Abstract
In this study, the effects of hypoxia-inducible factor-1α (HIF-1α) on gastric carcinoma (GC) drug resistance through apoptosis-related genes are investigated. First, HIF-1α-specific siRNA was synthetized and transfected into drug-resistant GC cell line OCUM-2MD3/L-OHP. Then MTT assay was applied to test the inhibition rate of GC cells by 5-fluorouracil (5-FU) and oxaliplatin (L-OHP). After that, flow cytometry (FCM) was applied to measure apoptosis rate. qPCR and Western blot assay were employed to detect HIF-1α and apoptosis-related genes. Results showed that HIF-1α in OCUM-2MD3/L-OHP cells was higher than that in OCUM-2MD3 and gastric epithelial cells. After HIF-1α-siRNA transfection, inhibition rates of 5-FU and L-OHP to tumor cells increased significantly. FCM results showed that apoptosis rate of OCUM-2MD3/L-OHP cells increased significantly. After HIF-1α-siRNA transfection, survivin and Bcl-2 decreased, whereas Bax, caspase 3, and caspase 8 increased significantly. Results from this study seem to confirm that HIF-1α getting involved in GC drug resistance is possibly due to its regulation of some apoptosis-related genes. HIF-1α may be a potential target to reverse drug resistance of GC.
Key words: Gastric carcinoma (GC), Hypoxia-inducible factor-1α, Drug resistance, Drug sensitivity test in vitro, Apoptosis-related genes
INTRODUCTION
Drug resistance is one of the main reasons contributing to poor prognosis of gastric carcinoma (GC) (1,2). Multiple genes have been found to be involved in drug resistance of GC with different mechanisms (3,4). Classical drug resistance genes, such as multidrug resistance gene 1 (MDR1) and multidrug resistance-associated protein 1 (MRP1), are well known for their function in drug resistance. However, the effects of many chemotherapy drugs are little related to classical drug resistance genes, for there are several other pathways working in cancer drug resistance besides classical drug resistance pathways. In these drug resistance pathways, the apoptotic pathway has been revealed to play an important role in GC drug resistance (5,6). Platinum and fluorouracil-based anticancer drugs are commonly used in chemotherapy for GC by inducing apoptosis of cancer cells, and drug resistance of cancer cells to these drugs was proven to be related to apoptosis-related genes (7,8). Regulating apoptosis-related genes could reverse resistance of GC cells to platinum and fluorouracil-based agents. However, specific regulatory mechanism and methods are poorly understood.
Hypoxia-inducible factor (HIF)-1α is a newly discovered transcription factor overexpressed in GC cells, and overexpressed HIF-1α is also found to be related to GC drug resistance (9). Results of Chen et al. (10) have shown that HIF-1α could regulate apoptosis-related genes, which then alter the apoptosis of cancer cells. However, it is still unclear whether HIF-1α could modify GC drug resistance by regulating apoptosis-related genes.
In order to confirm the molecular function of HIF-1α in GC drug resistance, we applied RNA interference technique to inhibit HIF-1α expression in oxaliplatin (L-OHP)-resistant GC cell line. Then the sensitivity of GC cells to chemotherapeutic agents was detected, and expression of apoptosis-related genes was also detected to confirm the effect of HIF-1α inhibition. Accordingly, related mechanisms of HIF-1α in GC drug resistance through apoptosis regulation were explored. Our results showed that after HIF-1α was downregulated in L-OHP-resistant cell line OCUM-2MD3/L-OHP, inhibition rates of 5-FU and L-OHP to tumor cells increased significantly and so did apoptosis rate of OCUM-2MD3/L-OHP cells. Besides, some apoptosis-related genes were regulated after HIF-1α was inhibited. Hence, we conclude that HIF-1α may reverse drug resistance of GC by regulating some apoptosis-related genes.
MATERIALS AND METHODS
Cell lines and Reagents
Gastric epithelial cell line GES-1 was purchased from Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Science (Shanghai, China). Poorly differentiated human GC peritoneal metastasis cell line OCUM-2MD3 was a gift from Professor Masakazu Yashiro (11). Oxaliplatin (L-OHP)-resistant cell line OCUM-2MD3/L-OHP was cultured in our laboratory (12). MTT was purchased from Sigma-Aldrich (USA); RPMI-1640 culture medium and trypsin were from Gibco Company; fluorescence quantitative RT-PCR kit was purchased from Promega (USA); Lipofectamine™ 2000 transfection kit was purchased from Invitrogen Corporation; rabbit-anti-human HIF-1α, caspase 3, caspase 8, β-actin polyclonal antibody, mouse-anti-human Bcl-2 and Bax monoclonal antibody were purchased from Santa Cruz (USA); PCR primers were synthesized by Shanghai Biological Engineering Co., and small interfering RNA by Shanghai Jikai Pharmaceutical Co. Fluorouracil (5-FU) was from Tianjin Jin-yao Pharmaceutical Company; oxaliplatin (L-OHP) was purchased from Jiangsu Hengrui Pharmaceutical Company; flow cytometry (Epics-XL type II) was a product of the USA. Our study was approved by the ethics committee of the Fourth Affiliated Hospital, Hebei Medical University.
Cell Culture
Cells were incubated with RPMI 1640 culture medium containing 10% fetal bovine serum. When drug-resistant GC cell line OCUM-2MD3/L-OHP was cultured, the culture medium was added with L-OHP at a concentration of 1.83 µg/ml, to maintain their resistance phenotype, and L-OHP was removed 1 week before beginning the experiments.
HIF-1α-siRNA Synthesis and Transfection
HIF-1α-specific small interfering RNA (HIF-1α-siRNA) sequences (13) were designed according to references. The sequence was as follows: CUGAUAACGUGAACAAAUAtt. Nonspecific control siRNA, (NS-siRNA) sequence was as follows: GGUCUCACUCCCCAUAGAGtt. Liposome-mediated method and Lipofectamine™ 2000 were used, and following the instructions, NS-siRNA (40 nM) or HIF-1α-siRNA was transfected into the cell line OCUM-2MD3/L-OHP.
Drug Sensitivity Test In Vitro by MTT Assay
GC cells (104) were seeded in 96-well plates with 200 µl in each cell. According to the instructions, NS-siRNA, HIF-1α-siRNA, or Lipofectamine™ 2000 was transfected into the cells, respectively. Then 200 µl of NS-siRNA, HIF-1α-siRNA, or Lipofectamine™ 2000 was added to each well, respectively, and six replicate wells were made in each group. After 6 h, culture medium was replaced with 200 µl of that containing calf serum and Pen/Strep antibiotics. Cells were cultured for 24 h, and then 5-FU (25 µg/ml) or L-OHP (8 µg/ml) was added to each well. The cells were cultured continuously for 8 h, with 20 µl MTT in each well. After 4 h, culture medium was discarded, and 150 µl dimethyl sulfoxide (DMSO) was added. Absorbance value (OD) at a wavelength of 490 nm was measured by a microplate reader. Inhibition rate (IR) = (1 − average A value in dosing hole/average A value in control well) × 100%.
Apoptosis Rate of Cells With Flow Cytometry (FCM) (Annexin V/PI Assay)
Quantitative apoptotic cell was measured by using an Annexin-V-FITC/PI detection kit (Jiamei, Beijing, China), according to the manufacturer’s instruction. Briefly, cells were harvested and resuspended in binding buffer (106 cells/ml). After addition of 5 µl Annexin-V-FITC and 10 µl of propidium iodide (PI) with mixing, the tubes were incubated for 15 min at room temperature in the dark. Annexin-V-FITC binding was detected by a FACSCalibur cytometer (Becton Dickinson, USA). The data were analyzed by Cell Quest software. The experiment was repeated three times.
mRNA Tested With qPCR
Total RNAs were isolated by one-step method using TRIzol reagent according to the instruction. The RNA samples were pretreated with RNase-free DNase, and 1 µg RNA of each sample was reverse-transcribed to synthesize first-strand cDNA using EasyScript First-Strand cDNA Synthesis Kit (TransGen Biotech, China). Then the cDNA templates were amplified by real-time quantitative PCR using Maxima SYBR Green/Fluorescein QPCR Master Mix (MBI, Canada) on an ABI-7300 PCR System (Applied Biosystems, USA). PCR reaction started with 1 cycle of 95°C for 10 min, followed by 40 cycles of three steps as 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. The fluorescence data were collected at 72°C step. The mRNA expression of the target gene was normalized to the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The mRNA relative expression levels of target gene were represented as 2-ΔΔCt, which were obtained by the software SDS v3.2 of ABI-7300 PCR system. PCR primers were designed using Primer Express Software V3.0 (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Sequences of primers were as follows:
HIF-1α: (F) 5′-TCGACACAGCCTGGATATGA-3′ and (R) 5′-CGGCT GCGGCCAGCAAAGT T-3′;
survivin: (F) 5′-GCCAGATTTGAATCGCGGGA-3′ and (R) 5′-GCAGTGGATGAAGCCAGCCT-3′;
Bcl-2: (F) 5′-TGTGTGGAGAGCGTCAACC-3′ and (R) 5′-TGGATCCAGGTGTGCAGGT-3’;
Bax: (F) 5′-TTTCTGACGGCAACTTCAAC-3′ and (R) 5′-AGTCCAATGTCCAGCCCAT-3′;
caspase 3: 5′-AGAGCTGGACTGCGGTATTGAG-3′ (F) and (R) 5′-GAACCATGACCCGTCCCTTG-3′;
caspase 8: 5′-GATGAGGCAGACTTTCTGCT-3′ (F) and (R) 5′-CATAGTTCACGCCAGTCAGGAT-3′;
GAPDH: (F) 5′-GACCCCTTCATTGACCTCAAC-3′ and (R) 5′-CGCTCCTGGAAGATGGTGAT-3′.
Proteins Tested With Western Blot
Cells were harvested and lysed on ice in RIPA buffer containing protease inhibitor. Protein concentration was determined using Bradford method (Bio-Rad, Hercules, CA, USA), and 40 µg of protein for each sample was separated by 10% SDS-PAGE, and then electrically transferred to PVDF membranes (Roche, Basel, Switzerland). Membranes were incubated overnight at 4°C with primary antibodies. β-Actin was utilized for an endogenous reference to standardize protein expression levels. Bands were detected with enhanced chemiluminescence (Pierce, USA). The density of bands was determined by Gelpro 4.0 software.
Statistical Analysis
SPSS13.0 statistical analysis software was used for the analysis of experimental data. Experimental results were expressed with means ± standard deviation, and t-test was used for comparison. A value of p < 0.05 indicated a statistically significant difference.
RESULTS
HIF-1α Expression in GC Cell Lines and Gastric Epithelial Cell Line
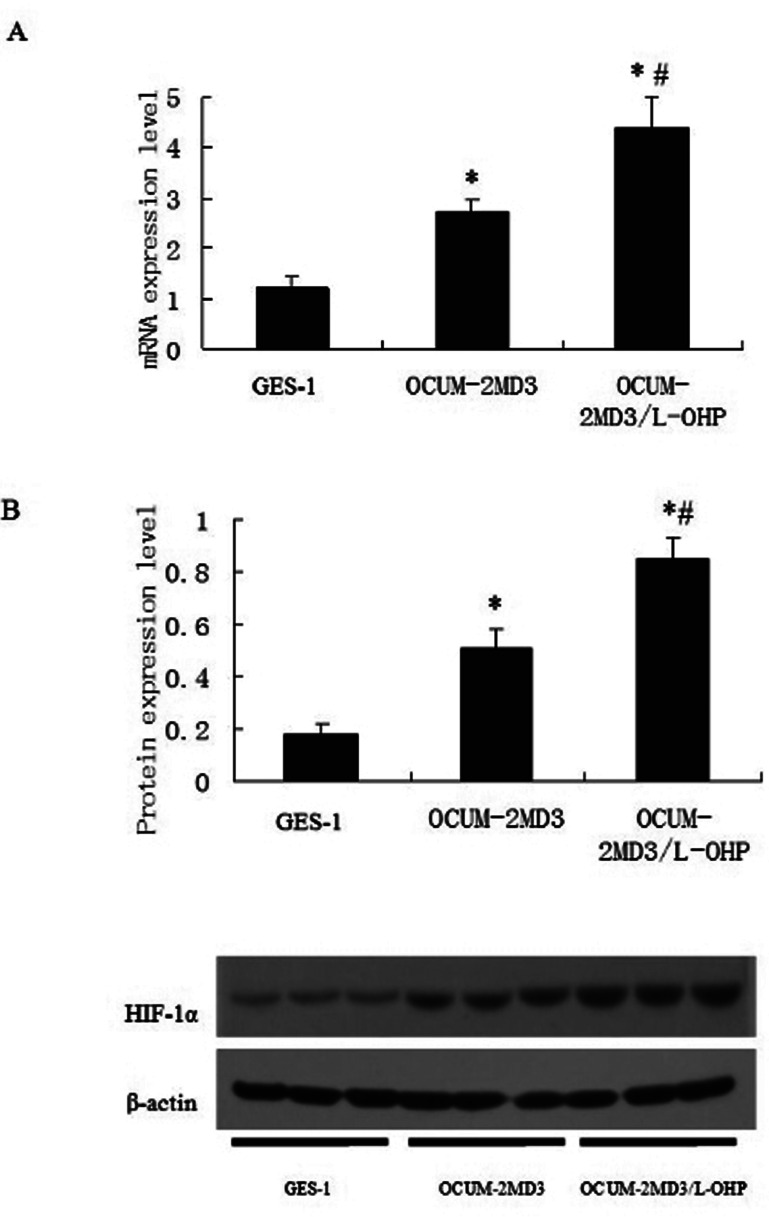
qPCR and Western blot were applied to detect HIF-1α expressions in OCUM-2MD3, OCUM-2MD3/L-OHP, and GES-1. The results showed that expressions of HIF-1α in OCUM-2MD3 and OCUM-2MD3/L-OHP were significantly higher than that in GES-1 (all p < 0.01), and HIF-1α in OCUM-2MD3/L-OHP was significantly higher than that in OCUM-2MD3 (Fig. 1).
Figure 1.
HIF-1α expression levels in gastric cell lines. Gastric cell lines GES1, OCUM-2MD3, and OCUM-2MD3/L-OHP were subjected to qPCR (A) and Western blot (B) assays to determine the expression of HIF-1α. Values are shown as means ± SD for cell samples n = 4 in each group. *p < 0.01 versus GES-1 group; #p < 0.01 versus OCUM-2MD3 group.
Effect of Transfection of HIF-1α-siRNA to OCUM-2MD3/L-OHP
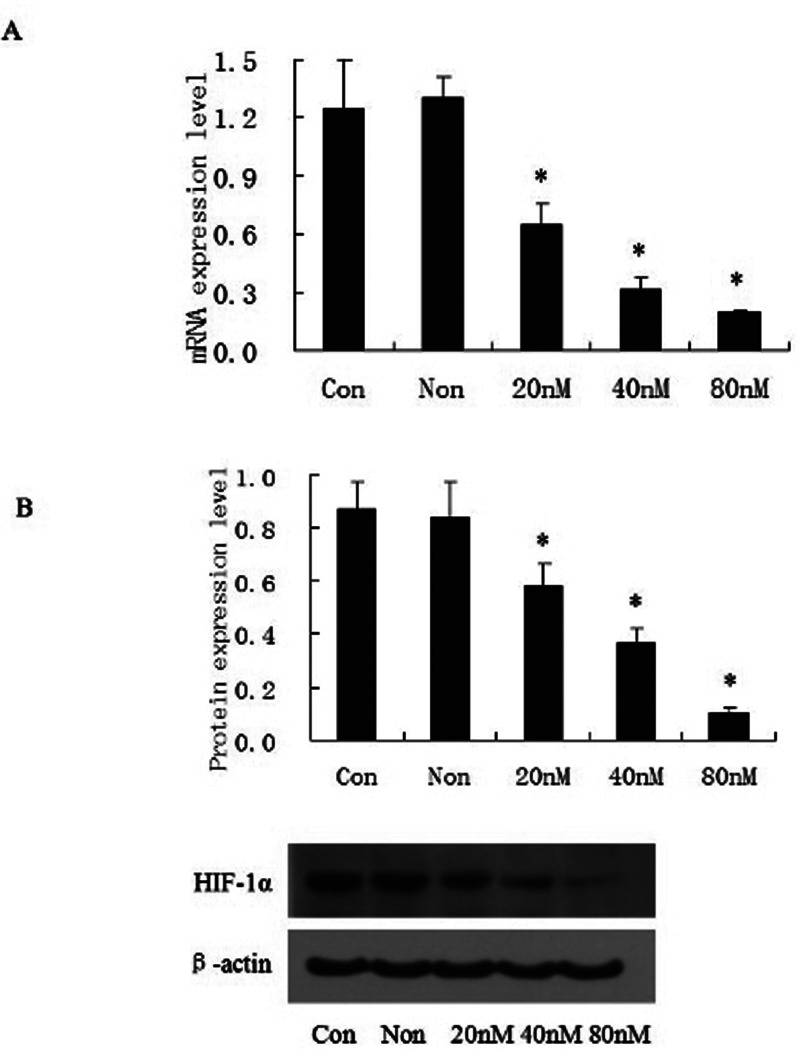
After HIF-1α-siRNA was transfected into OCUM-2MD3/L-OHP cells, HIF-1α was inhibited significantly. After 48 h, inhibition rate was 90% at the concentration of 80 nmol/L. Results of qPCR were in accordance with Western blot (all p < 0.01) (Fig. 2).
Figure 2.
HIF-1α-siRNA downregulated HIF-1α expression in CUM-2MD3/L-OHP cells. Cells were transfected with different amounts of sequence of HIF-1α-siRNA for 48 h (A, B); the expression of HIF-1α was identified by qPCR (A) as well as Western blot (B). *p < 0.01 versus NS-siRNA control group. Con, blank control group; Non, NS-siRNA control group.
Drug Sensitivity of GC Cells In Vitro With MTT Assay
The results showed that the inhibition rates of 5-FU and L-OHP to OCUM-2MD3/L-OHP cells in the control group were 35.85 ± 4.13% and 38.62 ± 4.46%, respectively. After transfection with HIF-1α-siRNA, inhibition rates of 5-FU and L-OHP to GC cells increased significantly (all p < 0.01) (Table 1).
Table 1.
Effect of HIF-1α-siRNA Transfection on Inhibition Rate of 5-FU and L-OHP to OCUM-2MD3/L-OHP Cells In Vitro (n = 6)
| 5-FU | L-OHP | |
|---|---|---|
| Transfection group | 80.24 ± 9.26% | 75.64 ± 8.86% |
| Control group | 35.85 ± 4.13% | 38.62 ± 4.46% |
| t | 10.72 | 9.14 |
| p | <0.001 | <0.001 |
Tumor Cell Apoptosis Tested With FCM Assay
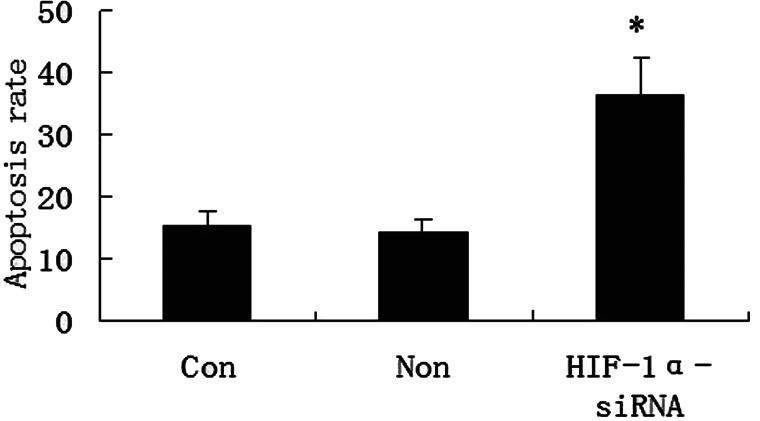
The antiapoptotic effects of HIF-1α-siRNA were studied using Annexin-V-FITC/PI assay. FCM showed that apoptosis rate (15.67 ± 2.84%) of tumor cells in transfection group was significantly higher than that (7.89 ± 1.03%) in control group (t = 6.31, p < 0.01) (Fig. 3).
Figure 3.
The effects of HIF-1α-siRNA on the apoptosis of gastric carcinoma cell line OCUM-2MD3/L-OHP with FCM. Cells were transfected with HIF-1α-siRNA or control NS-siRNA, and then tested by FCM. Apoptosis rates of three groups are shown. *p < 0.01 versus NS-siRNA control group. Con, blank control group; Non, NS-siRNA control group.
Effect of HIF-1α-siRNA Transfection to Expressions of Survivin, Bcl-2, Bax Caspase 3, and Caspase 8 in OCUM-2MD3/L-OHP Cells
The results of qPCR showed that, compared with those in the control group, after HIF-1α-siRNA was transfected, mRNA expressions of survivin and Bcl-2 in OCUM-2MD3/L-OHP cells were lowered (all p < 0.05), whereas mRNA expressions of Bax, caspase 3, and caspase 8 were significantly increased (all p < 0.05) (Fig. 4A). Western blot test results were in accordance with the qPCR results (Fig. 4B).
Figure 4.
The effects of HIF-1α-siRNA on the expression of apoptosis-related genes in gastric carcinoma cell OCUM-2MD3/L-OHP. Cells were transfected with HIF-1α-siRNA or control NS-siRNA and then subjected to qPCR (A) or Western blot assays (B) to detect mRNA or protein expression levels of survivin, Bcl-2, Bax caspase 3, and caspase 8. The mRNA or protein expression levels are represented as columns in (A) and (B). *p < 0.01 versus NS-siRNA control group. Con, blank control group; Non, NS-siRNA control group.
DISCUSSION
Although mechanisms of GC drug resistance have been studied extensively, what still remains unclear is the molecular regulation of drug resistance genes in GC. Hypoxia, a common phenomenon in the growth process of solid tumor, is also closely related to tumor drug resistance, and HIF-1α plays a key role in this process. Studies have shown that HIF-1α is related to drug resistance of malignancies, such as ovarian cancer, laryngeal cancer, breast cancer, lung cancer, GC, and colon cancer (14–19). It is reported that tumor cells with overexpressed HIF-1α have stronger ability of drug resistance. Our study also found that HIF-1α expression in L-OHP-resistant GC cell line was significantly increased compared with that in GC cell line and gastric epithelial cell line, suggesting that HIF-1α might be associated with GC drug resistance.
To confirm the effect of HIF-1α on GC drug resistance, we applied RNA interference technique to inhibit HIF-1α in drug-resistant GC cell line OCUM-2MD3/L-OHP. The results showed that after HIF-1α was effectively inhibited, the sensitivity of tumor cells to chemotherapeutic drugs 5-FU and L-OHP was significantly increased, indicating that the drug resistance of OCUM-2MD3/L-OHP cells had been effectively reversed, and that HIF-1α should be further studied as a target gene in GC drug-resistance reversal.
The mechanism of HIF-1α involved in GC drug resistance has been unclear until now. In our previous study, we found that inhibition of HIF-1α could simultaneously downregulate drug resistance-related genes, such as multidrug resistance gene 1 (MDR1) and GST-π (13), resulting in weakened drug resistance in GC cells. Considering that drug resistance genes, such as MDR1 and GST-π, only have effects on some chemotherapeutics, mostly alkaloids (20), these drugs are not commonly applied in GC chemotherapy. Currently, chemotherapy drugs for GC mainly involve 5-FU and L-OHP (21–23), which destroy cancer cells by inducing apoptosis. GC cells have the ability of apoptosis resistance, which makes chemotherapeutic drugs 5-FU and L-OHP that promote cell apoptosis less effective. Whether HIF-1α plays a role in this procedure needs to be confirmed. Therefore, we detected apoptosis-related genes that played a role in 5-FU and L-OHP-related drug resistance of GC in this study. Our results showed that after inhibition of HIF-1α expression, GC cell apoptosis rate was increased significantly, indicating that the antiapoptotic ability of tumor cells was decreased. Combined with increasing the sensitivity of tumor cells to chemotherapy drugs, HIF-1α inhibition could reverse drug resistance features by weakening the antiapoptotic ability of tumor cells.
To investigate apoptosis-related genes affected by HIF-1α, we tested survivin, Bcl-2, Bax, caspase 3, and caspase 8 genes after HIF-1α inhibition. Survivin, an important member of inhibitor of apoptosis protein (IAP) family, can directly inhibit members in the caspase family, resulting in enhanced antiapoptotic ability of tumor cells and increased drug-resistance characteristics (24,25); caspase 3 and caspase 8 can directly promote apoptosis as the core apoptotic genes (26,27); Bcl-2/Bax are important members of the mitochondrial pathway, both of which can be combined into dimers, the ratio of which may change, leading to changes in apoptosis ability (28). Our results showed that after inhibiting HIF-1α, expressions of survivin and Bcl-2 were downregulated, while expressions of Bax, caspase 3, and caspase 8 were significantly upregulated. We conclude that HIF-1α might modify apoptosis of OCUM-2MD3/L-OHP cells by upregulating survivin and Bcl-2 genes and downregulating Bax, caspase 3, and caspase 8 genes, thereby drug resistance of these cells to 5-FU and L-OHP will change.
The results from this study have confirmed that HIF-1α is involved in GC drug resistance by regulating some genes in apoptosis inhibition pathway as well as the mitochondrial pathway and that HIF-1α may be a target to reverse drug resistance of GC. However, the molecular regulation mechanism of HIF-1α to these genes needs to be confirmed by further research.
ACKNOWLEDGMENTS
Provincial Natural Science Foundation of Hebei Province. Item No. H2013206311.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Zhu C. Y.; Lv Y. P.; Yan D. F.; Gao F. L. Knockdown of MDR1 increases the sensitivity to adriamycin in drug resistant gastric cancer cells. Asian Pac. J. Cancer Prev. 14:6757–6760; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Yan L. H.; Wang X. T.; Yang J.; Kong F. B.; Lian C.; Wei W. Y.; Luo W.; Xie Y. B.; Xiao Q. Reversal of multidrug resistance in gastric cancer cells by E2F-1 downregulation in vitro and in vivo. J. Cell Biochem. 115:34–41; 2014. [DOI] [PubMed] [Google Scholar]
- 3. Kang J.; Zhao G.; Lin T.; Tang S.; Xu G.; Hu S.; Bi Q.; Guo C.; Sun L.; Han S.; Xu Q.; Nie Y.; Wang B.; Liang S.; Ding J.; Wu K. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 339:247–259; 2013. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y.; Liu L.; Liu X.; Zhang H.; Liu J.; Feng B.; Shang Y.; Zhou L.; Wu K.; Nie Y.; Zhang H.; Fan D. Shugoshin1 enhances multidrug resistance of gastric cancer cells by regulating MRP1, Bcl-2, and Bax genes. Tumour Biol. 34:2205–2214; 2013. [DOI] [PubMed] [Google Scholar]
- 5. Xie X.; Tang B.; Zhou J.; Gao Q.; Zhang P. Inhibition of the PI3K/Akt pathway increases the chemosensitivity of gastric cancer to vincristine. Oncol. Rep. 30:773–782; 2013. [DOI] [PubMed] [Google Scholar]
- 6. Zhang K. G.; Qin C. Y.; Wang H. Q.; Wang J. X.; Wang Q. M. The effect of TRAIL on the expression of multidrug resistant genes MDR1, LRP and GST-π in drug-resistant gastric cancer cell SGC7901/VCR. Hepatogastroenterology 59:2672–2676; 2012. [DOI] [PubMed] [Google Scholar]
- 7. Li Y.; Tan B. B.; Zhao Q.; Fan L. Q.; Liu Y.; Hao Y. J.; Zhao X. F. Tumor chemosensitivity is correlated with expression of multidrug resistance associated factors in variously differentiated gastric carcinoma tissues. Hepatogastroenterology 60:213–216; 2013. [DOI] [PubMed] [Google Scholar]
- 8. Gazzaniga P.; Gradilone A.; Petracca A.; Nicolazzo C.; Raimondi C.; Iacovelli R.; Naso G.; Cortesi E. Molecular markers in circulating tumour cells from metastatic colorectal cancer patients. J. Cell. Mol. Med. 14:2073–2077; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu L.; Sun L.; Zhang H.; Li Z.; Ning X.; Shi Y.; Guo C.; Han S.; Wu K.; Fan D. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int. J. Cancer 124:1707–1715; 2009. [DOI] [PubMed] [Google Scholar]
- 10. Chen M. H.; Ren Q. X.; Yang W. F.; Chen X. L.; Lu C.; Sun J. Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions in rats with spinal cord injury. Int. J. Clin. Exp. Pathol. 6:2312–2322; 2013. [PMC free article] [PubMed] [Google Scholar]
- 11. Yashiro M.; Chung Y. S.; Nishimura S.; Inoue T.; Sowa M. Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin. Exp. Metastasis. 14:43–54; 1996. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Q.; Zhang H.; Li Y.; Liu J.; Hu X.; Fan L. Anti-tumor effects of CIK combined with oxaliplatin in human oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J. Exp. Clin. Cancer Res. 29:118; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Q.; Li Y,; Tan B. B.; Fan L. Q.; Wang D.; Yang P. G. Effect of hypoxia inducible factor-lα on multidrug resistance of gastric cancer cells and mechanism. Chinese J. Exp. Surg. 29:2472–2474; 2012. [Google Scholar]
- 14. Xie Z.; Cao L.; Zhang J. miR-21 modulates paclitaxel sensitivity and hypoxia-inducible factor-1α expression in human ovarian cancer cells. Oncol. Lett. 6:795–800; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li D. W.; Dong P.; Wang F.; Chen X. W.; Xu C. Z.; Zhou L. Hypoxia induced multidrug resistance of laryngeal cancer cells via hypoxia-inducible factor-1α. Asian. Pac. J. Cancer Prev. 14:4853–4858; 2013. [DOI] [PubMed] [Google Scholar]
- 16. Li S. Z.; Li K.; Zhang J. H.; Dong Z. The effect of quercetin on doxorubicin cytotoxicity in human breast cancer cells. Anticancer Agent. Med. Chem. 13:352–355; 2013. [DOI] [PubMed] [Google Scholar]
- 17. Ye M. X.; Zhao Y. L.; Li Y.; Miao Q.; Li Z. K.; Ren X. L.; Song L. Q.; Yin H.; Zhang J. Curcumin reverses cisplatin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine 19:779–787; 2012. [DOI] [PubMed] [Google Scholar]
- 18. Chen M.; Huang S. L.; Zhang X. Q.; Zhang B.; Zhu H.; Yang V. W.; Zou X. P. Reversal effects of pantoprazole on multidrug resistance in human gastric adenocarcinoma cells by down-regulating the V-ATPases/mTOR/HIF-1α/P-gp and MRP1 signaling pathway in vitro and in vivo. J. Cell Biochem. 113:2474–2487; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y.; Tozzi F.; Chen J.; Fan F.; Xia L.; Wang J.; Gao G.; Zhang A.; Xia X.; Brasher H.; Widger W.; Ellis L. M.; Weihua Z. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 72:304–314; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao P.; Zhang Y.; Sun M.; He Y. Reversion of multidrug resistance in human glioma by RNA interference. Neurol. Res. 30:562–566; 2008. [DOI] [PubMed] [Google Scholar]
- 21. Zhao Q.; Li Y.; Tian Y.; Chen Y. N.; Tan B. B.; Zhao X. F.; Jiao Z. K.; Zhang Z. D.; Chang S. L. Histological complete response after neoadjuvant XELOX in advanced gastric carcinoma. Hepatogastroenterology 60:638–640; 2013. [DOI] [PubMed] [Google Scholar]
- 22. Chen S.; Feng X.; Li Y.; Yuan X.; Zhou Z.; Chen Y. Efficacy and safety of XELOX and FOLFOX6 adjuvant chemotherapy following radical total gastrectomy. Oncol. Lett. 3:781–786; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim G. M.; Jeung H. C.; Rha S. Y.; Kim H. S.; Jung I.; Nam B. H.; Lee K. H.; Chung H. C. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur. J. Cancer 48:518–526; 2012. [DOI] [PubMed] [Google Scholar]
- 24. Dong H.; Liu G.; Jiang B.; Guo J.; Tao G.; Yiu W.; Zhou J.; Li G. Overexpression of the Survivin gene in SGC7901 cell resistance to cisplatin. Oncol. Lett. 8:1953–1956; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan Z.; Khan N.; Varma A. K.; Tiwari R. P.; Mouhamad S.; Prasad G. B.; Bisen P. Oxaliplatin-mediated inhibition of survivin increases sensitivity of head and neck squamous cell carcinoma cell lines to paclitaxel. Curr. Cancer Drug Targets 10:660–669; 2010. [DOI] [PubMed] [Google Scholar]
- 26. Wittkopf N.; Günther C.; Martini E.; He G.; Amann K.; He Y. W.; Schuchmann M.; Neurath M. F.; Becker C. Cellular FLICE-like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase-8. Gastroenterology 145:1369–1379; 2013. [DOI] [PubMed] [Google Scholar]
- 27. Sikdar S.; Mukherjee A.; Ghosh S.; Khuda-Bukhsh A. R. Condurango glycoside-rich components stimulate DNA damage-induced cell cycle arrest and ROS-mediated caspase-3 dependent apoptosis through inhibition of cell-proliferation in lung cancer, in vitro and in vivo. Environ. Toxicol. Pharmacol. 37:300–314; 2013. [DOI] [PubMed] [Google Scholar]
- 28. Kontos C. K.; Christodoulou M. I.; Scorilas A. Apoptosis-related BCL2-family members: Key players in chemotherapy. Anticancer Agents Med. Chem. 14:353–374; 2014. [DOI] [PubMed] [Google Scholar]