Abstract
We evaluated the efficacy and feasibility of the combination of gemcitabine plus vinorelbine in patients with platinum-based chemotherapy-refractory esophageal cancer. We enrolled 35 patients who received gemcitabine plus vinorelbine as second-line treatment after platinum-based chemotherapy failure between May 2009 and April 2012. Dosage: gemcitabine 1,000 mg/m2 plus vinorelbine 25 mg/m2; all drugs were administered on days 1 and 8 of a 21-day cycle, and this was continued until failure or unacceptable toxicity. A total of 125 cycles of treatment were administered, and all patients received at least two cycles of treatment (two to five cycles; median number of cycles: three). Thirty-two patients were evaluable for response. The response rate was 31.3%, and the disease control rate (partial response plus stable disease) was 62.5%. The progression-free survival (PFS) was 4.3 ± 0.2 months [95% confidence interval (CI), 4.0–4.6], and the median overall survival (OS) was 7.3 ± 0.3 months (95% CI, 6.7–7.8). In the subgroup analysis, median PFS was 4.0 ± 0.2 months (95% CI, 3.6–4.3) in patients with high expression of miRNA-214, while it was 4.6 ± 0.3 months (95% CI, 4.1–5.1) in patients with low expression of miRNA-214 (log rank = 0.023). Myelosuppression with neutropenia and thrombocytopenia was the most common side effect observed with this combination regimen, and higher than grade 3 neutropenia and thrombocytopenia were observed in 10 (31.3%) and 8 patients (25.0%), respectively. Grade 3 fatigue was the most common nonhematologic toxicity, which was observed in 2 (6.1%) patients. The combination of gemcitabine plus vinorelbine was well tolerated as second-line treatment for platinum-based chemotherapy-refractory esophageal cancer patients and appeared to provide enhanced clinical activity especially in patients with low expression of miRNA-214.
Key words: Advanced esophageal cancer, Second-line treatment, Gemcitabine plus vinorelbine, Platinum-based chemotherapy, miRNA-214, Progression-free survival
INTRODUCTION
Esophageal cancer is a highly aggressive neoplasm with a strong tendency for invasion and metastasis1, and despite the use of multimodality therapy, it remains one of the leading causes of cancer-related deaths in the world2. Unfortunately, esophageal cancer rarely presents at an early stage, and patients are usually not diagnosed until the cancer has reached an advanced stage, at which point surgical resection with radical esophagectomy is not feasible. Therefore, chemotherapy plays an important role in patients with metastatic disease. The combination of cisplatin and 5-fluorouracil (5-FU) is considered the first-line standard regimen. This regimen has demonstrated an overall response rate of 25% to 45%, with a median survival of less than 1 year3–5. Unfortunately, patients experience recurrence or disease progression with standard chemotherapy. Therefore, it is important to establish second-line chemotherapy regimens after failure of the standard cisplatin and 5-FU chemotherapy.
Vinorelbine is a synthetic vinca alkaloid, and it has a favorable toxicity profile and activity against a wide range of human malignancies, including non-small cell lung cancer, breast cancer, ovarian cancer, and esophageal squamous cell carcinoma (ESCC)6. In a clinical trial conducted by the European Organisation for Research and Treatment of Cancer (EORTC), this drug was administered on a weekly basis at a dose of 25 mg/m2: 6 of 30 previously untreated patients (20%) and 1 of 16 previously treated patients (6%) had a major response7. Gemcitabine is a deoxycytidine analog, and it has also been tested in esophageal cancer, with significant clinical activity and manageable toxicity in patients with advanced esophageal cancer8,9. A phase II trial of gemcitabine and cisplatin in patients with recurrent or metastatic ESCC had shown an overall response rate of 42.1%. Median progression-free survival (PFS) and median survival for all patients were 4.1 and 10 months, respectively. Patients with a response had significantly longer median survival compared with patients without a response (11 months vs. 7.5 months, p = 0.0069). Overall survival (OS) at 1 year was 36.8%, at 2 years it was 10.5%, and at 5 years it was 5.3%. The most common grades 3–4 toxicity for all patients was leucopenia (44.7%)10. One clinical study of single-agent vinorelbine for pretreated or metastatic squamous cell carcinoma of the esophagus had shown that partial responses were observed in 4 of the 16 patients (25%). A significant improvement of dysphagia was obtained in 4 of 11 symptomatic patients. In general, toxicity was mild, with only one episode each of grade 4 neutropenia and constipation, respectively11.
MicroRNAs (miRNAs) are a group of small noncoding RNAs that regulate gene expression at the posttranscriptional level. There is evidence showing that differential expression of miRNAs was correlated with survival in patients with esophageal carcinoma12,13. miRNA expression profiles may become useful biomarkers for cancer diagnostics, prognosis, and prediction of response to treatment14. Recent evidence demonstrates that miRNAs may function similarly to oncogenes or tumor suppressors, suggesting that they may play an important role in tumorigenesis15. Zhang et al. confirmed that pri-miR-124-1 rs531564 and pri-miR-34 rs4938723 were associated with an increased risk of ESCC in Chinese patients16. Another study has shown that miR-335 expression is an independent prognostic factor for patients with esophageal cancer, thereby identifying it as a potential valuable biomarker for ESCC17.
MATERIALS AND METHODS
Patients
A total of 35 patients were enrolled between May 2009 and April 2012 at the Shandong Tumor Hospital in Jinan, China. The diagnosis of metastatic esophageal cancer was confirmed by histology and cytology prior to patients being treated with gemcitabine plus vinorelbine as second-line treatment after platinum-based chemotherapy failure. The patient selection criteria for this study were as follows: age 18–75 years; an Eastern Cooperative Oncology Group performance status of 0–2; life expectancy of ≥3 months and with one measurable lesion at least according to the modified response evaluation criteria in solid tumors (RECIST); adequate bone marrow function (white blood cell count ≥4.0 × 109/L, absolute neutrophil count ≥1.5 × 109/L, and platelet count ≥100 × 109/L); adequate renal function (serum creatinine level ≤1.5 mg/dl); adequate liver function [total bilirubin level ≤1.5 mg/dl, and aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase levels ≤2.5 × the upper limit of normal]. Patients were excluded from this trial with one of the following criteria: massive pleural effusion or ascites, active concomitant second malignancy, brain metastasis, prior systemic treatment with either gemcitabine or vinorelbine, pregnancy, or women who were breastfeeding. All patients had to give informed consent and to agree to be treated with this combination chemotherapy regimen. The clinical trial was authorized by the ethics committee of Shandong Tumor Hospital.
Treatment
The treatment schedule was as follows: gemcitabine was administered at a dose of 1,000 mg/m2 over 30 min and then followed by vinorelbine at a dose of 25 mg/m2 as a slow (10 min) intravenous bolus. Both drugs were administered on days 1 and 8 of a 21-day cycle. Each treatment cycle was repeated until the appearance of disease progression, unacceptable toxicity, or up to a total of six cycles. Prior to treatment, peripheral blood was collected in all patients for determination of serum miRNA levels.
Evaluation and Statistical Analysis
Tumor response was initially assessed after the second chemotherapy cycle by computed tomography (CT) scan using RECIST criteria and every two cycles thereafter. On the basis of the RECIST guideline, complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were determined. PFS was determined from the date of treatment initiation to documentation of disease progression or death. OS was determined from the time of treatment initiation with gemcitabine combined with vinorelbine until death or the last follow-up. The Kaplan–Meier method was utilized to construct the PFS and OS curves.
RESULTS
Patient Characteristics
A total of 35 patients were enrolled in this study between May 2009 and April 2012. Thirty-four of 35 patients had squamous cell carcinoma, with only 1 patient having adenocarcinoma. All patients had previously received platinum-based chemotherapy. In those patients who had been previously treated, 16 (45.7%) patients had undergone concurrent chemoradiotherapy, and 12 (34.3%) patients had received chemotherapy and radiotherapy, albeit not concurrently, including systemic chemotherapy alone in 7 patients. All patients had metastatic disease; 20 patients presented with metastases to a distant lymph node, 9 with lung metastases, and 4 with liver metastases. Sites of metastasis were confirmed by histology/cytology. The patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics (N = 35)
| Factor | Patients (%) |
|---|---|
| Age (median, 65.5; range, 39–74) | |
| <65 years | 15 (42.9) |
| ≥65 years | 20 (57.1) |
| Gender | |
| Male | 30 (85.7) |
| Female | 5 (14.3) |
| Performance status | |
| 0 | 9 (25.7) |
| 1 | 15 (42.9) |
| 2 | 11 (31.4) |
| Histology | |
| Squamous cell carcinoma | 34 (97.1) |
| Adenocarcinoma | 1 (2.9) |
| Prior treatment | |
| Concurrent chemoradiotherapy | 16 (45.7) |
| Chemotherapy plus radiotherapy | 12 (34.3) |
| Chemotherapy | 7 (20.0) |
| Sites of metastasis | |
| Distant lymph notes | 20 (57.1) |
| Lung | 9 (25.7) |
| Liver | 4 (11.4) |
| Both | 2 (5.7) |
Clinical Activity
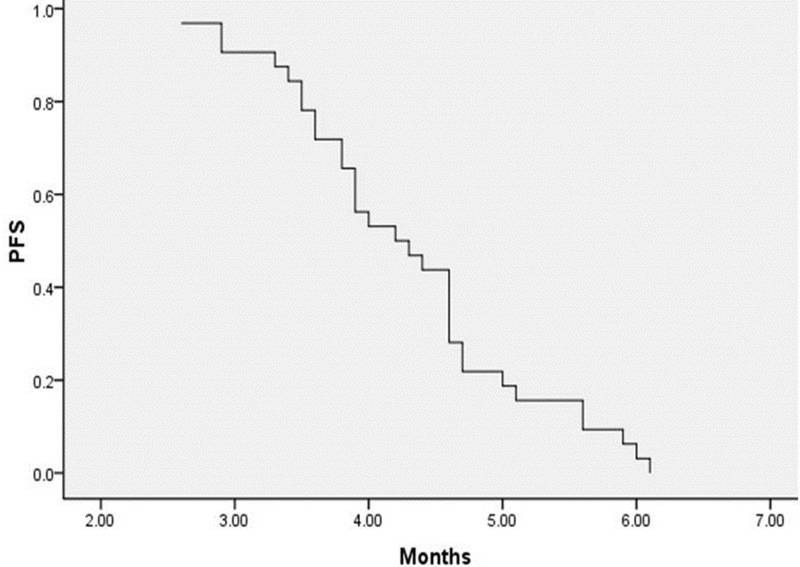
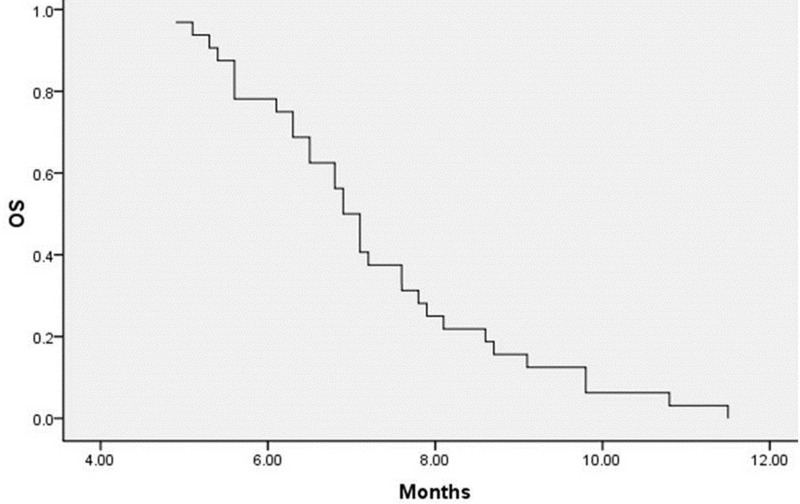
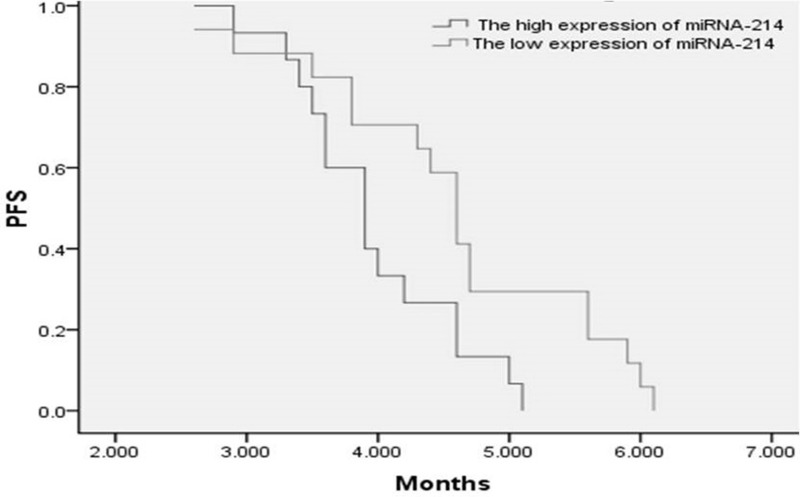
A total of 125 cycles of treatment were administered, and all patients received at least 2 cycles of therapy (2–5 cycles; median, 3). Thirty-two patients were evaluable for response. Of this group, 10 patients (31.3%) had a PR, 10 patients (31.3%) had an SD, and 12 patients (37.5%) had a PD. No CR was observed in this trial (Table 2). The overall response rate was 31.3%, and disease control rate (PR plus SD) was 62.5%. Median PFS was 4.3 ± 0.2 months [95% confidence interval (CI), 4.0–4.6] (Fig. 1), and the median OS was 7.3 ± 0.3 months (95% CI, 6.7–7.8) (Fig. 2). In the subgroup analysis, the PFS was significantly different in patients with high expression of miRNA-214 when compared to low expression. The PFS was 4.0 ± 0.2 months (95% CI, 3.6–4.3) and 4.6 ± 0.3 months (95% CI, 4.1–5.1) in high and low expression of miRNA-214, respectively (log rank = 0.023) (Fig. 3).
Table 2.
Clinical Activity of the Gemcitabine/Vinorelbine Combination Regimen
| Response | |
|---|---|
| CR [No. of patients (%)] | 0 |
| PR [No. of patients (%)] | 10 (31.3) |
| SD [No. of patients (%)] | 10 (31.3) |
| PD [No. of patients (%)] | 12 (37.5) |
| Response rate [No. of patients (%)] | 10 (31.3) |
| Disease control rate [No. of patients (%)] | 20 (62.5) |
| Median OS (95% CI) | 7.3 ± 0.3 (6.7–7.8) months |
| Median PFS (95% CI) | 4.3 ± 0.2 (4.0–4.6) months |
| PFS, high expression of miRNA-214 | 4.0 ± 0.2 (3.6–4.3) months |
| PFS, low expression of miRNA-214 | 4.6 ± 0.3 (4.1–5.1) months |
Figure 1.
PFS in population.
Figure 2.
OS calculated from the date of treatment with gemcitabine plus vinorelbine.
Figure 3.
PFS of high (dark gray line) and low (light gray line) expression of miRNA-214 (p = 0.02).
In general, the safety profile of the combination regimen was manageable, and no treatment-related deaths were observed. At the time of final analysis, all patients had discontinued treatment. Hematologic toxicity was the most important side effect, including neutropenia and thrombocytopenia. Grade 3/4 neutropenia and thrombocytopenia were observed in 10 (31.3%) and 8 (25.0%) patients, respectively. Nonhematologic side effects included diarrhea, nausea/vomiting, neurotoxicity, fatigue, alopecia, and constipation. Grade 3 fatigue was observed in two patients (6.1%). However, no grade 4 nonhematologic toxicity was observed in this study (Table 3).
Table 3.
Toxicity of the Gemcitabine/Vinorelbine Combination Regimen
| Toxicity | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|
| Neutropenia | 5 (15.6) | 9 (28.1) | 8 (25.0) | 2 (6.2) |
| Thrombocytopenia | 8 (25.0) | 6 (18.7) | 7 (21.8) | 1 (3.1) |
| Anemia | 10 (31.2) | 5 (15.6) | 0 | 0 |
| Febrile neutropenia | 6 (18.7) | 2 (6.2) | 1 (3.1) | 0 |
| Nausea/vomiting | 11 (34.4) | 6 (18.7) | 0 | 0 |
| Diarrhea | 2 (6.2) | 0 | 0 | 0 |
| Fatigue | 12 (37.5) | 5 (15.6) | 2 (6.2) | 0 |
| Constipation | 7 (21.8) | 2 (6.2) | 0 | 0 |
| Neurotoxicity | 5 (15.6) | 1 (3.1) | 0 | 0 |
| Alopecia | 8 (25.0) | 2 (6.2) | 0 | 0 |
| ALT/AST | 3 (9.4) | 0 | 0 | 0 |
| Nephrotoxicity | 4 (12.5) | 1 (3.1) | 0 | 0 |
DISCUSSION
The overall prognosis for patients with metastatic or recurrent esophageal cancer is extremely poor, with median survival in the range of 4–8 months18,19. Several different chemotherapy regimens have been associated with encouraging clinical activity as first-line chemotherapy for metastatic or recurrent esophageal cancer, but the median duration of response is generally short20,21. As such, there remains an urgent need to identify effective and well-tolerated second-line treatment regimens.
The vinca alkaloid vindesine has shown a 17% response rate in pretreated small cell esophageal carcinoma (SCEC) patients; however, this agent was associated with significant toxicity, with half of the patients experiencing peripheral neuropathy and one treatment-related death. In contrast, vinorelbine has shown a similar response rate, albeit with reduced toxicity7,23. In a study of previously treated metastatic squamous cell carcinoma of the esophagus, single-agent vinorelbine had a 25% response rate. Only one patient experienced grade 4 neutropenia and constipation22. A phase II study of vinorelbine plus cisplatin in previously untreated patients with metastatic squamous cell esophageal carcinoma showed a 33.8% response rate. This combination was associated with a median PFS of 3.6 months and a median OS of 6.8 months. Toxicity was mainly related to neutropenia23. The combination of docetaxel and vinorelbine in recurrent squamous cell esophageal carcinoma resulted in an overall response rate of 60%, which included 3 of 20 CRs (15%) and 9 of 20 PRs (45%). Median response duration was 7 months, and median OS was 10.5 months. Neutropenia was the most frequent and severe toxicity (grade 4 in 80%; grade 3 in 20%)24.
Single-agent irinotecan or the combination of irinotecan plus docetaxel in cisplatin-pretreated metastatic esophageal cancer was associated with a median PFS of 2 months and a median OS time of 4.5 months. The response rate was relatively low at 12.5% (95% CI 2.7–32.4%). The incidence of grade 3 hematologic toxicity was rare25,26. Several clinical studies have investigated docetaxel plus cisplatin or single-agent paclitaxel for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy, and the overall response rates have been in the 10.3–44.2% range, median PFS of 2.1–4.8 months, and OS of 7.2–10.4 months. The main grade 3/4 hematologic toxicities have been myelosuppression in the form of neutropenia (48.8–52.8%) and leucopenia (45.3–47.3%), respectively27,28.
An earlier study showed that miRNA expression correlated with response to therapy and overall prognosis in acute myelogenous leukemia29. Two studies have demonstrated aberrant expression of miRNAs in ESCC and that these miRNAs were involved in several biological processes by targeting different mRNAs30,31. In one study, the miRNA expression level was compared in matched ESCC tissues and normal tissues by quantitative polymerase chain reaction, and it was determined that expression levels of miR-98, miR-101, and miR-214 were significantly lower in tumor than in normal tissues32. A different study showed that overexpression of miR-214 decreased the sensitivity of the cells to gemcitabine and that miR-214 could induce cell survival and cisplatin resistance through targeting the phosphatase and tensin homolog (PTEN) pathway33.
In conclusion, the combination of gemcitabine plus vinorelbine was well tolerated in the second-line treatment of platinum-based chemotherapy-refractory esophageal cancer patients. Moreover, this combination appears to have improved clinical activity, at least in terms of PFS, in patients whose tumors express reduced levels of miRNA-214. One of the potential limitations of this study is the relatively small patient size. However, based on the promising clinical activity and manageable safety profile observed in this clinical study, the combination of gemcitabine plus vinorelbine merits further clinical investigation.
ACKNOWLEDGMENTS
The work is supported by the Science and Technology Department of Shandong Province.
REFERENCES
- 1. Lu C. L.; Ji Y.; Ge D.; Guo J.; Ding J. Y. The expression of CXCR4 and its relationship with matrix metalloproteinase-9/vascular endothelial growth factor in esophageal squamous cell cancer. Dis. Esophagus 24(4):283–290; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Malthaner R.; Wong R. K.; Spithoff K.; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Preoperative postoperative therapy for resectable oesophageal cancer: An updated practice guideline. Clin. Oncol. (R. Coll. Radiol.) 22(4):250–256; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Enzinger P. C.; Mayer R. J. Esophageal cancer. N. Engl. J. Med. 349(23):2241–2252; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Iizuka T.; Kakegawa T.; Ide H.; Ando N.; Watanabe H.; Tanaka O.; Takagi I.; Isono K.; Ishida K.; Arimori M. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: A Japanese Esophageal Oncology Group Trial. Jpn J. Clin. Oncol. 22(3):172–176; 1992. [PubMed] [Google Scholar]
- 5. Ilson D. H. Oesophageal cancer: New developments in systemic therapy. Cancer Treat. Rev. 29(6):525–532; 2003. [DOI] [PubMed] [Google Scholar]
- 6. Kulke M. H.; Muzikansky A.; Clark J.; Enzinger P. C.; Fidias P.; Kinsella K.; Michelini A.; Fuchs C. S. A phase II trial of vinorelbine in patients with advanced gastroesophageal adenocarcinoma. Cancer Invest. 24(4):346–350; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Conroy T.; Etienne P. L.; Adenis A.; Wagener D. J.; Paillot B.; François E.; Bedenne L.; Jacob J. H.; Seitz J. F.; Bleiberg H.; Van Pottelsberghe C.; Van Glabbeke M.; Delgado F. M.; Merle S.; Wils J. Phase II trial of vinorelbine in metastatic squamous cell esophageal carcinoma. European Organization for Research and Treatment of Cancer Gastrointestinal Treat Cancer Cooperative Group. J. Clin. Oncol. 14(1):164–170; 1996. [DOI] [PubMed] [Google Scholar]
- 8. Kroep J. R.; Pinedo H. M.; Giaccone G.; Van Bochove A.; Peters G. J.; Van Groeningen C. J. Phase II study of cisplatin preceding gemcitabine in patients with advanced oesophageal cancer. Ann. Oncol. 15(2):230–235; 2004. [DOI] [PubMed] [Google Scholar]
- 9. SWOG 0101. Evaluation of gemcitabine and irinotecan in esophageal cancer. Proceedings of the ASCO Gastrointestinal Cancers Symposium 2004; Abstr 25. [Google Scholar]
- 10. Huang J.; Fan Q. X.; Chen L.; Liu A. N.; Cai R. G.; Hao X. Z.; Wang J. W.; Sun Y. Long-term outcomes of gemcitabine and cisplatin in patients with recurrent or metastatic esophageal squamous cell carcinoma: A phase II trial. Chin. Med. J. (Engl.) 124(23):4012–4017; 2011. [PubMed] [Google Scholar]
- 11. Bidoli P.; Stani S. C.; De Candis D.; Cortinovis D.; Parra H. S.; Bajetta E. Single-agent chemotherapy with vinorelbine for pretreated or metastatic squamous cell carcinoma of the esophagus. Tumori 87(5):299–302; 2001. [DOI] [PubMed] [Google Scholar]
- 12. Guo Y.; Chen Z.; Zhang L.; Zhou F.; Shi S.; Feng X.; Li B.; Meng X.; Ma X.; Luo M.; Shao K.; Li N.; Qiu B.; Mitchelson K.; Cheng J.; He J. Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res. 68(1):26–33; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Mathe E. A.; Nguyen G. H.; Bowman E. D.; Zhao Y.; Budhu A.; Schetter A. J.; Braun R.; Reimers M.; Kumamoto K.; Hughes D.; Altorki N. K.; Casson A. G.; Liu C. G.; Wang X. W.; Yanaihara N.; Hagiwara N.; Dannenberg A. J.; Miyashita M.; Croce C. M.; Harris C. C. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: Associations with survival. Clin. Cancer Res. 15(19):6192–6200; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartel D. P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116(2):281–297; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Croce C. Introduction to the role of microRNAs in cancer diagnosis, prognosis, and treatment. Cancer J. 18(3):213–221; 2012. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J.; Huang X.; Xiao J.; Yang Y.; Zhou Y.; Wang X.; Liu Q.; Yang J.; Wang M.; Qiu L.; Zheng Y.; Zhang P.; Li J.; Wang Y.; Wei Q.; Jin L.; Wang J.; Wang M. Pri-miR-124 rs531564 and pri-miR-34b/c rs4938723 polymorphisms are associated with decreased risk of esophageal squamous cell carcinoma in Chinese populations. PLoS One 9(6):e100055; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang B. J.; Gong H. Y.; Zheng F.; Liu D. J.; Liu H. X. Up-regulation of miR-335 predicts a favorable prognosis in esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 7(9):6213–6218; 2014. [PMC free article] [PubMed] [Google Scholar]
- 18. Kunisaki C.; Makino H.; Takagawa R.; Yamamoto N.; Nagano Y.; Fujii S.; Kosaka T.; Ono H. A.; Otsuka Y.; Akiyama H.; Ichikawa Y.; Shimada H. Surgical outcomes in esophageal cancer patients with tumor recurrence after curative esophagectomy. J. Gastrointest. Surg. 12(5):802–810; 2008. [DOI] [PubMed] [Google Scholar]
- 19. Bagheri R.; Maddah G.; Saedi H. S.; Sadeghian M. H.; Roodbari S. Bone marrow involvement in esophageal cancer patients who underwent surgical resection. Eur. J. Cardiothorac. Surg. 40(2):343–346; 2011. [DOI] [PubMed] [Google Scholar]
- 20. Ilson D. H. Oesophageal cancer: Developments in systemic therapy. Cancer Treat. Rev. 29(6):525–532; 2003. [DOI] [PubMed] [Google Scholar]
- 21. Levard H.; Pouliquen X.; Hay J. M.; Fingerhut A.; Langlois-Zantain O.; Huguier M.; Lozach P.; Testart J. 5-Fluorouracil and cisplatin as palliative treatment of advanced oesophageal squamous cell carcinoma. A multicentre randomised controlled trial. The French Associations for Surgical Research. Eur. J. Surg. 164(11):849–857; 1998. [DOI] [PubMed] [Google Scholar]
- 22. Bidoli P.; Stani S. C.; De Candis D.; Cortinovis D.; Parra H. S.; Bajetta E. Single-agent chemotherapy with vinorelbine for pretreated or metastatic squamous cell carcinoma of the esophagus. Tumori 87(5):299–302; 2001. [DOI] [PubMed] [Google Scholar]
- 23. Conroy T.; Etienne P. L.; Adenis A.; Ducreux M.; Paillot B.; Oliveira J.; Seitz J. F.; Francois E.; Van Cutsem E.; Wagener D. J.; Kohser F.; Daamen S.; Praet M.; Gorlia T.; Baron B.; Wils J.; European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Vinorelbine and cisplatin in metastatic squamous cell carcinoma of the oesophagus: Response, toxicity, quality of life and survival. Ann. Oncol. 13(5):721–729; 2002. [DOI] [PubMed] [Google Scholar]
- 24. Airoldi M.; Cortesina G.; Giordano C.; Pedani F.; Bumma C.; Gabriele P. Docetaxel and vinorelbine: An effective regimen in recurrent squamous cell esophageal carcinoma. Med. Oncol. 20(1):19–24; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Burkart C.; Bokemeyer C.; Klump B.; Pereira P.; Teichmann R.; Hartmann J. T. A phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res. 27(4C):2845–2848; 2007. [PubMed] [Google Scholar]
- 26. Lordick F.; von Schilling C.; Bernhard H.; Pedani F.; Bumma C.; Gabriele P. Phase II trial of irinotecan plus docetaxel in cisplatin-pretreated relapsed or refractory oesophageal cancer. Br. J. Cancer 89(4):630–633; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shim H. J.; Cho S. H.; Hwang J. E.; Bae W. K.; Song S. Y.; Cho S. B.; Lee W. S.; Joo Y. E.; Na K. J.; Chung I. J. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. Am. J. Clin. Oncol. 33(6):624–628; 2010. [DOI] [PubMed] [Google Scholar]
- 28. Kato K.; Tahara M.; Hironaka S.; Muro K.; Takiuchi H.; Hamamoto Y.; Imamoto H.; Amano N.; Seriu T. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother. Pharmacol. 67(6):1265–1272; 2011. [DOI] [PubMed] [Google Scholar]
- 29. Garzon R.; Volinia S.; Liu C. G.; Fernandez-Cymering C.; Palumbo T.; Pichiorri F.; Fabbri M.; Coombes K.; Alder H.; Nakamura T.; Flomenberg N.; Marcucci G.; Calin G. A.; Kornblau S. M.; Kantarjian H.; Bloomfield C. D.; Andreeff M.; Croce C. M. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood 111(6):3183–3189; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu Y.; Correa A. M.; Hoque A.; Guan B.; Ye F.; Huang J.; Swisher S. G.; Wu T. T.; Ajani J. A.; Xu X. C. Prognostic significance of differentially expressed miRNAs in esophageal cancer. Int. J. Cancer 128(1):132–143; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang M.; Liu R.; Sheng J.; Liao J.; Wang Y.; Pan E.; Guo W.; Pu Y.; Yin L. Differential expression profiles of microRNAs as potential biomarkers for the early diagnosis of esophageal squamous cell carcinoma. Oncol. Rep. 29(1):169–176; 2013. [DOI] [PubMed] [Google Scholar]
- 32. Huang S. D.; Yuan Y.; Zhuang C. W.; Li B. L.; Gong D. J.; Wang S. G.; Zeng Z. Y.; Cheng H. Z. MicroRNA-98 and microRNA-214 post-transcriptionally regulate enhancer of zeste homolog 2 and inhibit migration and invasion in human esophageal squamous cell carcinoma. Mol. Cancer 11:51; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang H.; Kong W.; He L.; Zhao J. J.; O’Donnell J. D.; Wang J.; Wenham R. M.; Coppola D.; Kruk P. A.; Nicosia S. V.; Cheng J. Q. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68(2):425–433; 2008. [DOI] [PubMed] [Google Scholar]