Abstract
Drug resistance is the leading cause of chemotherapy failure in the treatment of ovarian cancer. So far, little is known about the mechanism of chemoresistance in ovarian cancer. In this study, we explored the mechanism that HSP27 was involved in cisplatin resistance of ovarian cancer both in vitro and clinically. HSP27 protein was found to be upregulated and expressed in cisplatin-resistant ovarian cancer cell line C13*, and HSP27 siRNA transfection reversed the chemoresistance of C13*. We found that HSP27 exerted its chemoresistant role by inhibiting p21 transferring from the nucleus to the plasma through the activation of phosphorylated-Akt pathway. These findings have implications for clinical trials aimed at a potential therapeutic target for ovarian tumors that are refractory to conventional treatment.
Key words: Cisplatin, Ovarian cancer, HSP27, p21
INTRODUCTION
Ovarian cancer is the sixth most frequently occurring cancer among women, and it leads to the highest mortality per year than any other gynecological cancer-related deaths (1). Survival rates have changed little since the early 1980s, with only 40% of all stages and 15–30% of patients with widespread metastatic disease surviving 5 years after the initial treatment (2,3). Platinum derivatives have played an important role in chemotherapeutic agents used for the treatment of ovarian cancer for a long time. Unfortunately, platinum-resistant relapse usually occurs, and patients succumb to their disease (2,4). To date, the mechanisms by which tumor cells develop resistance to cisplatin remain unclear, and current studies showed that cisplatin resistance is a complex phenomenon. New therapeutic approaches are needed to improve the outcome of women with ovarian cancer.
Among many novel molecular targets, heat shock protein 27 (HSP27) is deemed to be fascinating for therapeutic development. HSP27 is a kind of heat shock protein whose main function is to protect against protein aggregation (5–7). HSP27 exerts its antiapoptotic function in many ways, like binding and activating the antiapoptotic mediator protein kinase B/Akt (8,9). Overexpression of HSP27 was reported to dramatically enhance the tumorigenicity of cancer cells and induce chemotherapy resistance (5,6,8,10,11). However, the mechanism of HSP27-induced cisplatin resistance in ovarian cancer remains unknown.
Our study, along with previous studies, demonstrates that cytoplasmic p21 is a novel biomarker of cisplatin resistance, and it may represent a potential therapeutic target for ovarian tumors that are refractory to conventional treatment (12–14). In this article, we illustrate that HSP27 overexpression changed p21 cytosolic localization in an AKT-dependent manner and induced cisplatin resistance of ovarian cancer cells. Moreover, we revealed that HSP27 inhibition could restore the chemosensitivity of cisplatin-resistant cells to cisplatin and increase cytoplasmic p21 accumulation, which could be used as a novel therapeutic target to overcome cisplatin resistance in ovarian cancer.
MATERIALS AND METHODS
Reagents and Antibodies
Cisplatin (DDP) was purchased from Sigma-Aldrich (USA). RPMI-1640, fetal bovine serum (FBS), Lipofectamine™ 2000, and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA). The PI3K inhibitor (LY-294002) was purchased from Cell Signaling Technology (USA). HSP27 siRNA and matched negative control (NC) were synthesized by RiboBio Co. Ltd (Guangzhou, China). Antibodies to PARP, c-PARP, p-AKT, AKT, GAPDH, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies of p21, HSP27, and anti-cleaved caspase 3 were purchased from Cell Signaling Technology.
Two-Dimensional Gel Electrophoresis (2-DE)
2-DE was performed as previously described (15) and dealt with the samples according to isoelectric focusing on instrument operation guide. Specifically, 300-mg quantities of protein were diluted in the sample buffer with 2% IPG buffer up to 180 µl. Samples were added to pH 4–7, 11-cm IPG strips (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Rehydration and isoelectric focusing was carried out for 16 h at 20°C. The total voltage applied was 52,000–69,000 Vh (IPGphore Isoelectric Focusing Unit; Amersham Pharmacia Biotech). The IPG strips were equilibrated in buffer containing 6 mol/L urea, 30% glycerol, 1.5 mol/L Tris-base, pH 8.8, 2.5% acrylamide, and tributyl phosphine (Fluka, Buchs SG, Switzerland). After equilibration, SDS-PAGE was performed by 8–18% gradient gel without stacking gel via the Ettan Dalt system (Amersham Pharmacia Biotech). 2-DE was carried out overnight at 4 W per gel at 20°C. After finishing 2-DE, the proteins on gels were fixed with protein-fixing solution (40% methanol, 5% phosphoric acid) for 2 h and then stained using Coomassie G-250 (Bio-Rad, Hercules, CA, USA) for 3 h. GS710 2-DE gel scanner was used to scan the gel, and PDQuest software was used to analyze the digital image file.
Western Blot Analysis and Protein Extraction
Western blot was done as described previously (16). Cells were removed by trypsinization and centrifuged at 12,000 rpm at 4°C for 30 min to extract the supernatant. The protein concentration was measured by spectrophotometry using Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) based on the Bradford method. Antibody was detected with HRP-conjugated secondary antibody and developed with an enhanced chemiluminescence detection kit (Chemical Doc™ XRS+). Densitometric analysis using ImageLab™ software (NIH) was performed to interpret the difference in the results of Western blots.
Cell Viability Assay Using CCK-8
Cells were seeded into 96-well plates (1 × 104 cells/well) directly and allowed to attach overnight. Freshly prepared cisplatin was then added with different final concentrations. Forty-eight hours later, the IC50 (the concentration that inhibited growth by 50%) value was determined by assessing cell viability, which was measured using a Cell Counting Kit (CCK-8; Key Bio Tech, China). Cell viability was determined by measuring the optical absorbance of cells at 450 nm wavelength and normalizing the values to the corresponding controls.
Cell Lines and Cell Culture
Cisplatin-sensitive ovarian cancer cell line (OV2008), and its resistant variant (C13*) were gifts from Professor Benjamin K. Tsang in the Ottawa Health Research Institute, Ottawa, Canada, and the cells were cultured in Macoy’s 5A medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco) at 37°C in a humidified atmosphere of 5% CO2.
Immunohistochemical Staining and Analysis
Immunohistochemical (IHC) staining was performed according to standard procedures. Sections were stained with primary antibody HSP27 (dilution 1:50) from Cell Signaling Technology overnight at 4°C. The results for HSP27 staining were scored on the basis of the percentage of positively stained cells (score 0, no positive expression, score 1, ≤10% positive expression; score 2, 11–25% positive expression; score 3, 26–50% positive expression; and score 4, ≥51% positive expression). For further statistical analysis, scores 0 and 1 were categorized as low expression, and scores 2, 3, and 4 were categorized as high expression, as described before. The staining was determined separately by two independent experts simultaneously under the same conditions. In rare cases, discordant scores were reevaluated and scored on the basis of consensual opinion.
Analysis of Apoptosis by Flow Cytometry
Cells were harvested, washed with PBS, and stained with the Annexin-V/PI apoptosis kit according to manufacturer’s instructions. Analysis of apoptotic cells was performed using a FACS flow cytometer, and the data were analyzed using CellFIT software.
Transient Transfection for RNAi Targeting
For RNAi targeting, C13* cells cultured in six-well plates were transfected using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s protocol. After 4 h of incubation, the transfection solution was removed and replaced with fresh complete growth medium. Forty-eight hours posttransfection, the cells were assayed for the expression of HSP27 and treated with cisplatin for further experimentation.
Analysis of p21 Using Immunofluorescence Microscopy
Cells were grown on coverslips. After fixation for 30 min with 4% paraformaldehyde in PBS, they were permeabilized for 10 min with 1% Triton X-100. Cells were then blocked with 5% bovine serum albumin in PBS containing 0.1% Triton X-100 for 1 h at room temperature. Next, primary monoclonal p21 (1:100) antibody was prepared in PBS containing 0.1% Tween 20 buffer and incubated on the coverslips for 1 h at room temperature. After washing with PBS, fluorescent Texas red tag-conjugated goat anti-mouse IgG (1:200) secondary antibody was added, and the slides were incubated at room temperature for 1 h. Slides were further stained for nuclei with 4′,6-diamidino-2-phenylindole (DAPI: a fluorescent DNA stain to examine nuclear morphology). Images were acquired at room temperature with a Nikon fluorescence microscope E80i (Nikon) fitted with filters for Texas red and DAPI and processed using SPOT software (Diagnostic Instruments).
Statistical Analysis
Each experiment was repeated three times. The relationship between patients’ clinical characteristics and the results of HSP27 immunohistochemistry were assessed using the chi-squared test. HSP27 expression values were first analyzed as continuous variables, and Spearman rank correlation was used to determine the correlation between HSP27 expression and IC50 of cisplatin in ovarian cancer cells. Mann–Whitney U test was used to determine the differences in HSP27 expression between platinum-sensitive and platinum-resistant groups. Differences in progression-free survival (PFS) between different groups were examined with Kaplan–Meier curves with the log-rank test and Cox proportional hazards analysis. A two-sided value of p < 0.05 was considered statistically significant. All statistical analyses were done using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Two-Dimensional Gel Electrophoresis (2-DE) Analysis Between Cisplatin-Sensitive and Derived Resistant Ovarian Cancer Cells
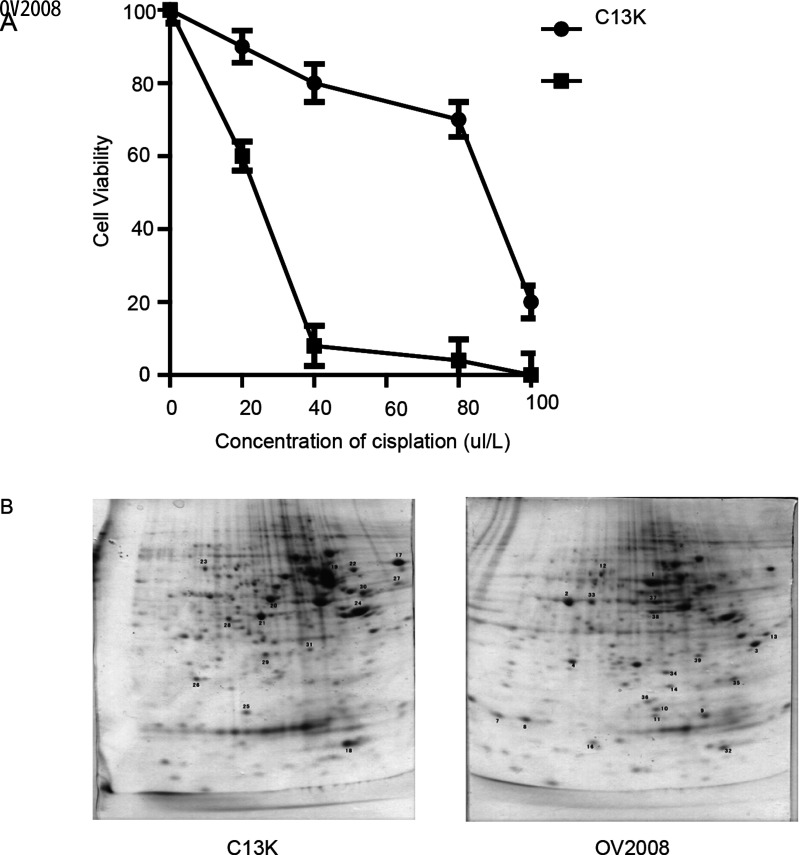
OV2008 and C13* ovarian cancer cells are one pair of cisplatin-sensitive and derived resistant human ovarian cancer cell lines (4). First, we tested the toxic effect of cisplatin on these paired cells using CCK-8 assay in vitro. Cells were exposed to 0, 20, 40, 80, 100 µmol/L cisplatin for 48 h. The results confirm that C13* is statistically significantly resistant to cisplatin compared to OV2008 (OV2008: mean IC50 = 22.11 µmol/L, 95% CI = 21.36 to 22.89 µmol/L; C13K: mean IC50 = 81.94 µmol/L, 95% CI = 63.38 to 105.9 µmol/L) (Fig. 1A). 2-DE in conjunction with mass spectrometry (MS) is commonly used to identify differential proteins of the interest sample, so we used this method to explore potential differentially expressed protein between OV2008 and C13*cells (Fig. 1B). The top five differentially expressed protein spots were separated for protein identification by mass spectrum analysis. The results demonstrated that HSP27 had more differentially expressed protein than any of the other proteins: TCTP-1, Oncoprotein-18, Erp-27, and Eif-5a. We thus hypothesized that the results from this trial show that chemotherapy resistance may be due to the antiapoptotic function of HSP27.
Figure 1.
Cisplatin-induced cytotoxicity and apoptosis in resistant (C13*) and sensitive cell lines (OV2008) and 2-DE gel electrophoresis results. (A) Cells were cultured in the presence of the indicated concentrations of cisplatin for 48 h. Cell viability in response to treatment with escalating doses of cisplatin was assessed by CCK-8. (B) Representative image of 2-DE urea gel electrophoresis of human ovarian cancer cells. Proteins were detected by Coomassie blue staining on the 2-DE images of the master gel.
The Correlation of HSP27 Expression and Cisplatin Sensitivity in Ovarian Cancer Cell Lines
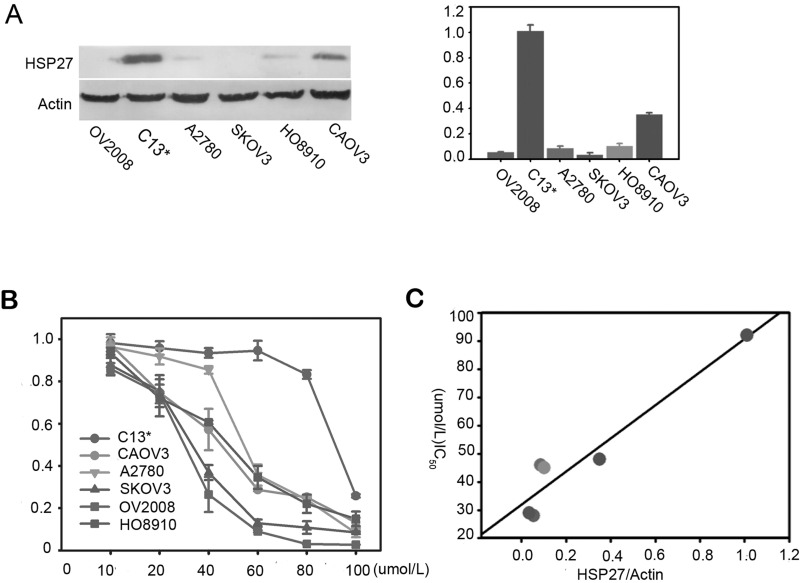
To determine whether the chemotherapy resistance of ovarian cancer cell lines may relate to the HSP27 expression, we investigated this correlation in a panel of ovarian cancer cells (OV2008, C13*, 2780, SKOV3, HO8910, and CaOV3). The relative densities of the Western blot bands were calculated, and the values are shown in Figure 2A. C13* expressed the highest level of HSP27, while OV2008, SKOV3, and A2780 cells expressed relatively lower levels. When the ovarian cancer cells were exposed to different doses of cisplatin for 48 h, as shown in Figure 2B, cisplatin decreased the viability of cells in a dose-dependent manner. The HSP27-deficient cell lines OV2008, SKOV3, and A2780 were significantly more sensitive to cDDP than HSP27-proficient C13* cells. Indeed, Pearson correlation analysis showed a significantly positive correlation of HSP27 protein level and IC50 of ovarian cancer cell lines, R 2 = 0.957 (p = 0.032) (Fig. 2C).
Figure 2.
The correlation of HSP27 expression and DDP sensitivity ovarian cancer cell line. (A) Expression of HSP27 protein in human ovarian cancer cell lines as depicted by Western blot analysis. β-Actin serves as a loading control. Right panel is the respective relative expression of HSP27 protein in ovarian cancer cell lines. Data are mean ± SEM. β-Actin was a normalization control. (B) Cell viability was assayed after treatment with increasing concentrations of cisplatin (cDDP) for 48 h by CCK-8. Data are shown as the mean ± 95% confidence interval from three independent experiments. (C) The IC50 analysis in these six ovarian cancer cell lines showed an inverse trend between cisplatin sensitivity and HSP27 expression. Data are means of three separated experiments ± SD.
Expression of HSP27 Is Associated With Cisplatin Response in Clinical Samples
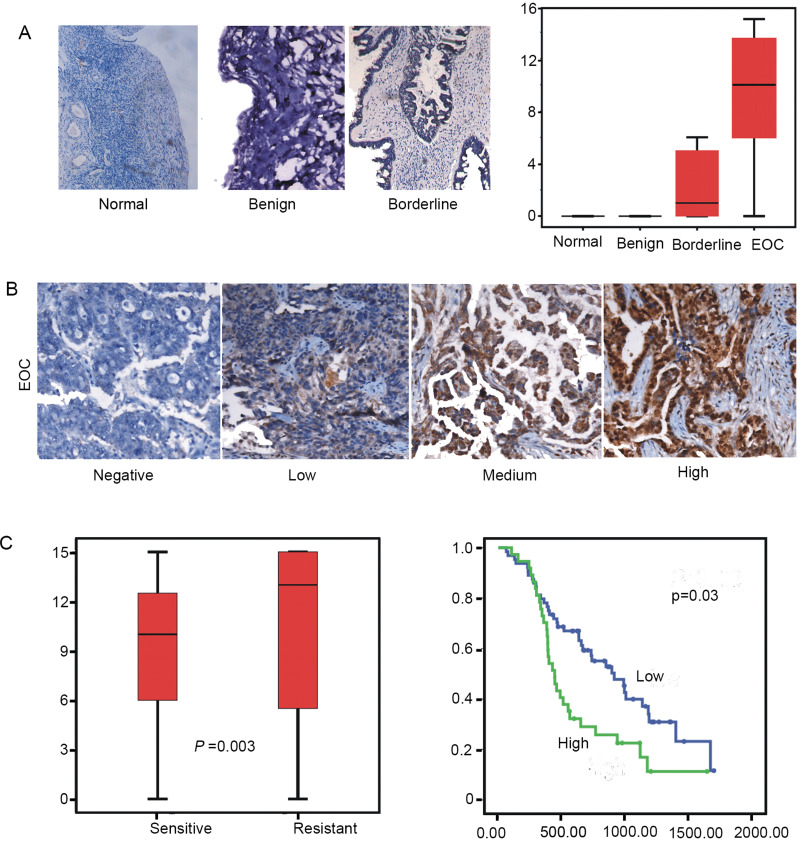
To better extrapolate the biological significance of HSP27 to clinical specimens, we detected the expression level of HSP27 by immunohistochemical analysis in normal benign tissue, borderline tumor, and epithelial ovarian cancer (EOC). We found that the level of HSP27 protein was significantly higher in EOC. In addition, our analyses showed that the level of HSP27 in EOC significantly correlated with a later stage of disease and grade of poorer differentiation (Fig. 3A, B).
Figure 3.
Expression of HSP27 is associated with cisplatin response in clinical samples (A, B) Immunohistochemical analysis of sections from paraffin-embedded blocks of tissues. Sections were stained with antibodies directed against HSP27 in normal and benign tissue or borderline tumor and (EOC). Anti-HSP27 antibody for immunohistochemical (IHC) analysis with scrambled probe and phosphate-buffered saline as a negative control, respectively. Representative photographs are shown. The differences of HSP27 expression between chemotherapy-sensitive and -resistant patients in ovarian cancer specimens is shown (p = 0.03, Mann–Whitney U test). (C) A Kaplan–Meier analysis of progression-free survival (PFS) for ovarian cancer patients with the corresponding expression profiles of HSP27 is shown (p = 0.03 and p = 0.01, respectively, log-rank test). All statistical tests were two sided.
We further explored the influence of expression protein of HSP27 on the cisplatin resistance of patients with EOC. According to high-grade ovarian serious carcinoma samples by immunohistochemical analysis, expression level of HSP27 protein was associated with platinum resistance or shorter progression-free survival (PFS) (p = 0.003). The results of these data indicated that expression of HSP27 may be associated with platinum resistance and poor prognosis of ovarian cancer patients (Fig. 3C, D). Collectively, these data support the hypothesis that high levels of HSP27 have a causal role in platinum resistance in EOC.
Knockdown of HSP27 Restores the Sensitivity to Cisplatin in C13* In Vitro
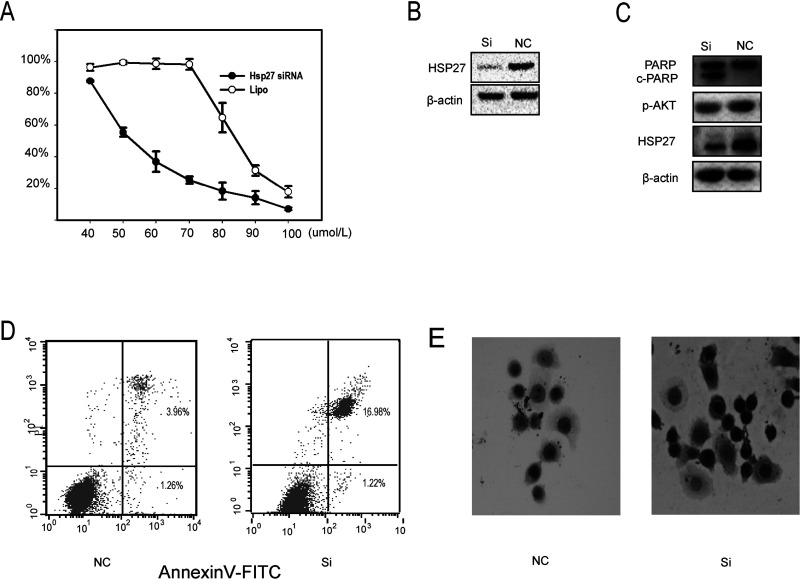
It has been demonstrated that HSP27 has the function of protecting cells from apoptosis and acts as a mediator of resistance to IR-induced apoptosis. To substantiate whether HSP27 deletion can reverse cisplatin resistance, small interference RNA (siRNA) assay was applied to suppress HSP27 in C13* ovarian cancer cells. After transfection, these cells were exposed to different concentrations of cisplatin for 48 h. CCK-8 assay revealed that HSP27 siRNA reversed cisplatin resistance in C13* ovarian cancer cells, and HSP27 siRNA transfection exhibited a dramatic decreased expression of HSP27 protein compared with a negative control group by Western blot (Fig. 4A, B). Moreover, downregulation of HSP27 expression in C13* cells with HSP27 siRNA resulted in enhanced PARP cleavage and lower phosphorylated p-AKT compared with control groups by Western blotting (Fig. 4C). In addition, flow cytometric analysis of C13* cells exposed to 80 µmol/L cisplatin for 48 h demonstrated that C13* cells transfected with HSP27 siRNA increased apoptosis rate than that of the nontransfected control group (16.98% ± 1.22% vs. 3.96% ± 1.26%) (p < 0.05) (Fig. 4D). In the meantime, C13* cells transfected with HSP27 siRNA and negative control group (NC) were stained with caspase 3, which is an important molecule in the cellular suicide cascade. Compared with NC (Fig. 4E), cell apoptosis rate in cells treated with HSP27 siRNA was significantly higher. Collectively, these data demonstrate that inhibition of HSP27 restores the sensitivity of resistant ovarian cells to cisplatin.
Figure 4.
Knockdown of HSP27 restores the sensitivity to cisplatin in C13* in vitro. (A) C13* cells were transfected with HSP27 small interfering RNA for 48 h. C13* cells were negative control (NC). Confirmation of HSP27 protein knockdown by Western blot (upper panel). Cell viability was assayed after treatment with increasing concentrations of cDDP for 48 h by CCK-8. Data are shown as the mean ± 95% confidence interval from two independent experiments. (B) Total protein was isolated 48 h posttransfection, and PARP, c-PARP, and p-AKT, HSP27 expression was analyzed by Western blotting. (C) The average percentage of apoptosis in the transfected cells was 10.87% for C13*, which was significantly greater than that found in the nontransfected multidrug-resistant controls 3.75%. (D) Expression of caspase 3 in si-HSP27 and negative control.
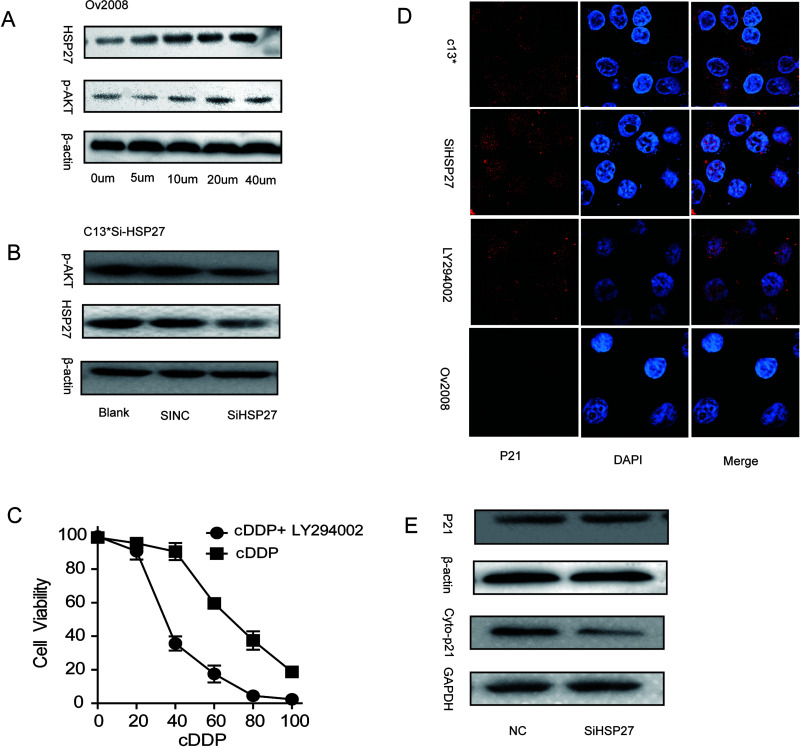
HSP27 Regulates Cytoplasmic p21 by Phosphatidylinositol 3-Kinase (PI3K)/Akt Signaling
Our laboratory has previously reported that control of the subcellular localization of p21 could represent an important regulatory switch from a nuclear tumor suppressor to a cytoplasmic oncogene phosphatidylinositol 3-kinase (PI3K)/Akt signaling (1). Moreover, recent articles showed that the interaction of HSP27 and Akt is necessary for Akt activation (9,17,18). Based on the above results, we intended to detect whether HSP27 regulated cytoplasm p21 via the phosphorylated AKT pathway in human ovarian cancer cells. First, the cisplatin-sensitive EOC cell line (OV2008) was treated with an increasing concentration of cisplatin for 48 h. Western blot demonstrated an increased expression level of HSP27 and p-AKT in a concentration-dependent manner (Fig. 5A). In addition, the increased trend of HSP27 was significantly positive when correlated with p-AKT levels. Furthermore, downregulation of HSP27 expression by siRNA in the cisplatin-resistant C13* cell line decreased p-AKT expression compared with control cells (Fig. 5B). PI3K inhibition with LY294002 sensitized C13* ovarian cancer cells to cisplatin (Fig. 5C). Additionally, the subcellular localization of p21 was investigated with immunofluorescence microscopy. In cells treated with HSP27 siRNA or LY-294002 for 48 h, p21 was localized in the nucleus, suggesting that the absence of HSP27 significantly promoted the translocation of p21 into the nucleus by PI3K/Akt pathway (Fig. 5D). In the meantime, total and cytoplasmic protein was extracted from the cells and analyzed by Western blot. In contrast to control cells, the cytoplasmic protein levels of p21 and total protein levels of p21 were significantly decreased in HSP27-siRNA-transfected cells (Fig. 5E). Therefore, these data show that HSP27 protein regulates the accumulation of p21 in the cytoplasm by activation of p-Akt and impairs the response of sensitive ovarian cells to cisplatin.
Figure 5.
HSP27 mediates drug resistance via phosphorylated-AKT pathway and induces p21 translocation into the cytoplasm. (A) Western blot analysis of HSP27, and p-AKT protein expression in response to cDDP (0–40 µm) in OV2008 cells. (B) Western blot analysis was used to detect the phosphorylation of AKT and HSP27 in HSP27 siRNA-treated C13* cells. (C) C13* cells were treated with LY294002 or negative control for 24 h. Cell viability was assayed after treatment with increasing concentrations of cDDP for 48 h by CCK-8. (D) Representative image of localization of p21 C13*, OV2008 and LY294002 (LY), HSP27-siRNA-treated C13* cells. (E) HSP27 suppression abolishes p21 expression and leads to downregulation of Cyto-p21compared with control. Data are represented as mean ± SD.
DISCUSSION
When platinum-resistant relapse occurs, patients usually succumb to ovarian cancer (2,4). To date, the mechanisms by which tumor cells develop resistance to cisplatin remain unclear, and current studies show that cisplatin resistance is a complex phenomenon. In proteomics, 2-DE in conjunction with mass spectrometry (MS) is a powerful tool, and its function is a way to quantify protein spots and will be crucial for the identification of molecular markers. In this study, we performed, to our knowledge for the first time, high throughput of 2-DE gel electrophoresis followed by matrix-assisted laser desorption/time of flight mass spectrometry (MALDI TOF-MS) to screen protein profiles in paired cisplatin-sensitive and -resistant ovarian cancer cell lines. We found that HSP27 was one of the most differently expressed proteins between C13* and its parental variant OV2008. By immunohistochemical analysis, we found that malignant tumors showed significantly higher levels of HSP27 than benign tumors and borderline tumors. Advanced-stage epithelial ovarian cancer (EOC stages II–IV) has a higher level of expression of HSP27 protein in the tissues.
Recently, several investigators showed that cancer cells with HSP27 overexpression have been associated with chemotherapeutic resistance (19–21) and inhibition of apoptosis in human bladder cancer cells (10). HSP27 is involved in the regulation of the serine/threonine kinase AKT (protein kinase B), an important signaling molecule for cell survival and proliferation of downstream growth factor stimulation (22), and degradation of certain client proteins. In agreement with these results, we found that, compared to those that were either static or cleanly responsive, HSP27 expression was significantly higher in tumors that had progressed on therapy. Collectively, these findings suggest that HSP27 knockdown with HSP27 siRNA combined therapy with cisplatin could be a novel strategy to inhibit the progression of ovarian cancer in the future. Moreover, overexpression of HSP27 in C13* cells accelerates cell growth and increases resistance against cisplatin, whereas HSP27 knockdown by HSP27 siRNA in vitro enhances the induction of apoptosis and chemosensitizes cells to cisplatin. These data indicate that HSP27 has both biological and clinical significance in the cisplatin resistance of ovarian cancer. Downregulation of HSP27 resulted in the sensitization of ovarian cancer cells to cisplatin, which hints at a highly significant relevance of HSP27 in cancer chemotherapy.
Furthermore, HSP27 is involved in the regulation of the serine/threonine kinase Akt(5), an important signaling molecule for cell survival. Our studies previously demonstrated that p21 is phosphorylated by Akt, which leads to increased p21 translocation into the cytoplasm. Previous studies demonstrated that p21 could act as a “tumor suppressor” by binding to cellular cyclin-dependent kinases (CDK) and proliferating cell nuclear antigen (PCNA), thereby inhibiting their function, leading to cell cycle arrest, blockade of DNA synthesis, and inhibition of cell proliferation (1,12,14). Even more, our studies demonstrated that p21 is phosphorylated by Akt, which leads to increased p21 stability as well as enhanced cell survival. AKT inactivity plays an essential role in apoptosis, and the stabilization of cytoplasmic p21 was found to be involved in this process. Akt regulates the balance of apoptosis and cell survival by phosphorylating proteins crucial in apoptotic and antiapoptotic mechanisms. We demonstrated here that localization of p21 in the cytoplasm was critical for cisplatin resistance, since relocalization of p21 to the nucleus by regulation of p-AKT through HSP27 inhibition sensitized EOC cell lines to cisplatin.
In summary, our in vitro experiments demonstrate that HSP27 is an effective biomarker in cisplatin resistance, and HSP27 inhibition can reverse the chemoresistance of cells to cisplatin in ovarian cancer. New therapeutic approaches are needed to improve the outcome of women with ovarian cancer, and according to our results, the neutralization of HSP27 is an attractive strategy for anticancer therapy. However, no small molecules are effectively available that selectively inhibit HSP27 so far in the clinic. Therefore, more research is needed to provide the potential to extend well-tolerated HSP27 inhibition to the treatment of HSP27-proficient cancers.
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (973 Program, 2015CB553903), the National High Technology Research and National Development Program of China (863 Program, 2012AA02A507), and National Nature and Science Foundation of China (81272859; 81372801; 81230038; 81501530).
REFERENCES
- 1. Xia X.; Ma Q.; Li X.; Ji T.; Chen P.; Xu H.; Li K.; Fang Y.; Weng D.; Weng Y.; Liao S.; Han Z.; Liu R.; Zhu T.; Wang S.; Xu G.; Meng L.; Zhou J.; Ma D. Cytoplasmic p21 is a potential predictor for cisplatin sensitivity in ovarian cancer. BMC Cancer 11:399; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaughan S.; Coward J. I.; Bast R. C.; Berchuck A.; Berek J. S.; Brenton J. D.; Coukos G.; Crum C. C.; Drapkin R.; Etemadmoghadam D.; Friedlander M.; Gabra H.; Kaye S. B.; Lord C. J.; Lengyel E.; Levine D. A.; Mcneish I. A.; Menon U.; Mills G. B.; Nephew K. P.; Oza A. M.; Sood A. K.; Stronach E. A.; Walczak H.; Bowtell D. D.; Balkwill F. R. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 11:719–725; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacob F.; Nixdorf S.; Hacker N. F.; Heinzelmann-Schwarz V. A. Reliable in vitro studies require appropriate ovarian cancer cell lines. J. Ovarian Res. 7:60; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun C.; Li N.; Yang Z.; Zhou B.; He Y.; Weng D.; Fang Y.; Wu P.; Chen P.; Yang X.; Ma D.; Zhou J.; Chen G. Mir-9 regulation of brca1 and ovarian cancer sensitivity to cisplatin and parp inhibition. J. Natl. Cancer Inst. 105:1750–1758; 2013. [DOI] [PubMed] [Google Scholar]
- 5. Hadchity E.; Aloy M. T.; Paulin C.; Armandy E.; Watkin E.; Rousson R.; Gleave M.; Chapet O.; Rodriguez-Lafrasse C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol. Ther. 17:1387–1394; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langdon S. P.; Rabiasz G. J.; Hirst G. L.; King R. J. B.; Hawkins R. A.; Smyth J. F.; Miller W. R. Expression of the heat shock protein hsp27 in human ovarian cancer. Clin. Cancer Res. 1:1603–1609; 1995. [PubMed] [Google Scholar]
- 7. Kim E. H.; Lee H. J.; Lee D. H.; Bae S.; Soh J. W.; Jeoung D.; Kim J.; Cho C. K.; Lee Y. J.; Lee Y. S. Inhibition of heat shock iprotein 27-mediated resistance to DNA damaging agents by a novel pkc delta-v5 heptapeptide. Cancer Res. 67:6333–6341; 2007. [DOI] [PubMed] [Google Scholar]
- 8. Kang S. H.; Kang K. W.; Kim K. H.; Kwon B.; Kim S. K.; Lee H. Y.; Kong S. Y.; Lee E. S.; Jang S. G.; Yoo B. C. Upregulated hsp27 in human breast cancer cells reduces herceptin susceptibility by increasing her2 protein stability. BMC Cancer 8:286; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanagasabai R.; Karthikeyan K.; Vedam K.; Qien W.; Zhu Q.; Ilangovan G. Hsp27 protects adenocarcinoma cells from uv-induced apoptosis by akt and p21-dependent pathways of survival. Mol. Cancer Res. 8:1399–1412; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamada M.; So A.; Muramaki M.; Rocchi P.; Beraldi E.; Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 6:299–308; 2007. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka Y.; Fujiwara K.; Tanaka H.; Maehata K.; Kohno I. Paclitaxel inhibits expression of heat shock protein 27 in ovarian and uterine cancer cells. Int. J. Gynecol. Cancer 14:616–620; 2004. [DOI] [PubMed] [Google Scholar]
- 12. Lin P. Y.; Fosmire S. P.; Park S. H.; Park J. Y.; Baksh S.; Modiano J. F.; Weiss R. H. Attenuation of pten increases p21 stability and cytosolic localization in kidney cancer cells: A potential mechanism of apoptosis resistance. Nat. Cell Biol. 3:245–252; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koster R.; Pietro A.; Timmer-Bosscha H.; Gibcus J. H.; Berg A.; Suurmeijer A. J.; Bischoff R.; Gietema J. A.; Jong S. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J. Clin. Invest. 120:3594–3605; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou B. P.; Liao Y.; Xia W.; Spohn B.; Lee M. C.; Hung M. C. Cytoplasmic localization of p21cip1/waf1 by akt-induced phosphorylation in her-2/neu-overexpressing cells. Nat. Cell Biol. 3:245–252; 2001. [DOI] [PubMed] [Google Scholar]
- 15. Gorg A.; Obermaier C.; Boguth G.; Harder A.; Scheibe B.; Wildgruber R.; Weiss W. The current state of two-dimensional electrophoresis with immobilized ph gradients. Electrophoresis 21(6):1037–1053; 2000. [DOI] [PubMed] [Google Scholar]
- 16. Liu Z. Q.; Mahmood T.; Yang P. C. Western blot: Technique, theory and trouble shooting. N. Am. J. Med. Sci. 6:160; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konishi H.; Matsuzaki H.; Tanaka M.; Takemura Y.; Kuroda S.; Ono Y.; Kikkawa U. Activation of protein kinase b (akt/rac-protein kinase) by cellular stress and its association with heat shock protein hsp27. FEBS Lett. 410:493–498; 1997. [DOI] [PubMed] [Google Scholar]
- 18. Vivanco I.; Sawyers C. L. The phosphatidylinositol3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2:489–501; 2002. [DOI] [PubMed] [Google Scholar]
- 19. Ciocca D. R.; Calderwood S. K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10:86–103; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berrieman H. K.; Cawkwell L.; O’kane S. L.; Smith L.; Lind M. J. Hsp27 may allow prediction of the response to single-agent vinorelbine chemotherapy in non-small cell lung cancer. Oncol. Rep. 15:283–286; 2006. [PubMed] [Google Scholar]
- 21. Vargas-Roig L. M.; Gago F. E.; Tello O.; Aznar J. C.; Ciocca D. R. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int. J. Cancer 79:468–475; 1998. [DOI] [PubMed] [Google Scholar]
- 22. Rane M. J.; Pan Y.; Singh S.; Powell D. W.; Wu R.; Cummins T.; Chen Q.; Mcleish K. R.; Klein J. B. Heat shock protein 27 controls apoptosis by regulating akt activation. J. Biol. Chem. 278:27828–27835; 2003. [DOI] [PubMed] [Google Scholar]