cancer. Until now, whether the poor prognosis of TNBC is related to the aggressive features or limited treatment options has not been certain. This TNBC cohort study examined the relationship between response to NACT and long-term survival and aimed to identify additional predictive factors for disease progression and ultimate survival other than pCR. These results may help researchers and practitioners to better understand TNBC and the clinical value of pCR.
In this study, the pCR rate was lower than some recent trials evaluating the efficacy of additional carboplatin and/or bevacizumab in neoadjuvant setting for TNBC (25,26). Two phase 2 trials from von Minckwitz et al. (25) and Sikov et al. (26) showed that even in the non-carboplatin-containing arms, the pCR rates in TNBC were higher than the present study at 36.9% and 41%, respectively. The gap might be caused by the low proportion of our patients compared with the two studies mentioned above. Meanwhile, the regimens are different. In our study, the main regimen was only anthracycline- and/or taxane-containing without additional carboplatin or targeted therapy, which may be related to the low pCR rate. Although our study failed to conclude significant difference among NACT regimens, a large-scale meta-analysis (27) reported that the pCR rates for anthracycline- and taxane-containing regimens were 26.8% and 30.5% and were much lower than platinum- and gemcitabine-containing regimens (44.2% and 44.5%, respectively). Furthermore, the total pCR rate in the TNBC group was similar to our study at 28.9%. All of these results implied that NACT regimens might be the main reasons compromising the pCR rates in our study.
Furthermore, TNBC tended to occur in overweight or obese women (median BMI, 27 kg/m2). The primary tumors slightly presented more on the left side with high grades and high NPI (after NACT), reflecting poor prognosis. Furthermore, regional radiotherapy was often given after surgery to treat residual tumors, while ACT was often omitted.
Other risk characteristics for TNBC, such as African race, younger age at menarche and diagnosis, and lower socioeconomic status, were mentioned in previous studies but not detected in the present study (7,9).
PFS Is Prolonged in Patients With pCR
Mounting evidence indicates that patients who obtain pCR to NACT have a better prognosis regardless of subtype, and increased pCR rates tend to occur more in aggressive subtypes, such as basal-like breast cancer and HER2-overexpressing subtype (15,16,21). Based on its outstanding prognostic value in TNBC patients, pCR to NACT is suggested to be a surrogate endpoint for OS in TNBC patients, which may allow rapid evaluation of clinical outcomes and further treatment decisions for patients without pCR (15,16,18). Interestingly, the relationship between pCR and long-term prognosis is discordant among age groups. Even though young TNBC patients are more likely to receive pCR after NACT, their PFS rates do not increase accordingly (1-year increase, HR = 0.98; 95% CI = 0.96–1.00) (5,19,28). Therefore, more neoadjuvant studies in TNBC patients are required to verify the clinical value of pCR. In the present study, high pCR rate and clinical objective response rate were basically coincident with previous studies, and no progression of primary tumors occurred during NACT (14,21). Univariate analysis demonstrated that the response to NACT influenced the long-term survival. Achievement of pCR, the most potent prognostic factor in TNBC, showed obvious prognostic value to OS but with a borderline value of p = 0.054. On the other hand, PFS rate in the pCR group was significantly higher than that in the non-pCR group (p = 0.013). A comprehensive interpretation of the results is that incidence of the first disease progression can be postponed in patients with pCR to NACT, but the benefit may be reduced over time, and fail to notably prolong the ultimate survival rates. Also, it is possible that this study is underpowered because of small sample size and variety of chemotherapy regimens.
On the other hand, there was no significant finding in PFS in multivariate analysis. The potential explanations are the low proportion of TNBC patients and the interaction among the factors involved in multivariate analysis. We wait for the final outcome data from GeparSixto and CALGB trials regarding long-term survival in correlation to pCR (25,26).
Higher BMI Harms pCR but Not Survival Rates
In all characteristics analyzed in this study, BMI was the only risk factor related to the pCR rates. Intriguingly, obesity in premenopausal women may be a protective factor for patients with ER/PgR-positive breast cancer due to the reduced exposure of estrogen secondary to anovulation (29). However, this benefit does not exist in patients with TNBC. Instead, obesity has been demonstrated as a risk factor of TNBC (30,31). Given that BMI is increasing among young women in Western countries, especially among African-Americans, and that BMI is related to high frequency of TNBC, more public attention should be focused on obesity in the management of breast cancer (4,32).
Although TNBC responds better to NACT compared with other subtypes, higher BMI is still associated with low percentage of pCR (13,19,30). In terms of prognosis, this study failed to identify significant harm of BMI to long-term survival, and the result is consistent with another TNBC study, which also showed no significant correlation between BMI category and recurrence-free survival or OS (31). Instead of observing TNBC only, previous studies enrolling overall population with breast cancer demonstrated higher risk of recurrence in the obese group (30,33).
Nevertheless, the prognostic value of BMI still cannot be explained easily because clinicians often intentionally reduce doses in overweight or obese women for fear of overdose toxicity, which may lead to less optimal efficacy and poorer prognosis (33). This study may be underpowered, since some data were collected from the earliest discharge records during treatment if the initial BMI was not available in diagnosis records. Hence, BMI in some cases might differ from the one at diagnosis because weight gain has been observed in women after breast cancer diagnosis (30).
Anthracycline/Taxane-Based Regimens Are Superior With Fewer Side Effects
Because of the lack of effective pharmacologic targets, chemotherapy is the only systematic treatment available for TNBC (5,13). TNBC is chemosensitive in a neoadjuvant setting with higher pCR rate compared with non-TNBC (18). Previous studies have demonstrated that anthracycline- and/or taxane-based regimens are especially active in TNBC and remain the mainstay of neoadjuvant agents in TNBC (12). In this study, E-CMF and FEC with/without T were two main regimens commonly delivered in neoadjuvant settings (35.8% and 35.9%, respectively). Comparing the anthracycline/taxanes group with anthracycline and taxanes group, the analysis did not show any relationship between NACT regimens and pCR rates (p = 0.838) or long-term prognosis (p = 0.230 for OS; p = 0.689 for PFS) in two groups. However, severe toxicity occurred in about 35.8% of patients (19/53), including five in the anthracycline/taxane group and 14 in the anthracyline and taxane group. Therefore, those patients receiving both anthracycline and taxane seemed to suffer from more severe side effects compared with their counterparts receiving only anthracycline-containing or taxane-containing regimens (OR = 3.719, 95% CI = 1.009–13.702). To the best of our knowledge, little attention was drawn to the difference between these two regimens in earlier studies, so this study may be an attempt to explore this issue, indicating that NACT regimens containing either anthracycline or taxanes were sufficient to deliver considerable clinical benefits without too much toxicity (13,20,34). Because only one patient received taxane-based NACT, comparison was not applied between anthracycline-based and taxane-based regimens. Larger sample studies may be required to monitor these three regimens.
The sensitivity of anthracycline-based regimens may be reduced in patients with BRCA1 deleterious mutations. In recent years, platinum and PARP1 inhibitors, which are potential agents against BRCA1-deficient tumors, have gained growing interest (18,35,36). Future studies about BRCA1-related TNBC may focus on these agents as an alternative to traditional anthracycline and taxanes.
Lymph Vascular Invasion Shows Strong Link With Long-Term Survival
LVI, which refers to peritumoral emboli, accounts for about 15% of invasive ductal breast cancer (37). It has been applied to predict risk of axillary lymph node involvement and distant metastasis. It is also taken into account in decisions on further treatment after surgery (38,39). In multivariate survival analysis, the superiority of the pCR group was not significant. Instead, LVI was strongly correlated with poor survival.
Mounting evidence proves that the existence of LVI is a crucial prognostic factor for women with invasive breast cancer regardless of their lymph node status (40–42). In the TNBC cohort, poorer disease-free survival was also detected in patients with extensive LVI (HR = 6.73; 95% CI = 1.50–30.3, p = 0.03). Similarly, univariate analysis showed that the existence of LVI significantly impacted OS (HR = 7.67; 95% CI = 2.65–22.21) and PFS (HR = 13.856; 95% CI = 4.68–41.04). However, the presence of LVI is not unique to breast cancer. Previous studies have demonstrated its prognostic significance in various malignancies, such as pancreatic cancer, cholangiocarcinoma, gastric cancer, and ovarian cancer (43–46). So LVI testing was added into St. Gallen Criteria for postoperative treatment selection in 2005 (47).
There are several potential mechanisms for LVI occurrence. One is vasculogenic mimicry. Previous studies have established several cancer xenograft models to show that tumor cells may differentiate into vascular channels directly through such a non-angiogenesis-dependent pathway (48,49). Another mechanism may be the recruitment of bone marrow-derived stem cells for tumor vasculature (50). For breast cancer, LVI might be also associated with putative gene mutations. Fidalgo et al. (49) detected a mild increased frequency of some gene alterations in LVI-present breast cancer.
However, in routine practice, detection of LVI is subject to interobserver variability, which may compromise its prognostic value (41). Also, LVI alone has proved to be insufficient to move patients from a low-risk recurrence group into a high-risk category, or omit ACT (40,41). Hence, therapeutic algorithms should cover both LVI and other prognostic factors. The interest of LVI also focuses on further distinguishing lymph invasion from vascular invasion to understand the contribution of both in the process of breast cancer metastasis (51,52).
Discordant Expression of Triple Receptors
It should be noted that the discordance of hormone receptors and HER-2 status between core needle biopsy and excisional biopsy was observed in seven patients (13.2%). Five patients experienced a negative-to-positive change in ER/PgR status. A literature review of 32 relevant studies concluded that discordance of hormone receptors after NACT with or without trastuzumab was reported in four of eight studies involving 8–33% of the patients, while HER-2 status was relatively stable (53). This phenomenon may be caused by the following confounders: (a) intratumor heterogeneity (PgR is more likely to concentrate diversely in the tumor); (b) intra- or interobserver variability, which may lead to different pathologic diagnosis in two tests; and (c) variation in tissue processing and fixation (54–56). NACT may also influence the expression of hormone status or HER-2. Possible mechanisms include (a) neoadjuvant treatment targets chemosensitive tumor cells but leaves insensitive tumor cells with different biology, (b) tumor cells switch expression status for better survival, and (c) ovarian suppression during and after chemotherapy results in low circulating levels of estrogen and progesterone (57).
Although neither this study nor previous research drew any conclusion about the prognostic value of such change, retesting of hormone receptors and HER-2 status after NACT was still suggested to tailor adjuvant endocrine or anti-HER-2 treatment after surgery (53,57).
Further Concern About the Application of pCR
Another point to be emphasized is that a clear definition of pCR should be declared in each neoadjuvant study because there is no standardized definition of pCR, and this may reduce the comparability between studies (15). In this study, the comparison of survival impacts among different definitions of pCR was limited to the small sample size. However, the controversy mainly focuses on the existence of carcinoma in situ (15,16). A retrospective analysis involving 2,302 NACT-treated breast cancer patients illustrates that residual ductal carcinoma in situ did not adversely affect long-term survival if the invasive breast cancer and lymph nodes were eradicated completely (58). However, another analysis of seven randomized trials including 6,377 patients concluded the opposite, that pCR should be defined as ypT0 ypN0 without any residual disease in breast or lymph nodes (16).
Limitations of This Study
This retrospective cohort study has several limitations. First, the sample size is small because of the low proportion of TNBC patients with NACT, which may compromise the reliability of research results. Second, the data collection strongly depended on clinical records in the past 9 years, which may be incomplete or contain some undiscovered manual errors and thus led to information bias. Also the treatment and diagnostic techniques have been improved in the past 9 years, and inter- and intraobserver variations exist during the treatment procedure, which may cause measurement and observer bias.
However, data collection, especially the statuses of hormone receptors and HER-2, has been double checked to avoid errors. To the best of our knowledge, limited studies have been implemented in the UK to evaluate the short- and long-term clinical outcomes of NACT in the TNBC group or comparing anthracycline/taxane-based regimens with anthracycline and taxane-based regimens. Thus, this study may be a fresh attempt to perform a comprehensive evaluation of NACT in patients with TNBC.
CONCLUSIONS
The difficulty of neoadjuvant study in TNBC patients is high because of low disease incidence and treatment limitation. However, it is of importance and interest to medical researchers.
pCR is considered the most potent indicator of the short-term outcomes of NACT, and its benefit can be translated to long-term survival (18,19). Our results relatively correspond with this point. However, the benefit may reflect more in the short term to delay the occurrence of further progression. Meanwhile, there is no difference in efficacy between anthracycline- or taxane-based regimens and regimens including both anthracycline and taxanes, but the single chemotherapy may be more acceptable with less toxicity. In addition, the existence of LVI showed superiority to pCR in prediction of long-term survival, which was not compared in earlier studies. Higher BMI, considered to be a risk factor of TNBC, is also related to poorer response to NACT. Although there is no sufficient evidence to prove its prognostic value, high BMI is still suggested to be a public health concern in Western countries.
The delivery of chemotherapy before surgery may decline the healing rate of subsequent surgery because of chemotherapy-induced immunosuppression or delay the best opportunity of radical surgery if patients have poor response to NACT. Therefore, further studies may concentrate more on exploring new prediction markers for tailored treatment, comprehensive comparison of NACT and ACT, and addition of other novel agents into traditional regimens (59,60). Furthermore, high-quality studies are required to validate the importance of BMI and LVI in TNBC patients, and the definition of pCR should be unified for neoadjuvant studies in TNBC (16).
ACKNOWLEDGMENTS
We thank Mr. Andrew Howman for his statistic consultation, and Sheena Collins and Margaret Cruise for conducting the use of clinical portal system and document search. We are grateful to Jean Assender, Yuk Ting Ma, Xun Ma, and Ping Zhou for their general support during data collection and manuscript drafting.
REFERENCES
- 1. Bosch A.; Eroles P.; Zaragoza R.; Viña J. R.; Lluch A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat. Rev. 36(3):206–215; 2010. [DOI] [PubMed] [Google Scholar]
- 2. Bryan B. B.; Schnitt S. J.; Collins L. C. Ductal carcinoma in situ with basal-like phenotype: A possible precursor to invasive basal-like breast cancer. Mod. Pathol. 19(5):617–621; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Thike A. A.; Cheok P. Y.; Jara-Lazaro A. R.; Tan B.; Tan P.; Tan P. H. Triple-negative breast cancer: Clinicopathological characteristics and relationship with basal-like breast cancer. Mod. Pathol. 23(1):123–133; 2010. [DOI] [PubMed] [Google Scholar]
- 4. Morris G. J.; Naidu S.; Topham A. K.; Guiles F.; Xu Y.; McCue P.; Schwartz G. F.; Park P. K.; Rosenberg A. L.; Brill K.; Mitchell E. P. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the national cancer institute’s surveillance, epidemiology, and end results database. Cancer 110(4):876–884; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Silver D. P.; Richardson A. L.; Eklund A. C.; Wang Z. C.; Szallasi Z.; Li Q.; Juul N.; Leong C. O.; Calogrias D.; Buraimoh A.; Fatima A.; Gelman R. S.; Ryan P. D.; Tung N. M.; De Nicolo A.; Ganesan S.; Miron A.; Colin C.; Sgroi D. C.; Ellisen L. W.; Winer E. P.; Garber J. E. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J. Clin. Oncol. 28(7):1145–1153; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Millikan R. C.; Newman B.; Tse C. K.; Moorman P. G.; Conway K.; Dressler L. G.; Smith L. V.; Labbok M. H.; Geradts J.; Bensen J. T.; Jackson S.; Nyante S.; Livasy C.; Carey L.; Earp H. S.; Perou C. M. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 109(1):123–139; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X. R.; Sherman M. E.; Rimm D. L.; Lissowska J.; Brinton L. A.; Peplonska B.; Hewitt S. M.; Anderson W. F.; Szeszenia-Dabrowska N.; Bardin-Mikolajczak A.; Zatonski W.; Cartun R.; Mandich D.; Rymkiewicz G.; Ligaj M.; Lukaszek S.; Kordek R.; García-Closas M. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol. Biomarkers Prev. 16(3):439–443; 2007. [DOI] [PubMed] [Google Scholar]
- 8. Onitilo A. A.; Engel J. M.; Greenlee R. T.; Mukesh B. N. Breast cancer subtypes based on er/pr and her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 7(1–2):4–13; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer K. R.; Brown M.; Cress R. D.; Parise C. A.; Caggiano V. Descriptive analysis of estrogen receptor (er)-negative, progesterone receptor (pr)-negative, and her2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer registry. Cancer 109(9):1721–1728; 2007. [DOI] [PubMed] [Google Scholar]
- 10. Bartlett J. M.; Brookes C. L.; Robson T.; van de Velde C. J.; Billingham L. J.; Campbell F. M.; Grant M.; Hasenburg A.; Hille E. T.; Kay C.; Kieback D. G.; Putter H.; Markopoulos C.; Kranenbarg E. M.; Mallon E. A.; Dirix L.; Seynaeve C.; Rea D. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: A prospectively powered pathology study in the tamoxifen and exemestane adjuvant multinational trial. J. Clin. Oncol. 29(12):1531–1538; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slamon D.; Eiermann W.; Robert N.; Pienkowski T.; Martin M.; Press M.; Mackey J.; Glaspy J.; Chan A.; Pawlicki M.; Pinter T.; Valero V.; Liu M. C.; Sauter G.; von Minckwitz G.; Visco F.; Bee V.; Buyse M.; Bendahmane B.; Tabah-Fisch I.; Lindsay M. A.; Riva A.; Crown J. Breast Cancer International Research Group. Adjuvant trastuzumab in her2-positive breast cancer. N. Engl. J. Med. 365(14):1273–1283; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isakoff S. J. Triple-negative breast cancer: Role of specific chemotherapy agents. Cancer J. 16(1):53–61; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pal S. K.; Childs B. H.; Pegram M. Triple negative breast cancer: Unmet medical needs. Breast Cancer Res. Treat. 125(3):627–636; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Badve S.; Dabbs D. J.; Schnitt S. J.; Baehner F. L.; Decker T.; Eusebi V.; Fox S. B.; Ichihara S.; Jacquemier J.; Lakhani S. R.; Palacios J.; Rakha E. A.; Richardson A. L.; Schmitt F. C.; Tan P. H.; Tse G. M.; Weigelt B.; Ellis I. O.; Reis-Filho J. S. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Mod. Pathol. 24(2):157–167; 2011. [DOI] [PubMed] [Google Scholar]
- 15. Cortazar P.; Zhang L.; Untch M.; Mehta K.; Costantino J. P.; Wolmark N.; Bonnefoi H.; Cameron D.; Gianni L.; Valagussa P.; Swain S. M.; Prowell T.; Loibl S.; Wickerham D. L.; Bogaerts J.; Baselga J.; Perou C.; Blumenthal G.; Blohmer J.; Mamounas E. P.; Bergh J.; Semiglazov V.; Justice R.; Eidtmann H.; Paik S.; Piccart M.; Sridhara R.; Fasching P. A.; Slaets L.; Tang S.; Gerber B.; Geyer C. E. Jr.; Pazdur R.; Ditsch N.; Rastogi P.; Eiermann W.; von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384(9938):164–172; 2014. [DOI] [PubMed] [Google Scholar]
- 16. von Minckwitz G.; Untch M.; Blohmer J. U.; Costa S. D.; Eidtmann H.; Fasching P. A.; Gerber B.; Eiermann W.; Hilfrich J.; Huober J.; Jackisch C.; Kaufmann M.; Konecny G. E.; Denkert C.; Nekljudova V.; Mehta K.; Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30(15):1796–1804; 2012. [DOI] [PubMed] [Google Scholar]
- 17. Parise C. A.; Bauer K. R.; Brown M. M.; Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (er), progesterone receptor (pr), and the human epidermal growth factor receptor 2 (her2) among women with invasive breast cancer in California, 1999–2004. Breast J. 15(6):593–602; 2009. [DOI] [PubMed] [Google Scholar]
- 18. von Minckwitz G.; Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC). Ann. Oncol. 23 (Suppl. 6):vi35–39; 2012. [DOI] [PubMed] [Google Scholar]
- 19. Liedtke C.; Mazouni C.; Hess K. R.; André F.; Tordai A.; Mejia J. A.; Symmans W. F.; Gonzalez-Angulo A. M.; Hennessy B.; Green M.; Cristofanilli M.; Hortobagyi G. N.; Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 26(8):1275–1281; 2008. [DOI] [PubMed] [Google Scholar]
- 20. Amos K. D.; Adamo B.; Anders C. K. Triple-negative breast cancer: An update on neoadjuvant clinical trials. Int. J. Breast Cancer 2012:385978; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carey L. A.; Dees E. C.; Sawyer L.; Gatti L.; Moore D. T.; Collichio F.; Ollila D. W.; Sartor C. I.; Graham M. L.; Perou C. M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 13(8):2329–2334; 2007. [DOI] [PubMed] [Google Scholar]
- 22. Kennedy C. R.; Gao F.; Margenthaler J. A. Neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer. J. Surg. Res. 163(1):52–57; 2010. [DOI] [PubMed] [Google Scholar]
- 23. Fisher C. S.; Ma C. X.; Gillanders W. E.; Aft R. L.; Eberlein T. J.; Gao F.; Margenthaler J. A. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann. Surg. Oncol. 19(1):253–258; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisenhauer E. A.; Therasse P.; Bogaerts J.; Schwartz L. H.; Sargent D.; Ford R.; Dancey J.; Arbuck S.; Gwyther S.; Mooney M.; Rubinstein L.; Shankar L.; Dodd L.; Kaplan R.; Lacombe D.; Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2):228–247; 2009. [DOI] [PubMed] [Google Scholar]
- 25. von Minckwitz G.; Schneeweiss A.; Loibl S.; Salat C.; Denkert C.; Rezai M.; Blohmer J. U.; Jackisch C.; Paepke S.; Gerber B.; Zahm D. M.; Kümmel S.; Eidtmann H.; Klare P.; Huober J.; Costa S.; Tesch H.; Hanusch C.; Hilfrich J.; Khandan F.; Fasching P. A.; Sinn B. V.; Engels K.; Mehta K.; Nekljudova V.; Untch M. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 15(7):747–56; 2014. [DOI] [PubMed] [Google Scholar]
- 26. Sikov W. M.; Berry D. A.; Perou C. M.; Singh B.; Cirrincione C. T.; Tolaney S. M.; Kuzma C. S.; Pluard T. J.; Somlo G.; Port E. R.; Golshan M.; Bellon J. R.; Collyar D.; Hahn O. M.; Carey L. A.; Hudis C. A.; Winer E. P. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33(1):13–21; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu K.; Yang Q.; Liu Y.; Wu A.; Yang Z. Meta-analysis on the association between pathologic complete response and triple-negative breast cancer after neoadjuvant chemotherapy. World J. Surg. Oncol. 12:95; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hess K. R.; Anderson K.; Symmans W. F.; Valero V.; Ibrahim N.; Mejia J. A.; Booser D.; Theriault R. L.; Buzdar A. U.; Dempsey P. J.; Rouzier R.; Sneige N.; Ross J. S.; Vidaurre T.; Gómez H. L.; Hortobagyi G. N.; Pusztai L. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J. Clin. Oncol. 24(26):4236–4244; 2006. [DOI] [PubMed] [Google Scholar]
- 29. Cleary M. P.; Maihle N. J. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc. Soc. Exp. Biol. Med. 216(1):28–43; 1997. [DOI] [PubMed] [Google Scholar]
- 30. Caan B. J.; Kwan M. L.; Hartzell G.; Castillo A.; Slattery M. L.; Sternfeld B.; Weltzien E. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19(10):1319–1328; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ademuyiwa F. O.; Groman A.; O’Connor T.; Ambrosone C.; Watroba N.; Edge S. B. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 117(18):4132–4140; 2011. [DOI] [PubMed] [Google Scholar]
- 32. Himes J. H. Examining the evidence for recent secular changes in the timing of puberty in us children in light of increases in the prevalence of obesity. Mol. Cell. Endocrinol. 254–255:13–21; 2006. [DOI] [PubMed] [Google Scholar]
- 33. Griggs J. J.; Sorbero M. E.; Lyman G. H. Undertreatment of obese women receiving breast cancer chemotherapy. Arch. Intern. Med. 165(11):1267–1273; 2005. [DOI] [PubMed] [Google Scholar]
- 34. Maur M.; Guarneri V.; Frassoldati A.; Conte P. F. Primary systemic therapy in operable breast cancer: Clinical data and biological fall-out. Ann. Oncol. 17(Suppl. 5):v158–v164; 2006. [DOI] [PubMed] [Google Scholar]
- 35. Liu M.; Mo Q. G.; Wei C. Y.; Qin Q. H.; Huang Z.; He J. Platinum-based chemotherapy in triple-negative breast cancer: A meta-analysis. Oncol. Lett. 5(3):983–991; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hiller D. J.; Chu Q. D. Current status of poly (adp-ribose) polymerase inhibitors as novel therapeutic agents for triple-negative breast cancer. Int. Breast Cancer 2012:829315; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heerma van Voss M. R.; van der Groep P.; Bart J.; van der Wall E.; van Diest P. J. Lympho-vascular invasion in BRCA related breast cancer compared to sporadic controls. BMC Cancer 10:145; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ejlertsen B.; Jensen M. B.; Rank F.; Rasmussen B. B.; Christiansen P.; Kroman N.; Kvistgaard M. E.; Overgaard M.; Toftdahl D. B.; Mouridsen H. T.; Danish Breast Cancer Cooperative Group. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J. Natl. Cancer Inst. 101(10):729–735; 2009. [DOI] [PubMed] [Google Scholar]
- 39. de Mascarel I.; MacGrogan G.; Debled M.; Sierankowski G.; Brouste V.; Mathoulin-Pélissier S.; Mauriac L. D2-40 in breast cancer: Should we detect more vascular emboli. Mod. Pathol. 22(2):216–222; 2008. [DOI] [PubMed] [Google Scholar]
- 40. Song Y. J.; Shin S. H.; Cho J. S.; Park M. H.; Yoon J. H.; Jegal Y. J. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J. Breast Cancer 14(3):198–203; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen J. W.; Bhandari M.; Astill D. S.; Wilson T. G.; Kow L.; Brooke-Smith M.; Toouli J.; Padbury R. T. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 12(2):101–108; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisher S. B.; Patel S. H.; Kooby D. A.; Weber S.; Bloomston M.; Cho C.; Hatzaras I.; Schmidt C.; Winslow E.; Staley C. A. 3rd; Maithel S. K. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: A multi-institution analysis. HPB (Oxford) 14(8):514–522; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li P.; He H. Q.; Zhu C. M.; Ling Y. H.; Hu W. M.; Zhang X. K.; Luo R. Z.; Yun J. P.; Xie D.; Li Y. F.; Cai M. Y. The prognostic significance of lymphovascular invasion in patients with resectable gastric cancer: A large retrospective study from Southern China. BMC Cancer 15:370; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuo K.; Yoshino K.; Hiramatsu K.; Banzai C.; Hasegawa K.; Yasuda M.; Nishimura M.; Sheridan T. B.; Ikeda Y.; Shiki Y.; Mabuchi S.; Enomoto T.; Kimura T.; Fujiwara K.; Roman L. D.; Sood A. K. Effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer. Obstet. Gynecol. 123(5):957–965; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldhirsch A.; Wood W. C.; Gelber R. D.; Coates A. S.; Thürlimann B.; Senn H. J. Meeting highlights: Updated international expert consensus on the primary therapy of early breast cancer. J. Clin. Oncol. 21(17):3357–3365; 2003. [DOI] [PubMed] [Google Scholar]
- 46. Shirakawa K.; Kobayashi H.; Sobajima J.; Hashimoto D.; Shimizu A.; Wakasugi H. Inflammatory breast cancer: Vasculogenic mimicry and its hemodynamics of an inflammatory breast cancer xenograft model. Breast Cancer Res. 5(3):136–139; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maniotis A. J.; Folberg R.; Hess A.; Seftor E. A.; Gardner L. M.; Pe’er J.; Trent J. M.; Meltzer P. S.; Hendrix M. J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 155(3):739–752; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peters B. A.; Diaz L. A.; Polyak K.; Meszler L.; Romans K.; Guinan E. C.; Antin J. H.; Myerson D.; Hamilton S. R.; Vogelstein B.; Kinzler K. W.; Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 11(3):261–262; 2005. [DOI] [PubMed] [Google Scholar]
- 49. Fidalgo F.; Rodrigues T. C.; Pinilla M.; Silva A. G.; Maciel Mdo S.; Rosenberg C.; de Andrade V. P.; Carraro D. M.; Krepischi A. C. Lymphovascular invasion and histologic grade are associated with specific genomic profiles in invasive carcinomas of the breast. Tumour Biol. 36(3):1835–1848; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van den Eynden G. G.; Van der Auwera I.; Van Laere S. J.; Colpaert C. G.; van Dam P.; Dirix L. Y.; Vermeulen P. B.; Van Marck E. A. Distinguishing blood and lymph vessel invasion in breast cancer: A prospective immunohistochemical study. Br. J. Cancer 94(11):1643–1649; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colleoni M.; Rotmensz N.; Maisonneuve P.; Sonzogni A.; Pruneri G.; Casadio C.; Luini A.; Veronesi P.; Intra M.; Galimberti V.; Torrisi R.; Andrighetto S.; Ghisini R.; Goldhirsch A.; Viale G. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann. Oncol. 18(10):1632–1640; 2007. [DOI] [PubMed] [Google Scholar]
- 52. Fujii T.; Yajima R.; Hirakata T.; Miyamoto T.; Fujisawa T.; Tsutsumi S.; Ynagita Y.; Iijima M.; Kuwano H. Impact of the prognostic value of vascular invasion, but not lymphatic invasion, of the primary tumor in patients with breast cancer. Anticancer Res. 34(3):1255–1259; 2014. [PubMed] [Google Scholar]
- 53. van de Ven S.; Smit V. T.; Dekker T. J.; Nortier J. W.; Kroep J. R. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat. Rev. 37(6):422–430; 2011. [DOI] [PubMed] [Google Scholar]
- 54. Arnedos M.; Nerurkar A.; Osin P.; A’Hern R.; Smith I. E.; Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann. Oncol. 20(12):1948–1952; 2009. [DOI] [PubMed] [Google Scholar]
- 55. Zidan A.; Christie Brown J. S.; Peston D.; Shousha S. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J. Clin. Pathol. 50(1):27–29; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gown A. M. Current issues in ER and HER2 testing by IHC in breast cancer. Mod. Pathol. 21:S8–S15; 2008. [DOI] [PubMed] [Google Scholar]
- 57. Tacca O.; Penault-Llorca F.; Abrial C.; Mouret-Reynier M. A.; Raoelfils I.; Durando X.; Achard J. L.; Gimbergues P.; Curé H.; Chollet P. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist 12(6):636–643; 2007. [DOI] [PubMed] [Google Scholar]
- 58. Mazouni C.; Peintinger F.; Wan-Kau S.; Andre F.; Gonzalez-Angulo A. M.; Symmans W. F.; Meric-Bernstam F.; Valero V.; Hortobagyi G. N.; Pusztai L. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J. Clin. Oncol. 25(19):2650–2655; 2007. [DOI] [PubMed] [Google Scholar]
- 59. Zhong T.; Hofer S. O.; McCready D. R.; Jacks L. M.; Cook F. E.; Baxter N. A comparison of surgical complications between immediate breast reconstruction and mastectomy: The impact on delivery of chemotherapy—an analysis of 391 procedures. Ann. Surg. Oncol. 19(2):560–566; 2012. [DOI] [PubMed] [Google Scholar]
- 60. Sapunar F.; Smith I. E. Neoadjuvant chemotherapy for breast cancer. Ann. Med. 32(1):43–50; 2000. [DOI] [PubMed] [Google Scholar]