Abstract
Relapsed acute myeloid leukemia (AML) represents a major therapeutic challenge. Achieving complete remission (CR) with salvage chemotherapy is the first goal of therapy for relapsed AML. However, there is no standard salvage chemotherapy. The current study evaluated outcomes and prognostic factors for achievement of CR in 91 AML patients in first relapse who were treated with the mitoxantrone–etoposide combination regimen. The overall response rate (CR and CRi) was 25%. Factors that were associated with a lower rate of CR included older age, shorter duration of first CR, low hemoglobin, and low platelet count. The median overall survival for all patients was 7.4 months. The survival of patients who achieved CR and underwent allogeneic hematopoietic cell transplantation (allo-HCT) was higher than those who achieved CR and did not undergo allo-HCT (35.3 months vs. 16.8 months, p = 0.057). The median duration of relapse-free survival was 12.7 months in the patients achieving CR. Older age at the time of AML relapse was associated with worse overall survival. The all-cause 4-week mortality rate was 4%, and the all-cause 8-week mortality rate was 13%. The findings of this study underscore the need for newer therapies, especially those that will improve the ability for patients with relapsed AML to achieve CR and to allow them to receive additional therapies.
Key words: Acute myeloid leukemia (AML), Relapse, Prognostic factors, Mitoxantrone, Etoposide
INTRODUCTION
Although up to 60–80% of patients newly diagnosed with acute myeloid leukemia (AML) are able to achieve hematologic complete remission (CR) with induction chemotherapy, the majority of patients relapse (1,2). Several studies have investigated the optimal treatment and prognostic factors after first relapse (3–5). These studies have shown that duration of first CR, cytogenetic risk category, and intensity of first-line treatment are recognized as prognostic factors in the relapsed setting. However, at present there is no standard salvage chemotherapy regimen for relapsed AML, as no study has shown any one regimen to be significantly superior. Mitoxantrone and etoposide are active agents in AML and have been used as monotherapy and in combination in refractory and relapsed AML patients (6–12). In the current study, we evaluated the efficacy and toxicity of a mitoxantrone–etoposide regimen in patients with AML in first relapse in a large cohort. We also assessed factors potentially predictive of response to mitoxantrone–etoposide and overall survival.
MATERIALS AND METHODS
Study Group
Subjects were identified as consecutive patients with AML in first relapse based on World Health Organization Classification of AML who were treated with the combination mitoxantrone–etoposide at the University of Pittsburgh Medical Center between January 2000 and January 2013. Treatment consisted of mitoxantrone 10 mg/m2 IV daily on days 1–5 concurrent with etoposide 100 mg/m2 IV daily on days 1–5. Cases of AML were classified as secondary on the basis of having a history of previous treatment with chemotherapy or radiotherapy or antecedent hematologic conditions including myelodysplasia and myeloproliferative neoplasms. The study was approved by the University of Pittsburgh Institutional Review Board according to institutional guidelines.
Cytogenetics
Cytogenetic abnormalities were defined based on published criteria (13,14). The favorable risk category included patients with abnormalities (abn) of inv(16)/t(16;16)/del(16q) or t(8;21) without del(9q) or as part of a complex karyotype. The intermediate risk category included patients characterized by +8, −Y, +6, del(12p), or normal karyotype. The unfavorable risk category was defined by the presence of one or more of −5/del(5q), −7/del(7q), inv(3q), abn 11q, 20q, or 21q, del(9q), t(6;9), t(9;22), abn 17p, or complex karyotype defined as 3 or more abnormalities.
Criteria for Response
Using established criteria (15), CR was defined by the presence of less than 5% blasts in the bone marrow, absence of extramedullary leukemia, and peripheral blood count recovery with a neutrophil count of at least 1 × 109/L and a platelet count of at least 100 × 109/L. CRi was defined by the presence of less than 5% blasts in the bone marrow, absence of extramedullary leukemia, incomplete platelet regeneration (<100 × 109/L), or neutrophil regeneration (<1 × 109/L).
Statistical Methods
Analyses based on logistic regression were conducted to assess the association between different factors and the occurrence of CR or CRi. Overall survival (OS) was measured from the date of relapse until the recorded date of death. Relapse-free survival (RFS) was measured from the date of CR until relapse. For patients who were alive at the time of analysis, follow-up was censored as of the date of last contact. The Kaplan–Meier method was used to estimate the survival distributions, which were then compared using the log-rank test. The relationship between OS and patient characteristics was evaluated using Cox proportional hazards regression. The corresponding relative mortality rates are summarized as hazard ratios (HR), with HR > 1.0 corresponding to increased mortality. Significance level was set at 0.05 and all p-values reported were two-sided. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient Characteristics
Ninety-four patients were identified who met the study criteria. Three patients were excluded because they proceeded directly to allogeneic hematopoietic cell transplantation (allo-HCT) before being evaluated for response to mitoxantrone–etoposide. Therefore, the study cohort consisted of 91 AML patients in first relapse. Patient demographics, baseline characteristics, and prior therapies are presented in Table 1. The median age at relapse was 56.8 years (range 21.9–75.8 years). Eight patients (9%) had secondary AML. The median time from AML diagnosis to first relapse was 10.5 months (1.3–37.5 months). Cytogenetic data was available for 77 patients at the time of relapse; 16 (21%) had an unfavorable risk karyotype, 55 (71%) had an intermediate risk karyotype, and 6 (8%) had a favorable risk karyotype.
Table 1.
Baseline Patient Characteristics (N = 91)
| Characteristic | n |
|---|---|
| Median age at AML diagnosis [years (range)] | 55 (20–74) |
| Median age at relapse [years (range)] | 56.8 (21.9–75.8) |
| Sex | |
| Male | 43 (47.25%) |
| Female | 48 (52.75%) |
| AML at diagnosis | |
| De novo | 83 (91.2%) |
| Secondary | 8 (8.8%) |
| Cytogenetic risk category at diagnosis | |
| Unfavorable | 16 (20%) |
| Intermediate | 57 (71.2%) |
| Favorable | 7 (8%) |
| WBC count at AML diagnosis [median (range) 109/L] | 16.5 (1.2–285) |
| Percent blasts in bone marrow at AML diagnosis [median (range)] | 57 (7.7–98) |
| Platelet count at AML diagnosis [median (range)] | 54 (11–403) |
| Hemoglobin at AML diagnosis [median (range)] | 9.15 (5.9–37) |
| Induction chemotherapy at AML diagnosis | |
| 73 patients (80%) received 1 course to achieve first CR* | |
| 18 patients (20%) received 2 courses to achieve first CR | |
| High dose cytarabine (HiDAC), consolidation prior to relapse | |
| 60 patients received HiDAC × 4 cycles | |
| 10 patients received HiDAC × 3 cycles | |
| 14 patients received HiDAC × 2 cycles | |
| 6 patients received HiDAC × 1 cycle | |
| Median time (months) from AML diagnosis to AML relapse (range) | 10.5 (1.3–37.5) |
| Cytogenetic group prognosis at relapse | |
| Unfavorable | 16 (20.8%) |
| Intermediate | 55 (71.4%) |
| Favorable | 6 (7.8%) |
| WBC count at AML relapse [median (range) × 109/L] | 3.95 (0.7–131) |
| Percent blasts in bone marrow prior to initiation of mitoxantrone + etoposide, median (range) | 51 (2.3–96) |
| Platelet count at AML relapse [median (range)] | 56.5 (6–454) |
| Hemoglobin at AML relapse [median (range)] | 10.8 (6.1–15) |
AML, acute myeloid leukemia; WBC, white blood cell.
Patients at AML diagnosis were initially treated with combination of cytarabine and an anthracycline (7 + 3). Mitoxantrone and etoposide were used in patients as second-course regimen.
Responses
All 91 patients completed treatment with mitoxantrone–etoposide. The overall response rate after therapy (CR and CRi) was 25%; 22 (24%) patients achieved CR, and 1 (1%) patient achieved CRi. Factors that were associated with a lower rate of CR included older age, shorter duration of first CR, low hemoglobin, and low platelet count (Table 2). Fifteen patients who achieved a CR after therapy with mitoxantrone–etoposide proceeded to allo-HCT. Fifty-two patients who did not respond to mitoxantrone–etoposide received additional therapies; 25 patients received another salvage chemotherapy regimen (e.g., fludarabine and cytarabine), 20 patients received another salvage chemotherapy regimen followed by allo-HCT, and 7 patients proceeded directly to allo-HCT with residual leukemia. Of these 52 patients, 12 patients (23%) achieved CR with subsequent therapy.
Table 2.
Responses
| Univariate OR (95% CI) | p | Multivariate OR (95% CI) | p | |
|---|---|---|---|---|
| Age at AML relapse (per year) | 0.96 (0.92–0.99) | 0.02 | 0.8 (0.8–0.9) | 0.002 |
| Length from AML diagnosis to relapse (per month) | 1.18 (1.075–1.3) | 0.0006 | 1.3 (1.0–1.6) | 0.004 |
| Cycles of consolidation therapy prior to relapse (per cycle) | 1.88 (1.03–3.4) | 0.0397 | 1.8 (0.7–4.4) | 0.2 |
| Percent blasts at AML relapse | 1.01 (0.997–1.038) | 0.0895 | 1.0 (1.0–1.1) | 0.002 |
| Hemoglobin at AML relapse | 1.21 (0.966–1.527) | 0.0966 | 1.7 (1.0–3.1) | 0.04 |
| Platelet count at AML relapse | 1.0 (0.99–1.0) | 0.5103 | 1.0 (1.0–1.03) | 0.003 |
| Primary versus secondary AML | 2.5 (0.29–21) | 0.39 | ||
| WBC at AML relapse | 1.0 (0.98–1.0) | 0.8661 | ||
| Cytogenetic risk at AML relapse | ||||
| Favorable versus unfavorable | 13 (1.4–133) | 0.02 | ||
| Intermediate versus unfavorable | 2.8 (0.5–14) | 0.6 |
OR, odds ratios; with OR >1.0 corresponding to increased odds of the occurrence of CR or CRi; CI, confidence interval.
Overall Survival
Eighty-two of the 91 patients had died by the time of this analysis. The median overall survival (OS) for all patients was 7.4 months [95% confidence interval (CI): 5–10.2 months] (Fig. 1A). The median OS for patients achieving CR after mitoxantrone–etoposide was significantly higher (20.1 months, range 12.4–41.1) than those without a CR (4.9 months, range 3.6–6.8, p < 0.001) (Fig. 1B). The median duration of RFS was 12.7 months (95% CI: 7.4–29.4 months) in the 23 patients achieving CR. The survival of patients who achieved CR and underwent allo-HCT (n = 15) was higher than those who achieved CR and did not undergo allo-HCT (n = 8) (35.3 months vs. 16.8 months, respectively, p = 0.057). Patients who relapsed within 12 months from AML diagnosis and those who relapsed after 12 months of AML diagnosis had significantly different OS (p = 0.0003). The estimated median time for patients who relapsed within 12 month of AML diagnosis was 4.7 months, (95% CI: 3.551, 6.773) and 13.6 months (95% CI: 9.271, 24.855) for patients who relapsed after 12 months of AML diagnosis. No significant difference in survival was observed in patients who achieved CR after mitoxantrone–etoposide and in those who achieved CR after additional therapies (20.1 vs. 20.4 months, p = 0.4). Cox regression analyses showed that worse OS was associated with older age at the time of AML relapse and lower hemoglobin (Table 3).
Figure 1.
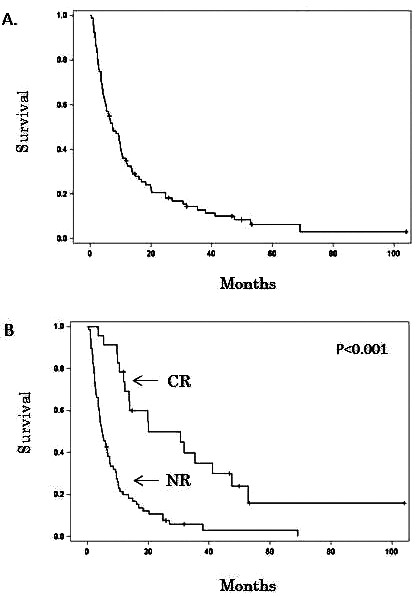
(A) Overall survival. (B) Comparison of survival between patients in complete remission (CR) and patients not responsive to mitoxantrone–etoposide (NR).
Table 3.
Overall Survival
| Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p | |
|---|---|---|---|---|
| Age at AML relapse (per year) | 1.0 (1.004–1.042) | 0.01 | 1.0 (1.001–1.039) | 0.04 |
| Time from AML diagnosis to relapse (per month) | 0.9 (0.8–0.9) | 0.0021 | 0.97 (0.926–1.023) | 0.2 |
| Cycles of consolidation therapy prior to relapse (per cycle) | 0.7 (0.5–0.9) | 0.004 | 0.8 (0.6–1.1) | 0.2 |
| Hemoglobin at AML relapse | 0.8 (0.79–0.97) | 0.01 | 0.8 (0.7–0.9) | 0.01 |
| Primary versus secondary AML | 0.6 (0.2–1.3) | 0.2 | ||
| Percent blasts at AML relapse | 0.99 (0.99–1.007) | 0.67 | ||
| WBC at AML relapse | 0.99 (0.986–1.008) | 0.57 | ||
| Platelet count at AML relapse | 0.99 (0.99–1.003) | 0.69 | ||
| Cytogenetic risk at AML relapse | 0.6 | |||
| Favorable versus unfavorable | 0.6 (0.25–1.6) | |||
| Intermediate versus unfavorable | 0.9 (0.5–1.7) |
HR, hazard ratios; with HR >1.0 corresponding to increased mortality; CI, confidence interval.
Toxicity
All patients developed grades 3–4 myelosuppression in the form of neutropenia and thrombocytopenia, with complete responders requiring a median of 35 days (range 21–63) from the completion of treatment to recover neutrophils (neutrophil count ≥1 × 109/L) and a median of 40 days (range 21–62) to recover platelets (platelet count ≥100 × 109/L). The all-cause 4-week mortality rate was 4%, and the all-cause 8-week mortality rate was 13%. Causes of death included sepsis with multiorgan failure (n = 6), progressive AML (n = 4), and intracranial hemorrhage (n = 1). No grade 3 or 4 hepatic toxicities were observed during mitoxantrone–etoposide therapy.
DISCUSSION
Leukemia relapse continues to be a formidable clinical challenge. Relapsed AML is associated with poor outcomes with reported median OS of less than 12 months (16,17). Therefore, when evaluating patients with AML relapse some of the challenges include assessing prognosis, choosing the appropriate salvage regimen, and identifying patients that are candidates for allo-HCT. The latter issue is important as outcomes for allo-HCT are better when the allo-HCT is performed in CR2 rather than during first relapse (18–20). Thus, achieving second CR is the first goal of therapy for relapsed AML. Furthermore, with the advancement of new immunotherapeutic strategies, including bispecific T-cell engager (BITE) antibodies and checkpoint inhibitors, relapsed AML patients that are not allo-HCT candidates may benefit from these therapies as a form of post-CR consolidation.
Despite extensive clinical research over the last three decades using mainly cytarabine-based combination regimens, there is no standard accepted chemotherapy regimen for patients in first relapse; salvage chemotherapy leads to CR rates up to 60% (17,21–23). Mitoxantrone and etoposide have been shown to be active as monotherapy in AML (6,11,12). The combination of mitoxantrone and etoposide has been used in studies that included both refractory and relapsed AML patients with reported CR rates ranging from 16% to 61% (7–10). In the current study, we evaluated the efficacy and toxicity of mitoxantrone–etoposide in a large group of patients with AML in first relapse. The CR rate was similar to previous reported studies. Of note, the median duration of first CR in this cohort was only 10.5 months, and CR rates are historically low in patients with duration of first remission <12 months. Treatment-related mortality was low, with 4-week and 8-week mortality rates that compare favorably to historical outcomes, especially given this older patient population. Furthermore, the study confirmed prognostic factors associated with responses and survival including duration of first CR and older age. As expected, the patients who achieved CR and were able to proceed to allo-HCT had a better overall survival compared to those who did not undergo allo-HCT. Of note, for patients who did not achieve a CR with mitoxantrone–etoposide, 23% were able to achieve CR with subsequent therapies, and their overall survival was not different from patients who achieved CR after mitoxantrone–etoposide. Therefore, additional therapies should be considered in AML patients not responding to first salvage chemotherapy.
Attempts to improve upon the efficacy of mitoxantrone–etoposide have been made by adding cytarabine in the regimen (24–27). Cytarabine is one of the most highly effective cytotoxic agents in the treatment of AML. Cytarabine is approved for AML in combination with other chemotherapy agents for remission induction, and it has been the mainstay of AML therapy, used in both induction and consolidation regimens. A recent comparative retrospective analysis recently evaluated the role of adding cytarabine to mitoxantrone–etoposide in patients with relapsed/refractory AML (27). The CR rate with the addition of cytarabine was 59% compared to 34%, which is a significant improvement. In addition, there was not any apparent additional toxicity. However, overall survival was not different between the two groups. A subset analysis identified patients with unfavorable risk cytogenetics, and patients <60 years old were more likely to achieve CR with the combination of mitoxantrone–etoposide–cytarabine compared to mitoxantrone–etoposide.
An inherent limitation of this single institution retrospective study was the use of the mitoxantrone–etoposide regimen as opposed to alternative treatment regimens. The lack of a control group prevents any comparisons. Furthermore, selection of relapsed AML patients with good performance status able to tolerate mitoxantrone–etoposide could potentially introduce selection biases and influence the outcomes of this study. Molecular studies were performed in only 22 patients since the study included patients from 2000, at a time when molecular studies were not routinely performed; 11 patients harbored FMS-like tyrosine kinase 3 (FLT-3) mutation, 5 patients had mutated nucleophosmin (NPM1), and 6 patients did not have FLT-3 or NPM1 mutations. Among the 11 patients with FLT3+ mutations only 2 patients achieved CR after mitoxantrone–etoposide, and 1 out of the 5 patients with mutated NPMI achieved CR. Due to the small number of patients with information on molecular studies, it is difficult to make any definitive conclusions on the role of molecular mutations and responses to mitoxantrone–etoposide. However, one of the important strengths of our study is the relative large number of AML patients in first relapse and the inclusion of all data on key risk factors for response and survival.
With the rapid development of novel agents and strategies using targeted therapies or immunotherapy, it is likely that the approach to the treatment of relapsed AML will continue to evolve (22,28–30). Emerging novel cytotoxic agents have been used in patients with refractory and relapse AML with CR rates ranging from 23% to 75% (31–34) (Table 4). In addition, new agents in AML have also been used in early clinical trials as monotherapy demonstrating some efficacy in the relapse setting. SGI-110 is a novel subcutaneous hypomethylating agent designed as a dinucleotide of decitabine and deoxyguanosine that is resistant to degradation by cytidine deaminase and results in prolonged in vivo exposure to its active moiety decitabine. In a clinical trial using SGI-110 in 50 patients with refractory/relapsed AML, there were eight complete remissions (16% with 95% CI: 7–29%) (35). SGN-CD33A is a CD33-directed antibody conjugated to two molecules of a pyrrolobenzodiazepine (PBD) dimer. Upon binding, 33A is internalized and transported to the lysosomes where PBD dimer is released via proteolytic cleavage of the linker, cross-linking DNA, and leading to cell death. In a phase I clinical trial, 87 AML patients, including 34 with relapsed AML, were treated with SGN-CD33A. Across all dose levels, the CR + CRi rate was 86% in the patients with NPM1+/FLT3− disease (n = 7). Of the 21 efficacy evaluable patients treated at 40 µg/kg, 3 patients achieved a best clinical response of CR, 4 achieved CRi, and 5 had morphologic leukemia-free state (36). ABT-199 is a selective, potent, orally bioavailable small molecule BCL-2 inhibitor. In a phase 2 clinical trial, 32 AML patients including 30 with relapsed/refractory AML were treated with ABT-199. The overall response rate (CR/CRi) was 15.5% (5/32) (37). Finally, ongoing work in the refinement of immunotherapy in AML will likely change the landscape of AML therapies, where combination of chemotherapy and immune modulation may finally improve our current outcomes with chemotherapy alone.
Table 4.
Selected Chemotherapy Regimens in Relapsed/Refractory AML
| Regimen | N | Age (Median and Range) | Adverse Cytogenetics | CR | LFS (Median) | OS [median (95% CI)] | Ref. |
|---|---|---|---|---|---|---|---|
| FLAM | 62 | 58 (23–73) | 50% | 75% in first relapse patients; 15% in primary refractory patients | 11 months (2.5–26.5+) | 8 months (0.3–30+) | 32 |
| GCLAC | 50 | 53 (19–69) | 40% | 46% | n/a | 9 months (5.2–13) | 31 |
| Elacytarabine | 191 | 62 (22–89) | 40% | 23% | 5.1 months (3.3–7.8) | 3.5 months (2.8–4.8) | 34 |
| Vosaroxin + cytarabine | 356 | 64 (20–80) | 24% | 30% | 11 months (8.3–NR) | 7.5 months (6.4–8.5) | 33 |
CR, complete remission; LFS, leukemia-free survival; OS, overall survival; N, numbers of patients; FLAM, flavopiridol, cytarabine, mitoxantrone; GCLAC, granulocyte colony-stimulating factor, clofarabine, cytarabine; NR, not reached; n/a, not available.
In conclusion, mitoxantrone–etoposide is a well-established regimen that is safe and active, albeit with still relatively low CR rates. It should be noted that other salvage regimens have not yet proven to be superior in large randomized clinical studies, and this regimen continues to be an option recommended for AML in relapse (38). The findings of this study underscore the need for newer therapies, especially those that will improve the ability for patients with relapsed AML to achieve CR and that will also allow them to receive additional therapies.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ferrara F.; Schiffer C. A. Acute myeloid leukaemia in adults. Lancet 381:484–95; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Dohner H.; Weisdorf D. J.; Bloomfield C. D. Acute myeloid leukemia. N. Engl. J. Med. 373:1136–1152; 2015. [DOI] [PubMed] [Google Scholar]
- 3. Breems D. A.; Van Putten W. L.; Huijgens P. C.; Ossenkoppele G. J.; Verhoef G. E.; Verdonck L. F.; Vellenga E.; De Greef G. E.; Jacky E.; Van der Lelie J.; Boogaerts M. A.; Lowenberg B. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 23:1969–1978; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Estey E.; Kornblau S.; Pierce S.; Kantarjian H.; Beran M., Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood 88:756; 1996. [PubMed] [Google Scholar]
- 5. Kurosawa S.; Yamaguchi T.; Miyawaki S.; Uchida N.; Sakura T.; Kanamori H.; Usuki K.; Yamashita T.; Okoshi Y.; Shibayama H.; Nakamae H.; Mawatari M.; Hatanaka K.; Sunami K.; Shimoyama M.; Fujishima N.; Maeda Y.; Miura I.; Takaue Y.; Fukuda T. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 95:1857–1864; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett J. M.; Lymann G. H.; Cassileth P. A.; Glick J. H., Oken M. M. A phase II trial of VP 16-213 in adults with refractory acute myeloid leukemia. An Eastern Cooperative Oncology Group study. Am. J. Clin. Oncol. 7:471–473; 1984. [DOI] [PubMed] [Google Scholar]
- 7. Ho A. D.; Lipp T.; Ehninger G.; Illiger H. J.; Meyer P.; Freund M.; Hunstein W. Combination of mitoxantrone and etoposide in refractory acute myelogenous leukemia—An active and well-tolerated regimen. J. Clin. Oncol. 6:213–217; 1988. [DOI] [PubMed] [Google Scholar]
- 8. Lazzarino M.; Morra E.; Alessandrino E. P.; Orlandi E.; Pagnucco G.; Merante S.; Bernasconi P.; Inverardi D.; Bonfichi M.; Bernasconi C. Mitoxantrone and etoposide: An effective regimen for refractory or relapsed acute myelogenous leukemia. Eur. J. Haematol. 43:411–416; 1989. [DOI] [PubMed] [Google Scholar]
- 9. O’Brien S.; Kantarjian H.; Estey E.; Koller C.; Beran M.; McCredie K.; Keating M. Mitoxantrone and high-dose etoposide for patients with relapsed or refractory acute leukemia. Cancer 68:691–694; 1991. [DOI] [PubMed] [Google Scholar]
- 10. Paciucci P. A.; Davis R. B.; Holland J. F.; Martelo O.; Schiffer C. A. Mitoxantrone and constant infusion etoposide for relapsed and refractory acute myelocytic leukemia. Am. J. Clin. Oncol. 13:516–519; 1990. [DOI] [PubMed] [Google Scholar]
- 11. Paciucci P. A.; Ohnuma T.; Cuttner J.; Silver R. T.; Holland J. F. Mitoxantrone in patients with acute leukemia in relapse. Cancer Res. 43:3919–3922; 1983. [PubMed] [Google Scholar]
- 12. Smith I. E.; Gerken M. E.; Clink H. M.; McElwain T. J. VP 16-213 in acute myelogenous leukaemia. Postgrad. Med. J. 52:66–70; 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byrd J. C.; Mrozek K.; Dodge R. K.; Carroll A. J.; Edwards C. G.; Arthur D. C.; Pettenati M. J.; Patil S. R.; Rao K. W.; Watson M. S.; Koduru P. R.; Moore J. O.; Stone R. M.; Mayer R. J.; Feldman E. J.; Davey F. R.; Schiffer C. A.; Larson R. A.; Bloomfield C. D. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461). Blood 100:4325–4336; 2002. [DOI] [PubMed] [Google Scholar]
- 14. Slovak M. L.; Kopecky K. J.; Cassileth P. A.; Harrington D. H.; Theil K. S.; Mohamed A.; Paietta E.; Willman C. L.; Head D. R.; Rowe J. M.; Forman S. J.; Appelbaum F. R. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96:4075–4083; 2000. [PubMed] [Google Scholar]
- 15. Cheson B. D.; Bennett J. M.; Kopecky K. J.; Buchner T.; Willman C. L.; Estey E. H.; Schiffer C. A.; Doehner H.; Tallman M. S.; Lister T. A.; Lo-Coco F.; Willemze R.; Biondi A.; Hiddemann W.; Larson R. A.; Lowenberg B.; Sanz M. A.; Head D. R.; Ohno R.; Bloomfield C. D. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 21:4642–4649; 2003. [DOI] [PubMed] [Google Scholar]
- 16. Keating M. J.; Kantarjian H.; Smith T. L.; Estey E.; Walters R.; Andersson B.; Beran M.; McCredie K. B.; Freireich E. J. Response to salvage therapy and survival after relapse in acute myelogenous leukemia. J. Clin. Oncol. 7:1071–1080; 1989. [DOI] [PubMed] [Google Scholar]
- 17. Litzow M. R.; Othus M.; Cripe L. D.; Gore S. D.; Lazarus H. M.; Lee S. J.; Bennett J. M.; Paietta E. M.; Dewald G. W.; Rowe J. M.; Tallman M. S. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: A report from the Eastern Cooperative Oncology Group. Br. J. Haematol. 148:217–225; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armistead P. M.; de Lima M.; Pierce S.; Qiao W.; Wang X.; Thall P. F.; Giralt S.; Ravandi F.; Kantarjian H.; Champlin R.; Estey E. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biol. Blood Marrow Transplant. 15:1431–1438; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlenk R. F.; Dohner K.; Mack S.; Stoppel M.; Kiraly F.; Gotze K.; Hartmann F.; Horst H. A.; Koller E.; Petzer A.; Grimminger W.; Kobbe G.; Glasmacher A.; Salwender H.; Kirchen H.; Haase D.; Kremers S.; Matzdorff A.; Benne,r A.; Dohner H. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J. Clin. Oncol. 28:4642–4648; 2010. [DOI] [PubMed] [Google Scholar]
- 20. Sierra J.; Storer B.; Hansen J. A.; Bjerke J. W.; Martin P. J.; Petersdorf E. W.; Appelbaum F. R.; Bryant E.; Chauncey T. R.; Sale G.; Sanders J. E.; Storb R.; Sullivan K. M.; Anasetti C. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: The effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood 89:4226–4235; 1997. [PubMed] [Google Scholar]
- 21. Estey E.; Plunkett W.; Gandhi V.; Rios M. B.; Kantarjian H.; Keating M. J. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk. Lymphoma 9:343–350; 1993. [DOI] [PubMed] [Google Scholar]
- 22. Thol F.; Schlenk R. F.; Heuser M.; Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 126:319–327; 2015. [DOI] [PubMed] [Google Scholar]
- 23. Vogler W. R.; McCarley D. L.; Stagg M.; Bartolucci A. A.; Moore J.; Martelo O.; Omura G. A. A phase III trial of high-dose cytosine arabinoside with or without etoposide in relapsed and refractory acute myelogenous leukemia. A Southeastern Cancer Study Group trial. Leukemia 8:1847–1853; 1994. [PubMed] [Google Scholar]
- 24. Amadori S.; Arcese W.; Isacchi G.; Meloni G.; Petti M. C.; Monarca B.; Testi A. M.; Mandelli F. Mitoxantrone, etoposide, and intermediate-dose cytarabine: An effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J. Clin. Oncol. 9:1210–1214; 1991. [DOI] [PubMed] [Google Scholar]
- 25. Kohrt H. E.; Patel S.; Ho M.; Owen T.; Pollyea D. A.; Majeti R.; Gotlib J.; Coutre S.; Liedtke M.; Berube C.; Alizadeh A. A.; Medeiros B. C. Second-line mitoxantrone, etoposide, and cytarabine for acute myeloid leukemia: A single-center experience. Am. J. Hematol. 85:877–881; 2010. [DOI] [PubMed] [Google Scholar]
- 26. Spadea A.; Petti M. C.; Fazi P.; Vegna M. L.; Arcese W.; Avvisati G.; Aloe Spiriti M. A.; Latagliata R.; Meloni G.; Testi A. M. Mitoxantrone, etoposide and intermediate-dose Ara-C (MEC): An effective regimen for poor risk acute myeloid leukemia. Leukemia 7:549–552; 1993. [PubMed] [Google Scholar]
- 27. Trifilio S. M.; Rademaker A. W.; Newman D.; Coyle K.; Carlson-Leuer K.; Mehta J.; Altman J.; Frankfurt O.; Tallman M. S. Mitoxantrone and etoposide with or without intermediate dose cytarabine for the treatment of primary induction failure or relapsed acute myeloid leukemia. Leuk. Res. 36:394–396; 2012. [DOI] [PubMed] [Google Scholar]
- 28. Jamieson K.; Odenike O. Late-phase investigational approaches for the treatment of relapsed/refractory acute myeloid leukemia. Expert Opin. Pharmacother. 13:2171–2187; 2012. [DOI] [PubMed] [Google Scholar]
- 29. Ramos N. R.; Mo C. C.; Karp J. E.; Hourigan C. S. Current approaches in the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Med. 4:665–695; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sasine J. P.; Schiller G. J. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: Novel agents and approaches currently in clinical trials. Blood Rev. 29:1–9; 2015. [DOI] [PubMed] [Google Scholar]
- 31. Becker P. S.; Kantarjian H. M.; Appelbaum F. R.; Petersdorf S. H.; Storer B.; Pierce S.; Shan J.; Hendrie P. C.; Pagel J. M.; Shustov A. R.; Stirewalt D. L.; Faderl S.; Harrington E.; Estey E. H. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br. J. Haematol. 155:182–189; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karp J. E.; Smith B. D.; Levis M. J.; Gore S. D.; Greer J.; Hattenburg C.; Briel J.; Jones R. J.; Wright J. J.; Colevas A. D. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: A phase II trial in adults with poor-risk acute myelogenous leukemia. Clin. Cancer Res. 13:4467–4473; 2007. [DOI] [PubMed] [Google Scholar]
- 33. Ravandi F.; Ritchie E. K.; Sayar H.; Lancet J. E.; Craig M. D.; Vey N.; Strickland S. A.; Schiller G. J.; Jabbour E.; Erba H. P.; Pigneux A.; Horst H. A.; Recher C.; Klimek V. M.; Cortes J.; Roboz G. J.; Odenike O.; Thomas X.; Havelange V.; Maertens J.; Derigs H. G.; Heuser M.; Damon L.; Powell B. L.; Gaidano G.; Carella A. M.; Wei A.; Hogge D.; Craig A. R.; Fox J. A.; Ward R.; Smith J. A.; Acton G.; Mehta C.; Stuart R. K.; Kantarjian H. M. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): A randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 16:1025–1036; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roboz G. J.; Rosenblat T.; Arellano M.; Gobbi M.; Altman J. K.; Montesinos P.; O’Connell C.; Solomon S. R.; Pigneux A.; Vey N.; Hills R.; Jacobsen T. F.; Gianella-Borradori A.; Foss O.; Vetrhusand S.; Giles F. J. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J. Clin. Oncol. 32:1919–1926; 2014. [DOI] [PubMed] [Google Scholar]
- 35. Kantarjian H. M.; Jabbour E.; Yee K.; Kropf P.; O’Connell C.; Stock W.; Tibes R.; Rizzieri D.; Walsh K.; Griffiths E. A.; Roboz G.; Savona M.; Ervin T.; Podoltsev N. A.; Pemmaraju N.; Daver N.; Garcia-Manero G.; Borthakur G.; Wierda W. G.; Ravandi F.; Cortes J. E.; Brandwein J. M.; Odenike O.; Feldman E. J.; Chung W.; Naim S.; Choy G.; Taverna P.; Hao Y.; Dimitrov G.; Mohammad A.; Pierre Issa J. First clinical results of a randomized phase 2 study of SGI-110, a novel subcutaneous (SQ) hypomethylating agent (HMA), in adult patients with acute myeloid leukemia (AML). Blood 122; 2013. [Google Scholar]
- 36. Stein A. S.; Walter R. B.; Erba H. P.; Fathi A. T.; Advani A. S.; Lancet J. E.; Ravandi F. R.; Kovacsovics T. J.; DeAngelo D. J.; Bixby D.; Faderl S.; Jillella A. P.; O’Meara M. M.; Zhao B.; Stein E. M. A phase 1 trial of SGN-CD33A As monotherapy in patients with CD33-positive acute myeloid leukemia (AML). Blood 126; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konopleva M.; Pollyea D. A.; Potluri J.; Chyla B. J.; Busman T.; McKeegan E.; Salem A.; Zhu M.; Ricker J. L.; Blum W.; DiNardo C. D.; Dunbar M.; Kirby R.; Falotico N.; Leverson J. D.; Humerickhouse R. A.; Mabry M.; Stone R. M.; Kantarjian H. M.; Letai A. G. A phase 2 study of ABT-199 (GDC-0199) in patients with acute myelogenous leukemia (AML). Blood 124; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dohner H.; Estey E. H.; Amadori S.; Appelbaum F. R.; Buchner T.; Burnett A. K.; Dombret H.; Fenaux P.; Grimwade D.; Larson R. A.; Lo-Coco F.; Naoe T.; Niederwieser D.; Ossenkoppele G. J.; Sanz M. A.; Sierra J.; Tallman M. S.; Lowenberg B.; Bloomfield C. D. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood 115:453–474; 2010. [DOI] [PubMed] [Google Scholar]


