Abstract
Hematopoietic pre-B-cell leukemia transcription factor (PBX)-interacting protein (HPIP/PBXIP1) is a nucleo-cytoplasmic shuttling protein, and its expression is associated with cancer aggressiveness. However, the role of HPIP in ovarian cancer is still unclear. Here, we aimed to clarify the role of HPIP in epithelial–mesenchymal transition (EMT) process of ovarian cancer cells, stimulated by transforming growth factor (TGF)-β1. In this study, we found that HPIP was highly expressed in ovarian cancer cells, and TGF-β1 treatment induced HPIP expression in ovarian cancer cells. In addition, knockdown of HPIP suppressed TGF-β1-induced EMT and migration/invasion in ovarian cancer cells. Moreover, knockdown of HPIP significantly blocked the phosphorylated pattern of both PI3K and Akt induced by TGF-β1 in SKOV3 cells. In conclusion, the present study showed that HPIP silencing might prevent TGF-β1-induced EMT in ovarian cancer cells. Thus, HPIP may be a potential therapeutic target for the treatment of ovarian cancer.
Key words: HPIP, Ovarian cancer, Epithelial–mesenchymal transition (EMT), Transforming growth factor (TGF)-β1
INTRODUCTION
Ovarian cancer is the second leading cancer in women and the incidence rate has been increasing annually (1,2). Owing to a lack of definitive early symptoms and appropriate markers for ovarian cancer diagnosis at an early stage, patients with metastatic disease have poor outcomes, with the average overall 5-year survival rate of approximately 30% (3,4). Thus, elucidation of the molecular mechanisms underlying ovarian cancer tumorigenesis and progression is critical to treatment of ovarian cancer.
Epithelial–mesenchymal transition (EMT) is known to be a central mechanism responsible for invasiveness and metastasis of various cancers. It is characterized by morphological changes attributable to loss of epithelial features, acquisition of mesenchymal features, dissolution of cell–cell interactions, and increased cell motility (5). Transforming growth factor (TGF)-β1, a multipotent cytokine, induces EMT, activating several transcription factors, such as Snail, Slug, Twist1, and zinc finger E-box-binding homeobox 1 (ZEB1), in ovarian cancer (6). Thus, inhibiting TGF-β1-induced EMT of ovarian cancer cells may be a therapeutic method for human ovarian cancer.
Hematopoietic pre-B-cell leukemia transcription factor (PBX)-interacting protein (HPIP/PBXIP1), a corepressor for the transcription factor PBX, is a nucleo-cytoplasmic shuttling protein (7). A growing body of evidence indicates that HPIP is highly expressed in various tumors, including gastric cancer, colorectal cancer, breast infiltrative ductal carcinoma, and astrocytoma. HPIP can promote proliferation and migration/invasion of tumor cells, thereby contributing to tumorigenesis (8–10). However, the role of HPIP in ovarian cancer is still unclear. Here we aimed to clarify the role of HPIP in EMT process of ovarian cancer cells, stimulated by TGF-β1.
MATERIALS AND METHODS
Cell Culture and Treatment
Human ovarian cancer cell lines (CAOV3, SKOV3, and HO-8910) and the normal epithelial ovarian cell line (Moody) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). They were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD, USA), 100 U/ml streptomycin and penicillin (Gibco) in a humidified atmosphere of 5% CO2 at 37°C. Cells were treated by recombinant human TGF-β1 (5 ng/ml) for different times.
RNA Interference and Transfection
Small interfering RNA (siRNA) targeting HPIP and its negative control (mock) were purchased from GenePharma Co., Ltd. (Shanghai, China). For in vitro transfection, SKOV3 cells were seeded in 24-well plates in triplicate containing DMEM supplemented with 10% FBS. Then SKOV3 cells were transfected with siRNA-HPIP or mock using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Depletion of the targeted genes was confirmed with Western blot.
RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated from ovarian cancer cells using the RNA plus kit (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized with the Prime Script RT reagent kit (Takara Bio Inc., Otsu, Japan). PCR amplification was carried out by ABI PRISM 7900 thermocycler using SYBR Premix Taq (Applied Biosystems). The specific primers for HPIP were forward 5′-GTCCCCTCGAGGAGTTGTGT-3′ and reverse 5′-ATCTTCCATCATCTGAGGGC-3′ and for β-actin were forward 5′-AAATCGTGCGTGACATCAAAGA-3′ and reverse 5′-GGCCATCTCCTGCTCGAA-3′. The reaction conditions was as follows: an initial denaturation step at 95°C for 3 min, followed by 35 cycles at 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s, and a final elongation step at 72°C for 5 min. Relative levels of gene expression was expressed relative to β-actin and calculated using the 2−ΔΔCt method. The relative quantification of gene expression level was compared with the internal referee β-actin using the 2−ΔΔCt method.
Western Blot
Total protein was extracted from ovarian cancer cells using RIPA lysis buffer (Beyotime, Nantong, China) according to the manufacturer’s instructions. Protein concentration was measured by BCA kit. Total proteins of 30 µg were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Sigma-Aldrich, St. Louis, MO, USA). The membranes were treated using the following procedure with shaking and blocking at room temperature with 5% nonfat dry milk in Tris-buffered saline (TBS) for 1 h followed by incubation in the primary antibodies (anti-HPIP, anti-E-cadherin, anti-N-cadherin, anti-PI3K, anti-p-PI3K, anti-Akt, anti-p-Akt, and anti-GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. After washing with TBST buffer, the blots were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Then target proteins were detected by enhanced chemiluminescence reagents ECL.
Cell Migration and Invasion Assay
In vitro Transwell migration assay was performed in Boyden chambers with 8-µm pore filter inserts in 24-well plates (BD Biosciences, Eugene, OR, USA). Briefly, SKOV3 cells at a density of 1 × 105 cells per well were suspended in serum-free medium and loaded onto the upper compartment of the chamber, and the lower chamber contained 750 µl culture medium supplemented with 10% FBS. After incubation at 37°C for 24 h, cells on the upper surface of the microporous membrane were wiped off with a cotton swab. The cells on the lower surface of the membrane were fixed in 95% ethanol and stained with 0.1% crystal violet, then counted under a light microscope. The invasion assay was done by the same procedure, except that the filters were precoated with 100 ml Matrigel.
Statistical Analysis
Data are expressed as mean ± SD of triplicate samples. Statistical comparisons were performed using one-way analysis of variance followed by Student’s t-test. Statistical significance was declared with a value of p < 0.05.
RESULTS
HPIP Is Highly Expressed in Ovarian Cancer Cells
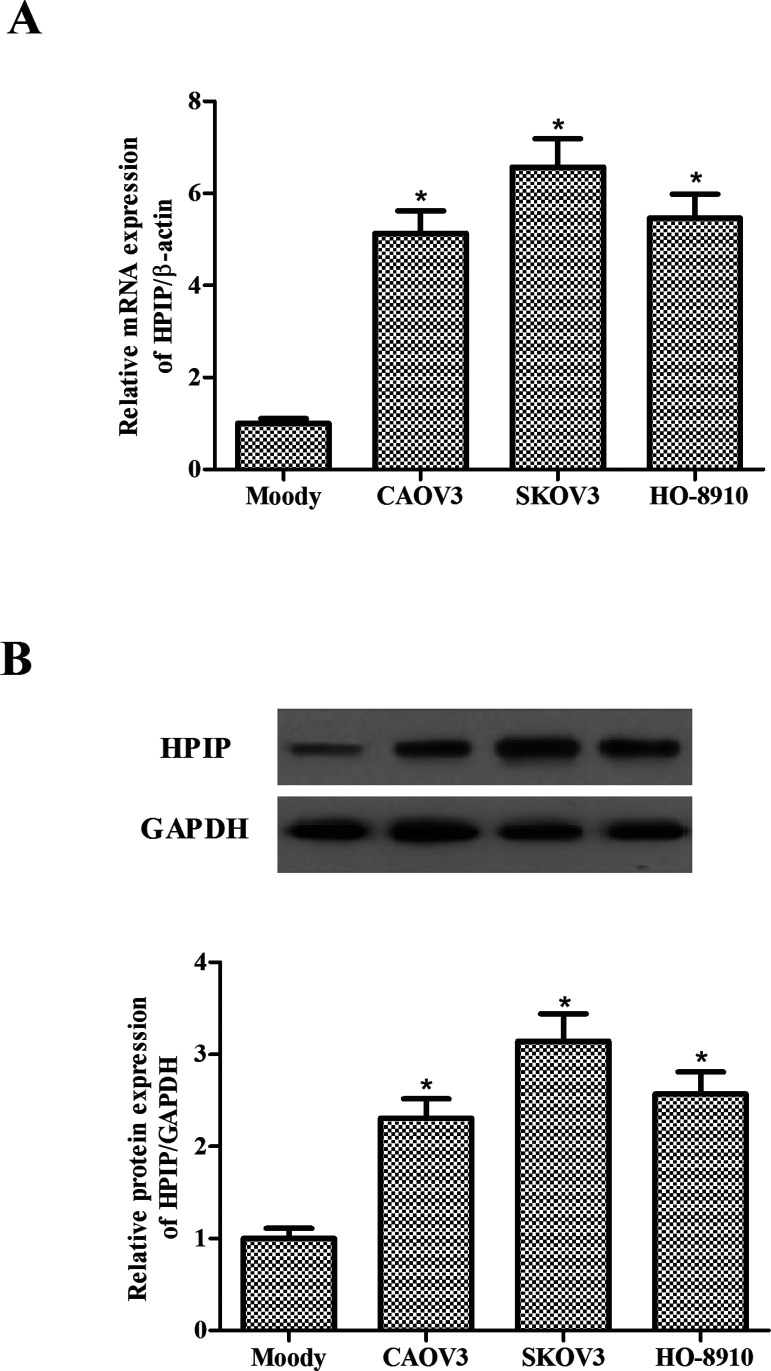
First, we detected the HPIP mRNA and protein levels in three ovarian cancer cell lines (CAOV3, SKOV3, and HO-8910) and normal epithelial ovarian cells (Moody) using RT-qPCR and Western blot, respectively. The results showed that both mRNA (Fig. 1A) and protein levels (Fig. 1B) were significantly higher in three ovarian cancer cell lines compared with normal epithelial ovarian cells, suggesting that HPIP was overexpressed in the progression of ovarian cancer.
Figure 1.
HPIP is highly expressed in ovarian cancer cells. (A) mRNA expression of HPIP was analyzed by RT-qPCR in human ovarian cancer cell lines CAOV3, SKOV3, and HO-8910. (B) Representative Western image of HPIP protein in human ovarian cancer cells. All experiments were repeated at least three times. The values shown represent the mean ± SD. *p < 0.05 compared to the Moody group.
TGF-β1 Induces HPIP Expression in Ovarian Cancer Cells
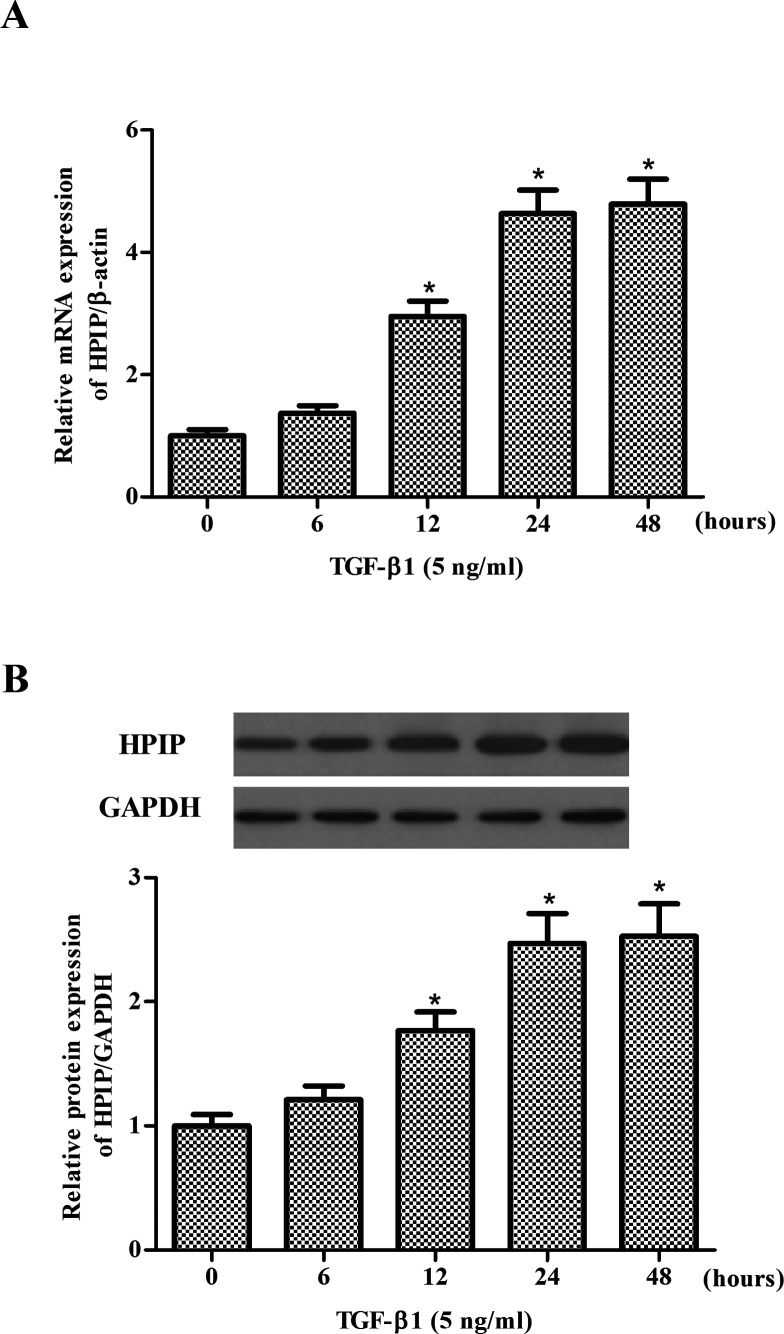
We explored the effect of TGF-β1 on the expression levels of HPIP in SKOV3 cells. The results of RT-qPCR showed that TGF-β1 treatment considerably increased the mRNA expression of HPIP in SKOV3 cells (Fig. 2A). Western blot analysis demonstrated that the expression of HPIP protein was upregulated in response to TGF-β1 in a time-dependent manner (Fig. 2B).
Figure 2.
TGF-β1 induces HPIP expression in ovarian cancer cells. (A) SKOV3 cells were treated with TGF-β1 (5 ng/ml) for 6, 12, 24, and 48 h. The mRNA (A) and protein (B) levels of HPIP were determined by RT-qPCR and Western blot, respectively. All experiments were repeated at least three times. The values shown represent the mean ± SD. *p < 0.05 compared to the control group.
Knockdown of HPIP Inhibits TGF-β1-Induced EMT in Ovarian Cancer Cells
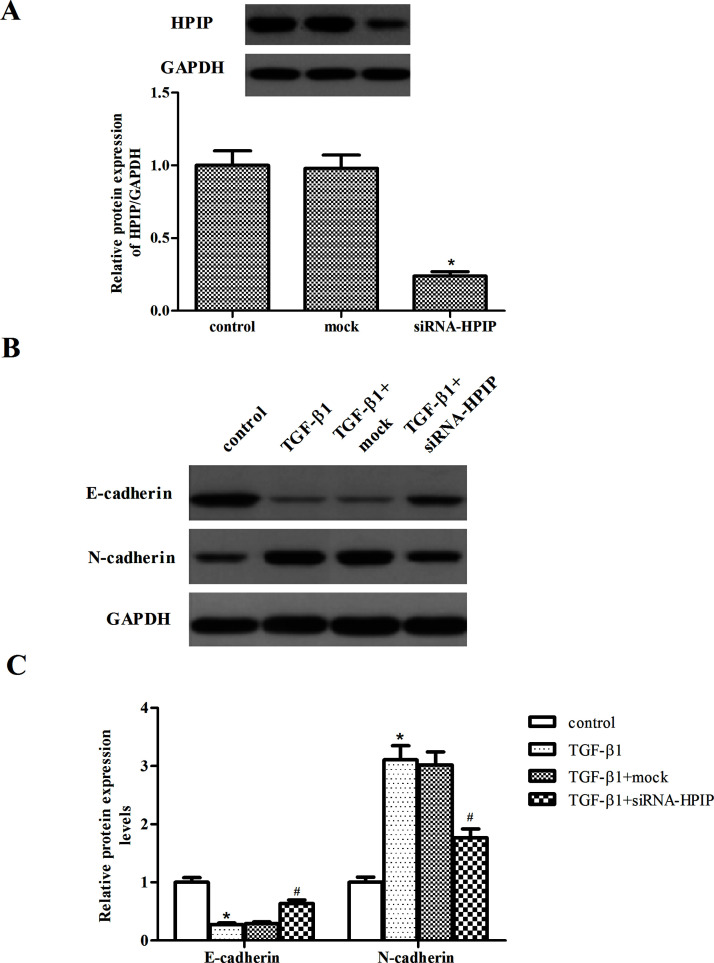
To investigate the effect of HPIP on the EMT process, we used siRNA to knockdown the expression of HPIP in SKOV3 cells. As indicated in Figure 3A, in comparison with the control and mock, the expression level of HPIP protein was significantly reduced in SKOV3 cells after siRNA-HPIP transfection. As indicated in Figure 3B, treatment with TGF-β1 induced the expression level of N-cadherin and decreased the expression of E-cadherin. However, HPIP knockdown partially restored the protein expression of E-cadherin and decreased N-cadherin by the stimulation of TGF-β1 in SKOV3 cells.
Figure 3.
Knockdown of HPIP inhibits TGF-β1-induced EMT in ovarian cancer cells. (A) SKOV3 cells were transfected with siRNA-HPIP or mock for 48 h, and the efficiency of transfection was determined by Western blot. (B) SKOV3 cells transfected with siRNA-HPIP or mock were treated with TGF-β1 for 48 h. The E-cadherin and N-cadherin expression levels were analyzed via Western blot. The relative protein expression levels of E-cadherin and N-cadherin were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. All experiments were repeated at least three times. The values shown represent the mean ± SD. *p < 0.05 compared to the control group, #p < 0.05 compared to the TGF-β1 + mock group.
Knockdown of HPIP Inhibits TGF-β1-Induced Ovarian Cancer Cell Migration and Invasion
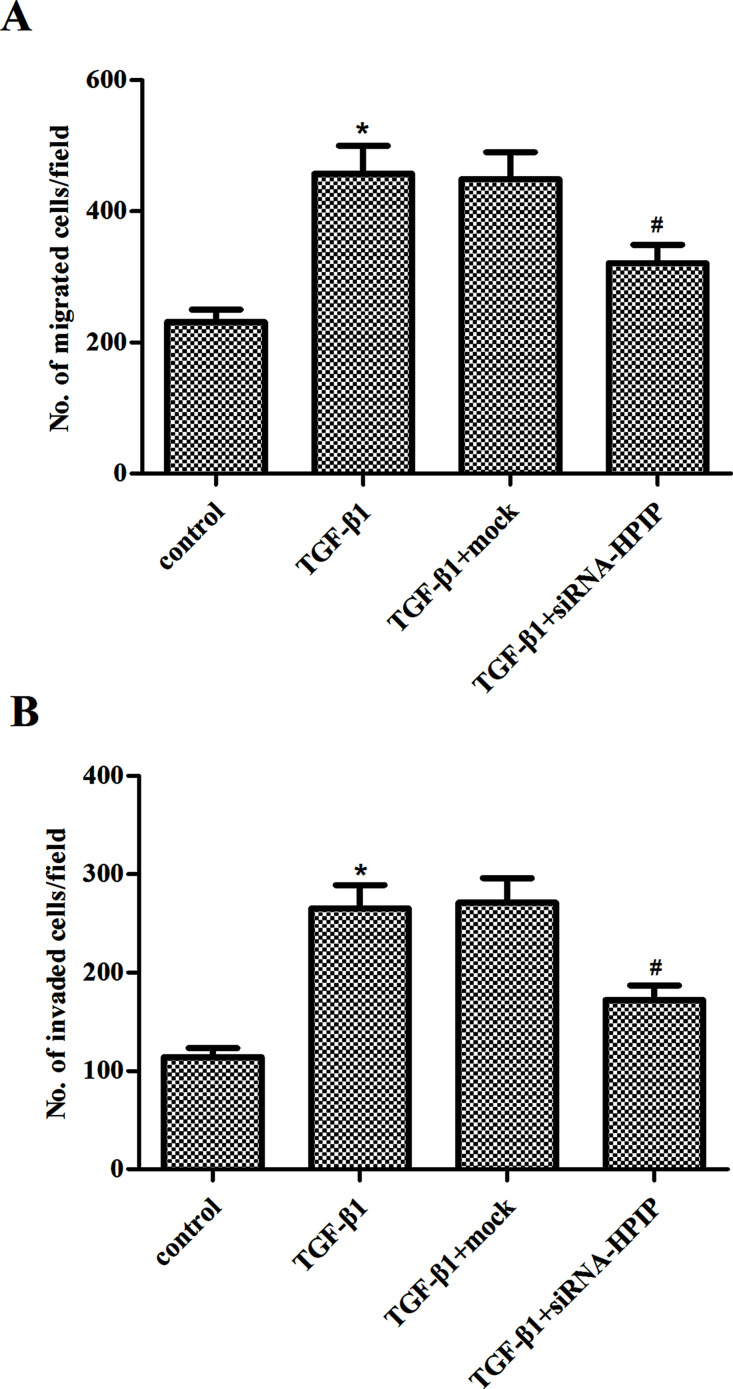
Then we investigated the effect of HPIP on migration and invasion of SKOV3 cells under TGF-β1 by Transwell migration and Matrigel invasion assays. As shown in Figure 4A, in response to TGF-β1, SKOV3 cells exhibited enhanced migratory ability. Knockdown of HPIP greatly decreased the migration of SKOV3 cells, compared to mock-transfected cells in the presence of TGF-β1. Similarly, knockdown of HPIP also prevented TGF-β1-induced invasion in SKOV3 cells (Fig. 4B).
Figure 4.
Knockdown of HPIP inhibits TGF-β1-induced ovarian cancer cell migration and invasion. SKOV3 cells transfected with siRNA-HPIP or mock were treated with TGF-β1 for 48 h. (A) Knockdown of HPIP inhibited the TGF-β1-induced migration of SKOV3 cells. (B) Knockdown of HPIP inhibited the TGF-β1-induced invasion of SKOV3 cells. All experiments were repeated at least three times. The values shown represent the mean ± SD. *p < 0.05 compared to the control group, #p < 0.05 compared to the TGF-β1 + mock group.
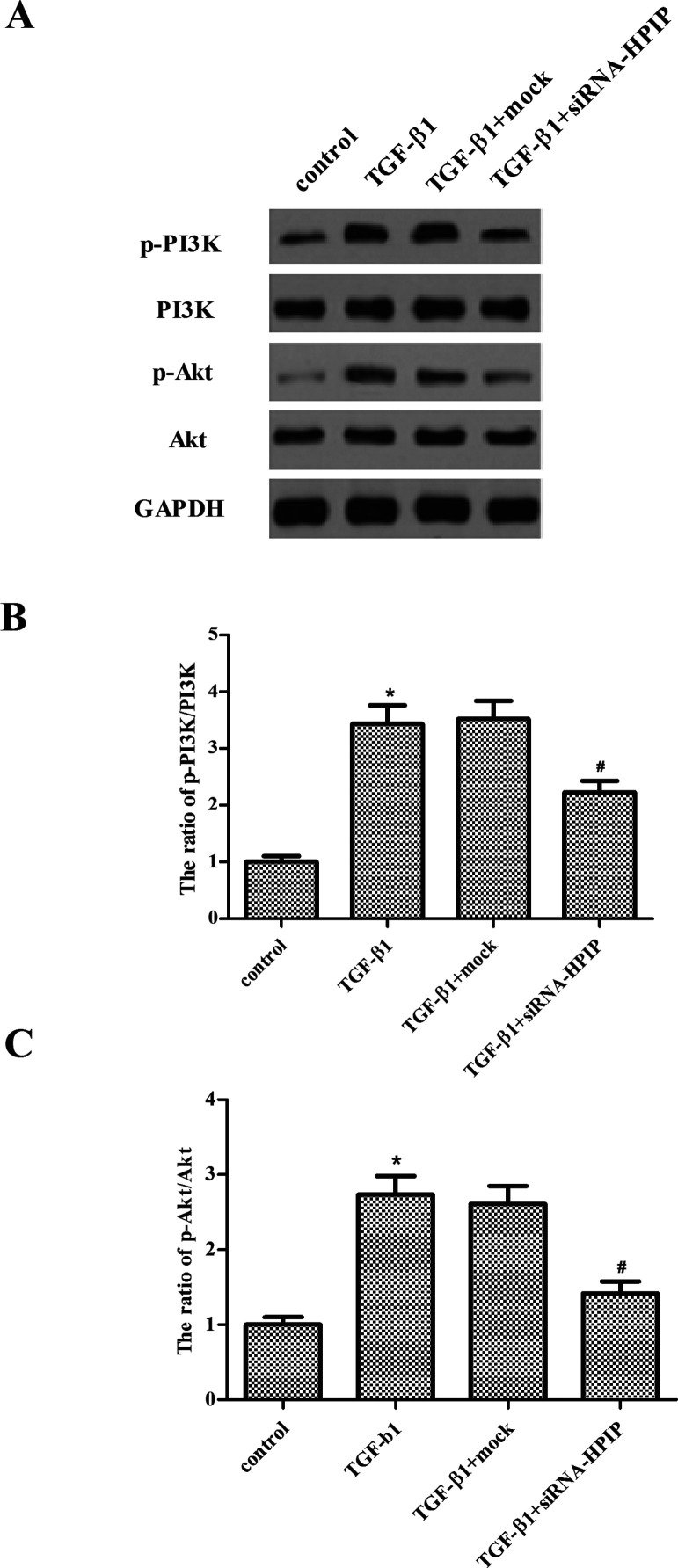
Knockdown of HPIP Suppresses TGF-β1-Induced Activation of PI3K/Akt Signaling Pathway in Ovarian Cancer Cells
To investigate the molecular mechanism for HPIP-regulated EMT process, we assessed the activation of PI3K/Akt signaling pathway in SKOV3 cells. As shown in Figure 5, the results showed an increased phosphorylated pattern of both PI3K and Akt under treatment with TGF-β1, compared with the control group. However, knockdown of HPIP significantly blocked such activation in SKOV3 cells under treatment with TGF-β1.
Figure 5.
Knockdown of HPIP suppresses TGF-β1-induced activation of PI3K/Akt signaling pathway in ovarian cancer cells. SKOV3 cells transfected with siRNA-HPIP or mock were treated with TGF-β1 for 48 h. (A) The protein levels of phosphorylated PI3K, total PI3K, phosphorylated Akt, and total Akt were measured by Western blotting. (B, C) The relative protein expression levels of p-PI3K and p-Akt were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. All experiments were repeated at least three times. The values shown represent the mean ± SD. *p < 0.05 compared to the control group, #p < 0.05 compared to the TGF-β1 + mock group.
DISCUSSION
In this study, we found that HPIP was highly expressed in ovarian cancer cells, and TGF-β1 treatment induced HPIP expression in ovarian cancer cells. In addition, knockdown of HPIP suppressed TGF-β1-induced EMT and migration/invasion in ovarian cancer cells. Moreover, knockdown of HPIP significantly blocked the phosphorylated pattern of both PI3K and Akt induced by TGF-β1 in SKOV3 cells.
Upregulation of HPIP is associated with various malignant tumors, but its role in ovarian cancer has not been reported. In this study, we found that HPIP was highly expressed in ovarian cancer cells, and TGF-β1 treatment induced HPIP expression in ovarian cancer cells. These data suggest that HPIP may act as a critical regulator in promoting EMT and ovarian cancer progression.
EMT, an important cellular process, involves a morphological change in epithelial cells, from an epithelial phenotype to a mesenchymal phenotype. During this process, epithelial cells downregulate the expression of cell adhesion molecules including E-cadherin, dissolve cell–cell junctions, lose their apical–basal polarity, and acquire migratory and invasive behavior (11). E-cadherin is an epithelial cell–cell adhesion molecule and loss of its expression is a hallmark of the EMT (12). Previous studies showed that the expression level of E-cadherin expression was reduced in various invasive tumors (13–16). It has been reported that TGF-β1 promoted tumor invasion and metastasis by inducing the EMT process, and the suppression of E-cadherin expression is an important and essential event in the induction of the EMT by TGF-β1 (17). Consistent with these results, we found that treatment with TGF-β1 obviously induced the expression of N-cadherin and decreased the expression of E-cadherin as well as the migration/invasion in SKOV3 cells. However, we found that knockdown of HPIP significantly prevented the EMT process and migration/invasion induced by TGF-β1 in SKOV3 cells. This result is also in accordance with previous reports indicating the antimetastasis potential of siRNA-HPIP in other types of cancer cells (9,18,19). These data suggest that siRNA-HPIP attenuates TGF-β1-induced increased invasion and migration via inhibiting EMT in ovarian cancer cells.
The PI3K/Akt signaling pathway plays an important role in human cancer initiation and progression (20–22). Growing evidence suggests that PI3K/Akt activation is vital to the induction of EMT in tumor cells (2,23,24). The oncogenic serine/threonine kinase Akt, a downstream effector of PI3K, is involved in many cellular processes, including cell cycle progression, cell proliferation, cell survival, metabolism, and EMT (25). Activated Akt produced a transcription factor Snail, which is known to promote EMT via the repression of E-cadherin gene (25). Interestingly, the administration of TGF-β1 activates FAK and subsequent downstream signaling transducers such as Ras, Src, or PI3K/Akt pathways (26,27). Most recently, Wang et al. reported that knockdown of HPIP inhibits the proliferation, migration, and EMT by suppressing the PI3K/Akt pathway in thyroid carcinoma cells (9). Similarly, in this study we found that knockdown of HPIP significantly blocked the phosphorylated pattern of both PI3K and Akt induced by TGF-β1 in SKOV3 cells.
In conclusion, the present study showed that HPIP silencing might prevent TGF-β1-induced EMT in ovarian cancer cells. Thus, HPIP may be a potential therapeutic target for the treatment of ovarian cancer.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Menon U.; Gentry-Maharaj A.; Jacobs I. Ovarian cancer screening and mortality. JAMA 306:1544–1545; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Lee R. Y. A.; Hong S. E.; Brose M. S.; Michels D. L. Principles of medical oncology. Asian Pac. J. Surg. Oncol. 1:39–46; 2015. [Google Scholar]
- 3. Aldea M. W. M.; Dedivitis R. A. Minimally invasive surgical oncology: State of the art. Asian Pac. J. Surg. Oncol. 2:163–172; 2016. [Google Scholar]
- 4. Winter W. E.; Maxwel G. L.; Tian C.; Carlson J. W.; Ozols R. F.; Rose P. G.; Markman M.; Armstrong D. K.; Muggia F.; McGuire W. P. Prognostic factors for stage III epithelial ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 25:3621–3627; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Huber M. A.; Kraut N.; Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558; 2005. [DOI] [PubMed] [Google Scholar]
- 6. Cao L.; Shao M.; Schilder J.; Guise T.; Mohammad K.; Matei D. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 31:2521–2534; 2012. [DOI] [PubMed] [Google Scholar]
- 7. Abramovich C.; Chavez E. A.; Lansdorp P. M.; Humphries R. K. Functional characterization of multiple domains involved in the subcellular localization of the hematopoietic Pbx interacting protein (HPIP). Oncogene. 21:6766–6771; 2002. [DOI] [PubMed] [Google Scholar]
- 8. Xu X.; Jiang C.; Wang S.; Tai Y.; Wang T.; Kang L.; Fan Z.; Li S.; Li L.; Fu J. HPIP is upregulated in liver cancer and promotes hepatoma cell proliferation via activation of G2/M transition. IUBMB Life 65:873–882; 2013. [DOI] [PubMed] [Google Scholar]
- 9. Wang S. C.; Chai D. S.; Chen C. B.; Wang Z. Y.; Wang L. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/Akt signaling pathway. Biomed. Pharmacother. 75:33–39; 2015. [DOI] [PubMed] [Google Scholar]
- 10. Feng Y.; Li L.; Zhang X.; Zhang Y.; Liang Y.; Lv J.; Fan Z.; Guo J.; Hong T.; Ji B. Hematopoietic pre-B cell leukemia transcription factor interacting protein is overexpressed in gastric cancer and promotes gastric cancer cell proliferation, migration, and invasion. Cancer Sci. 106:1313–1322; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiery J. P.; Sleeman J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7:131–142; 2006. [DOI] [PubMed] [Google Scholar]
- 12. Thiery J. P.; Acloque H.; Huang R. Y.; Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Onder T. T.; Gupta P. B.; Mani S. A.; Yang J.; Lander E. S.; Weinberg R. A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68:3645–3654; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Sawada K.; Mitra A. K.; Radjabi A. R.; Bhaskar V.; Kistner E. O.; Tretiakova M.; Jagadeeswaran S.; Montag A.; Becker A.; Kenny H. A. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 68:2329–2339; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poser I.; Domínguez D.; de Herreros A. G.; Varnai A.; Buettner R.; Bosserhoff A. K. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J. Biol. Chem. 276:24661–24666; 2001. [DOI] [PubMed] [Google Scholar]
- 16. Christofori G.; Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 24:73–76; 1999. [DOI] [PubMed] [Google Scholar]
- 17. Massagué J.; Blain S. W.; Lo R. S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 103:295–309; 2000. [DOI] [PubMed] [Google Scholar]
- 18. Pan J.; Qin Y.; Zhang M. HPIP promotes non-small cell lung cancer cell proliferation, migration and invasion through regulation of the sonic hedgehog signaling pathway. Biomed. Pharmacother. 77:176–181; 2016. [DOI] [PubMed] [Google Scholar]
- 19. Wang D.; Wang L.; Zhou Y.; Zhao X.; Xiong H. The involvement of hematopoietic pre-B cell leukemia transcription factor-interacting protein in regulating epithelial-mesenchymal transition of human spinal glioblastoma. Tumor Biol. 1–7; 2015. [DOI] [PubMed] [Google Scholar]
- 20. Altomare D. A.; Wang H. Q.; Skele K. L.; De Rienzo A.; Klein-Szanto A. J.; Godwin A. K.; Testa J. R. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 23:5853–5857; 2004. [DOI] [PubMed] [Google Scholar]
- 21. Lee S.; Choi E. J.; Jin C.; Kim D. H. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol. Oncol. 97:26–34; 2005. [DOI] [PubMed] [Google Scholar]
- 22. Meng Q.; Xia C.; Fang J.; Rojanasakul Y.; Jiang B. H. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70s6K1 pathway. Cell Signal. 18:2262–2271; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Larue L.; Bellacosa A. Epithelial–mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24:7443–7454; 2005. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y.; Zhang J.; Zhu Y.; Zhang Z.; Sun H.; Feng Y. Follicle-stimulating hormone induced epithelial-mesenchymal transition of epithelial ovarian cancer cells through follicle-stimulating hormone receptor PI3K/Akt-Snail signaling pathway. Int. J. Gynecol. Cancer 24:1564–1574; 2014. [DOI] [PubMed] [Google Scholar]
- 25. Grille S. J.; Bellacosa A.; Upson J.; Klein-Szanto A. J.; Van Roy F.; Lee-Kwon W.; Donowitz M.; Tsichlis P. N.; Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172–2178; 2003. [PubMed] [Google Scholar]
- 26. Moreno-Bueno G.; Peinado H.; Molina P.; Olmeda D.; Cubillo E.; Santos V.; Palacios J.; Portillo F.; Cano A. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nat. Protoc. 4:1591–1613; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Pechkovsky D. V.; Scaffidi A. K.; Hackett T. L.; Ballard J.; Shaheen F.; Thompson P. J.; Thannickal V. J.; Knight D. A. Transforming growth factor β1 induces αvβ3 integrin expression in human lung fibroblasts via a β3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J. Biol. Chem. 283:12898–12908; 2008. [DOI] [PubMed] [Google Scholar]