Abstract
Armadillo repeat-containing protein 8 (ARMC8) plays an important role in regulating cell migration, proliferation, tissue maintenance, signal transduction, and tumorigenesis. However, the expression pattern and role of ARMC8 in osteosarcoma are still unclear. In this study, our aims were to examine the effects of ARMC8 on osteosarcoma and to explore its underlying mechanism. Our results demonstrated that ARMC8 was overexpressed in osteosarcoma cell lines. Knockdown of ARMC8 significantly inhibited osteosarcoma cell proliferation in vitro and markedly inhibited xenograft tumor growth in vivo. ARMC8 silencing also suppressed the epithelial–mesenchymal transition (EMT) phenotype, as well as inhibited the migration and invasion of osteosarcoma cells. Furthermore, knockdown of ARMC8 obviously inhibited the expression of β-catenin, c-Myc, and cyclin D1 in MG-63 cells. In conclusion, this report demonstrates that ARMC8 silencing inhibits proliferation and invasion of osteosarcoma cells. Therefore, ARMC8 may play an important role in the development and progression of human osteosarcoma and may represent a novel therapeutic target in the treatment of osteosarcoma.
Key words: Armadillo repeat-containing protein 8 (ARMC8), Osteosarcoma, Proliferation, Invasion
INTRODUCTION
Osteosarcoma is the most common primary malignant bone tumor and a leading cause of cancer death in adolescents (1). During the last decade, several strategies have been suggested for the treatment of osteosarcoma patients, but unfortunately some patients died of pulmonary metastases (2–5). Therefore, it is urgent to explore the molecular mechanisms underlying osteosarcoma carcinogenesis and progression in order to improve the prevention and treatment of this disease.
Armadillo repeat-containing protein 8 (ARMC8) proteins are novel proteins that are conserved in eukaryotes. Increasing evidence has reported that ARMC8 plays an important role in regulating cell migration, proliferation, tissue maintenance, signal transduction, and tumorigenesis (6–8). Jiang et al. reported that the expression level of ARMC8 was obviously higher in colon cancer in comparison to that in the adjacent normal colon tissues, and ARMC8 expression was associated with TNM stage, lymph node metastasis, and poor prognosis of colon cancer (9). Another study demonstrated that ARMC8 was also highly expressed in human non-small cell lung cancer tissues, and overexpression of ARMC8α significantly promoted growth, colony formation, and invasion in H1299 lung cancer cells (10). However, the expression pattern and role of ARMC8 in osteosarcoma are still unclear. In this study, our aims were to examine the effects of ARMC8 on osteosarcoma and to explore its underlying mechanism.
MATERIALS AND METHODS
Cell Culture
The human osteosarcoma cell lines (U2OS and SaOS2) and the normal osteoblast cell line (hFOB1.19) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin at 37°C with 5% CO2 in an incubator (Life Technologies, Baltimore, MD, USA).
RNA Interference and Transfection
The short hairpin RNA targeting ARMC8 (ARMC8-shRNA) and its negative control were synthesized by RiBo Biotech (GuangZhou RiBo Biotech). The ARMC8-shRNA or scr-shRNA was transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) when the cell density reached 70%–80%. The cells were harvested for assays 48 h after transfection.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was isolated from human osteosarcoma cells using TRIzol reagent (Invitrogen). About 5 μg of total RNA for each sample was reverse transcribed into first-strand cDNA for qRT-PCR analysis. The primer sequences used for PCR amplification are ARMC8, 5′-GTGTAGAGCTGGAGCAATTCGG-3′ (forward) and 5′-AGGCAAGTGTCTCAGCTCCTTCGTCCCCTCGAGGAGTTGTGT-3′ (reverse); β-actin, 5′-ATCACCATTGGCAATGAGCG-3′ (forward) and 5′-TTGAAGGTAGTTTCGTGGAT-3′ (reverse). The amplification protocol used was as follows: an initial 3-min denaturation at 94°C, followed by 40 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 45 s. β-Actin was used as internal control. The relative amount of each cDNA was analyzed by means of 2−ΔΔCt.
Western Blot
Cells were lysed using RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, and 0.1% sodium dodecyl sulfate) on ice for 30 min, and then the protein was quantified by a BCA assay (Pierce, Rockford, IL, USA). A total of 20 µg of protein was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane (Amersham, Little Chalfont, UK). The membrane was blocked with 2% nonfat dry milk in Tris-buffered saline (TBS) for 1 h at room temperature (RT) followed by incubation in primary antibodies (antibodies against ARMC8, E-cadherin, N-cadherin, β-catenin, c-Myc, cyclin D1, and β-actin; diluted 1:1,000; all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) in the blocking buffer overnight at 4°C. Subsequently, the blots were washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG. Protein bands were visualized by enhanced chemiluminescence (Millipore, Boston, MA, USA) according to the manufacturer’s protocol.
Cell Proliferation Assay
Cell proliferation was measured using Cell Counting Kit-8 (CCK-8) (DOJINDO, Kumamoto, Japan) according to the manufacturer’s protocol. Briefly, osteosarcoma cells (1 × 105 cells per well) transfected with ARMC8-shRNA or scr-shRNA were seeded in 96-well flat-bottom microtiter plates (Pierce) and then cultured at 24-h intervals for 4 days. Cell proliferation was then evaluated using the CCK-8 (Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer’s instructions. The absorbance value was read spectrophotometrically at 450 nm on a microtiter plate reader (Merek, Whitehouse Station, NJ, USA).
Cell Migration and Invasion Assay
The cell migration capability was measured using the Transwell assay. In brief, osteosarcoma cells transfected with ARMC8-shRNA or scr-shRNA suspended in RPMI medium were added to the upper chamber, and the lower chamber of the Transwell plates was filled with 500 µl of RPMI medium containing 10% FBS (Invitrogen). After 24 h of incubation, the cells that migrated through the membrane were fixed in 4% paraformaldehyde for 20 min, stained with 0.1% crystal violet for 30 min, and counted under the microscope (Olympus, Tokyo, Japan) by counting five independent visual fields. The invasion assay was done by the same procedure, except that the membrane was coated with Matrigel to form a matrix barrier.
In Vivo Xenograft Tumor Assay
All mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee of Huaihe Hospital of Henan University. MG-63 cells (1 × 106 cells/0.1 ml) transfected with ARMC8-shRNA or scr-shRNA were injected subcutaneously into the flank of nude mice. About 5 weeks after inoculation, mice were euthanized by subcutaneous injection with sodium pentobarbital (40 mg/kg), and the tumors were weighed. Tumor size was measured every 7 days using a caliper and calculated using the formula: volume = length × width2 × π/6.
Statistical Analysis
All statistical analyses were performed using the SPSS software (ver. 13.0; SPSS, Chicago, IL, USA), and all results are expressed as mean ± SD. Statistical significance was analyzed with one-way analysis of variance followed by the Student’s t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
ARMC8 Is Overexpressed in Human Osteosarcoma Cell Lines
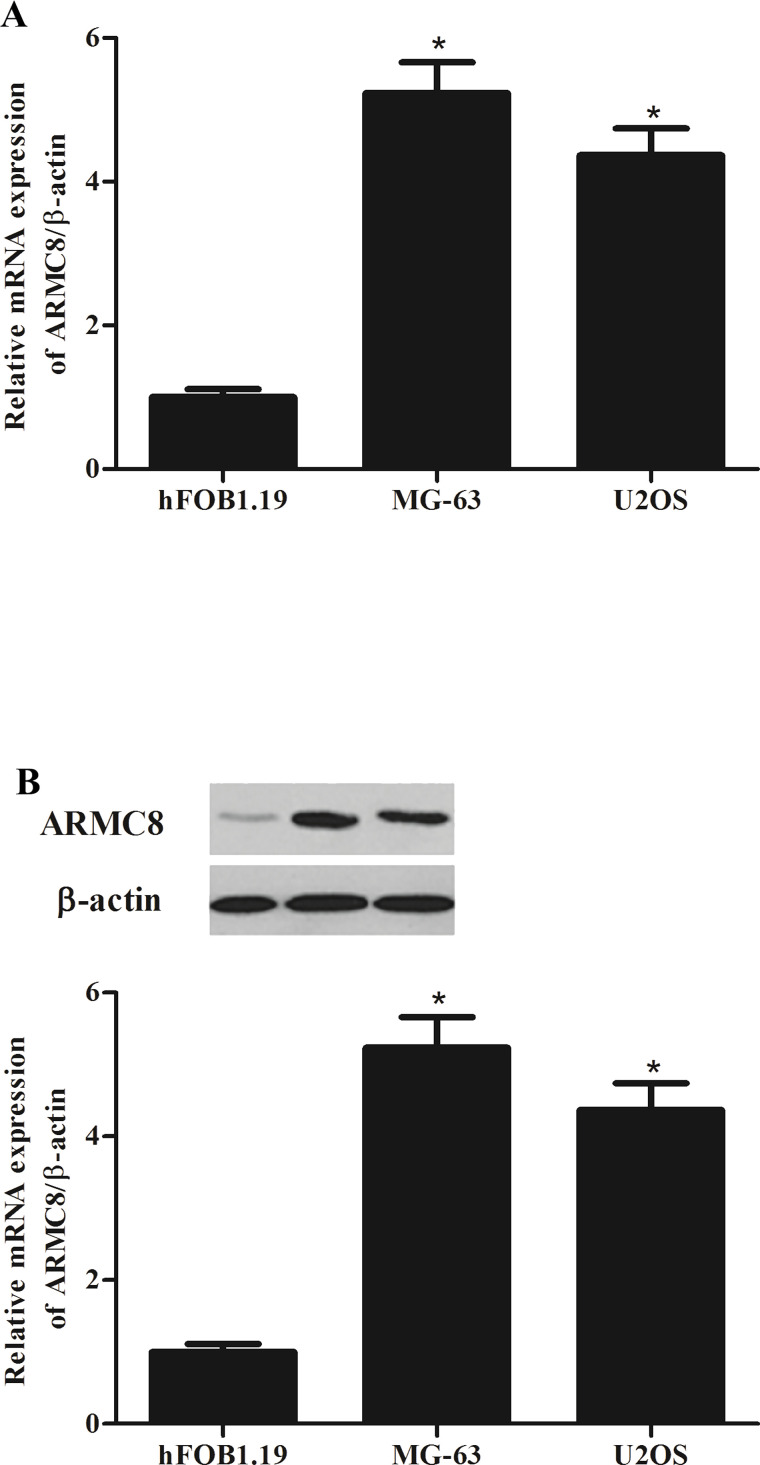
To verify the expression of ARMC8 in osteosarcoma, we detected the mRNA level and protein level of ARMC8 in different osteosarcoma cell lines using qRT-PCR and Western blot assays. The results of the qRT-PCR analysis demonstrated that compared with the normal cells, the mRNA expression of ARMC8 was overexpressed in osteosarcoma cell lines (Fig. 1A). Consistent with the results of the qRT-PCR, Western blot analysis also showed that the protein expression of ARMC8 was higher in osteosarcoma cell lines than that in normal cells (Fig. 1B).
Figure 1.
Armadillo repeat-containing protein 8 (ARMC8) is overexpressed in human osteosarcoma cell lines. (A) Representative mRNA expression of ARMC8 in human osteosarcoma cell lines. (B) Representative Western blot image of ARMC8 protein in human osteosarcoma cells lines. Data represent mean ± SD from three independent experiments. *p < 0.05.
ARMC8 Silencing Inhibits Osteosarcoma Cell Proliferation In Vitro
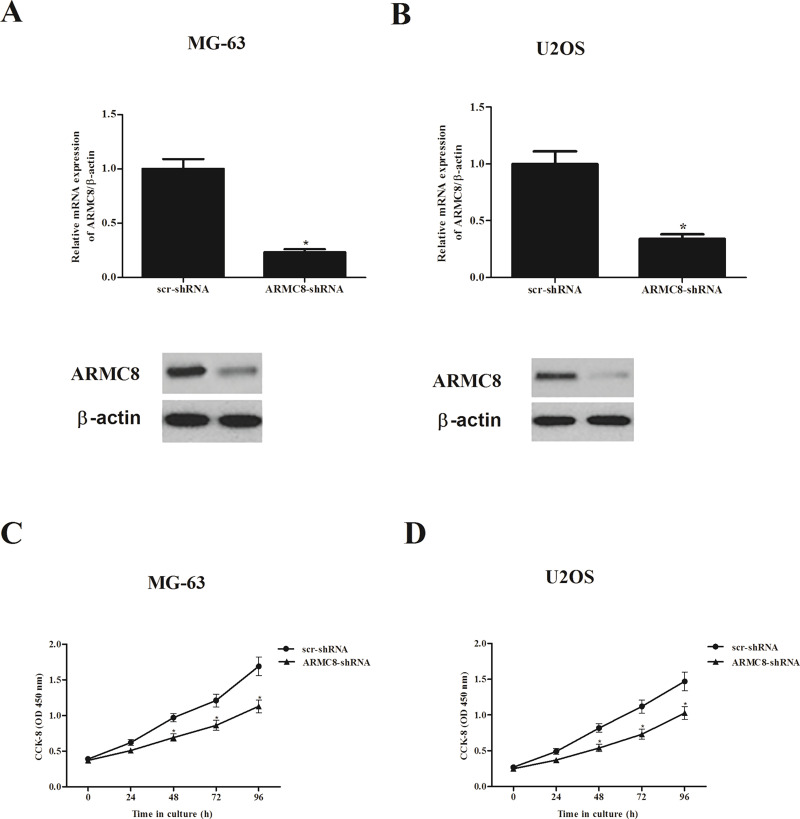
To investigate the biological role of ARMC8 in osteosarcoma tumorigenesis, we generated ARMC8 silencing osteosarcoma cell lines of MG-63 and U2OS. The transfection efficiency was confirmed by qRT-PCR and Western blot. The results indicated that the mRNA and protein expression levels of ARMC8 were significantly reduced in MG-63 (Fig. 2A) and U2OS (Fig. 2B) cells, respectively. We then observed the effect of ARMC8 silencing on osteosarcoma cell proliferation. The results of the CCK-8 assay showed that knockdown of ARMC8 significantly inhibited the growth of MG-63 cells (Fig. 2C). Similarly, knockdown of ARMC8 also suppressed the proliferation in U2OS cells (Fig. 2D).
Figure 2.
ARMC8 silencing inhibits osteosarcoma cell proliferation in vitro. (A) mRNA and protein levels of ARMC8 in ARMC8-shRNA-transfected MG-63 cells. (B) mRNA and protein levels of ARMC8 in ARMC8-shRNA-transfected U2OS cells. (C) Knockdown of ARMC8 inhibited the proliferation of MG-63 cells in a time-dependent manner. (D) Knockdown of ARMC8 inhibited the proliferation of U2OS cells in a time-dependent manner. Data represent mean ± SD from three independent experiments. *p < 0.05.
ARMC8 Silencing Inhibits the Growth of Osteosarcoma In Vivo
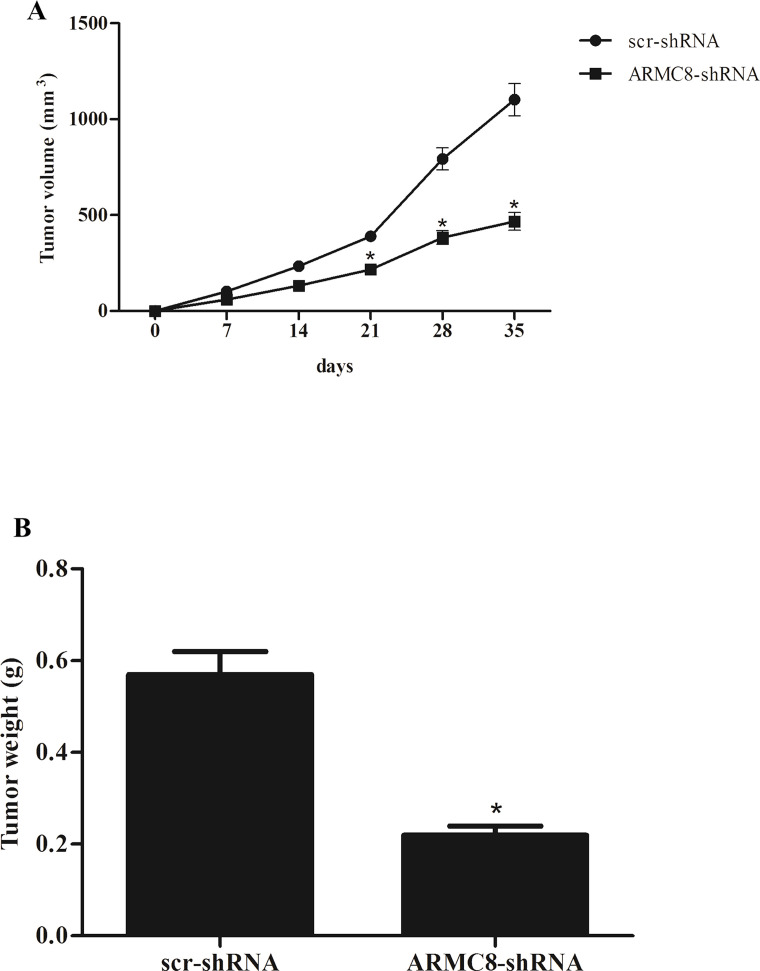
Furthermore, we examined the effect of ARMC8 silencing on osteosarcoma growth in vivo. We found that knockdown of ARMC8 significantly reduced tumor volume compared to control mice (Fig. 3A). In addition, knockdown of ARMC8 obviously reduced the weight of the tumor, compared with the control tumor (Fig. 3B).
Figure 3.
ARMC8 silencing inhibits the growth of osteosarcoma in vivo. MG-63 cells (1 × 106 cells/0.1 ml) transfected with ARMC8-shRNA or scr-shRNA were injected subcutaneously into the flank of nude mice. (A) The tumor volumes were calculated in each group every 7 days from day 0 to day 35. (B) The tumor weights were measured at day 35. Data represent mean ± SD. *p < 0.05.
ARMC8 Silencing Inhibits the Epithelial–Mesenchymal Transition (EMT) Process in Osteosarcoma Cells
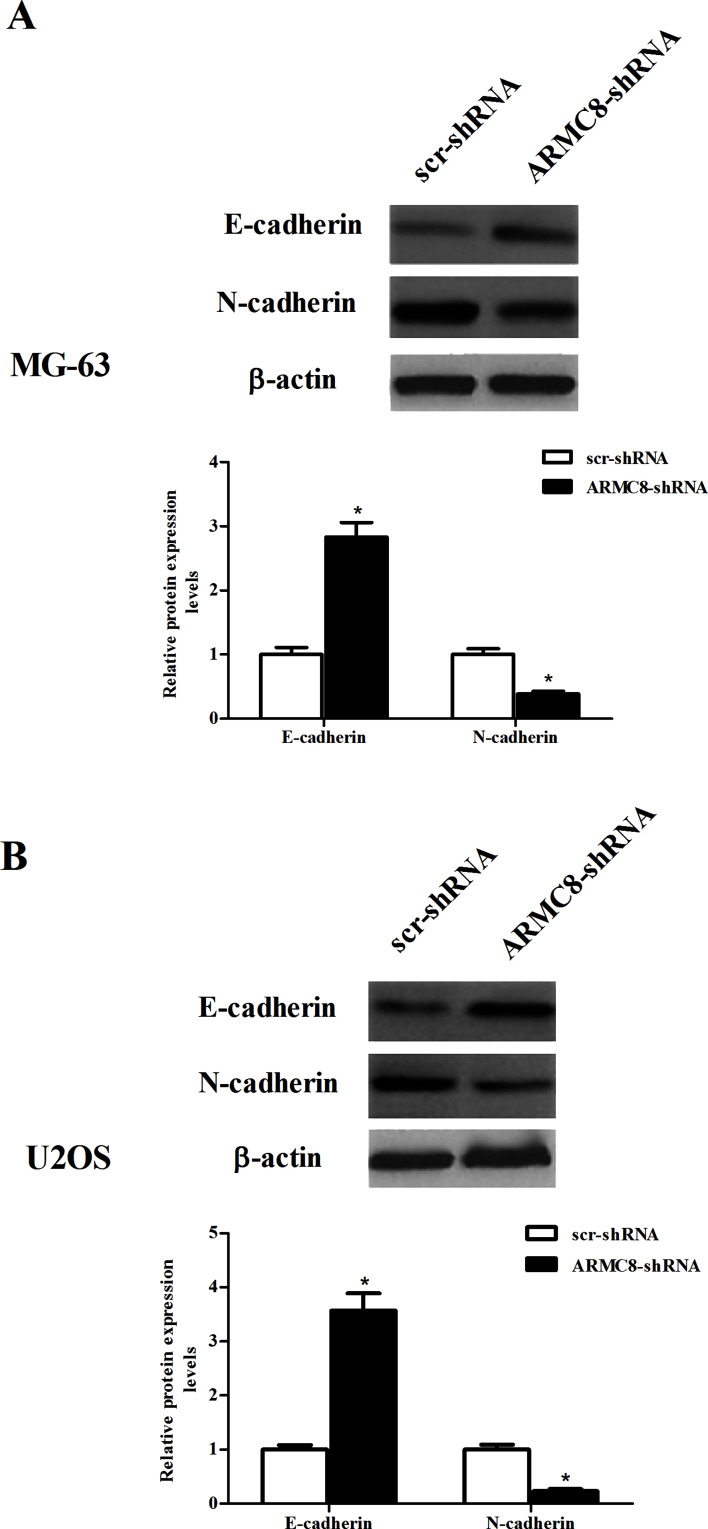
We investigated the effect of ARMC8 silencing on EMT phenotype in osteosarcoma cells. The results uncovered that knockdown of ARMC8 in MG-63 cells induced an increase in the protein expression of E-cadherin, paralleling with a decrease in the protein expression level of N-cadherin (Fig. 4A). Similar results were observed in U2OS cells (Fig. 4B).
Figure 4.
ARMC8 silencing inhibits the EMT process in osteosarcoma cells. MG-63 or U2OS cells were transfected with ARMC8-shRNA or scr-shRNA for 24 h. (A) The protein expression levels of E-cadherin and N-cadherin were detected by Western blot in MG-63 cells and quantification of protein levels from three independent experiments. (B) The protein expression levels of E-cadherin and N-cadherin were measured by Western blot in U2OS cells and quantification of protein levels from three independent experiments. Data represent mean ± SD. *p < 0.05.
ARMC8 Silencing Inhibits OS Cell Migration and Invasion In Vitro
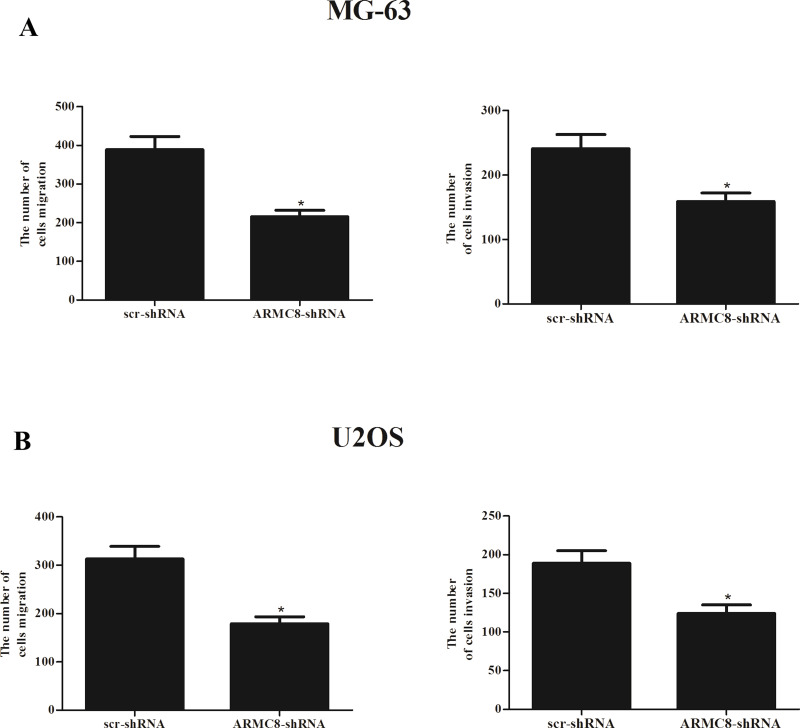
We next investigated the effect of ARMC8 silencing on the migration and invasion of OS cells. As shown in Figure 5A, for MG-63 cells, there was a significantly lower number of migrated cells in ARMC8-shRNA-transfected cells than in scr-shRNA-transfected cells. Furthermore, the Matrigel assays showed that the number of cells that passed through the Matrigel-coated membrane into the lower chamber was significantly lower in the ARMC8-shRNA-transfected cells than in empty scr-shRNA-transfected cells. At the same time, we observed that knockdown of ARMC8 greatly suppressed migration and invasion of U2OS cells (Fig. 5B).
Figure 5.
ARMC8 silencing inhibits OS cell migration and invasion in vitro. MG-63 or U2OS cells were transfected with ARMC8-shRNA or scr-shRNA for 24 h. (A) The number of migrated/invaded cells in the ARMC8-shRNA-transfected MG-63 cells was obviously decreased, compared with the scr-shRNA group. (B) Knockdown of ARMC8 significantly inhibited the migration and invasion of U2OS cells. Data represent mean ± SD from three independent experiments. *p < 0.05.
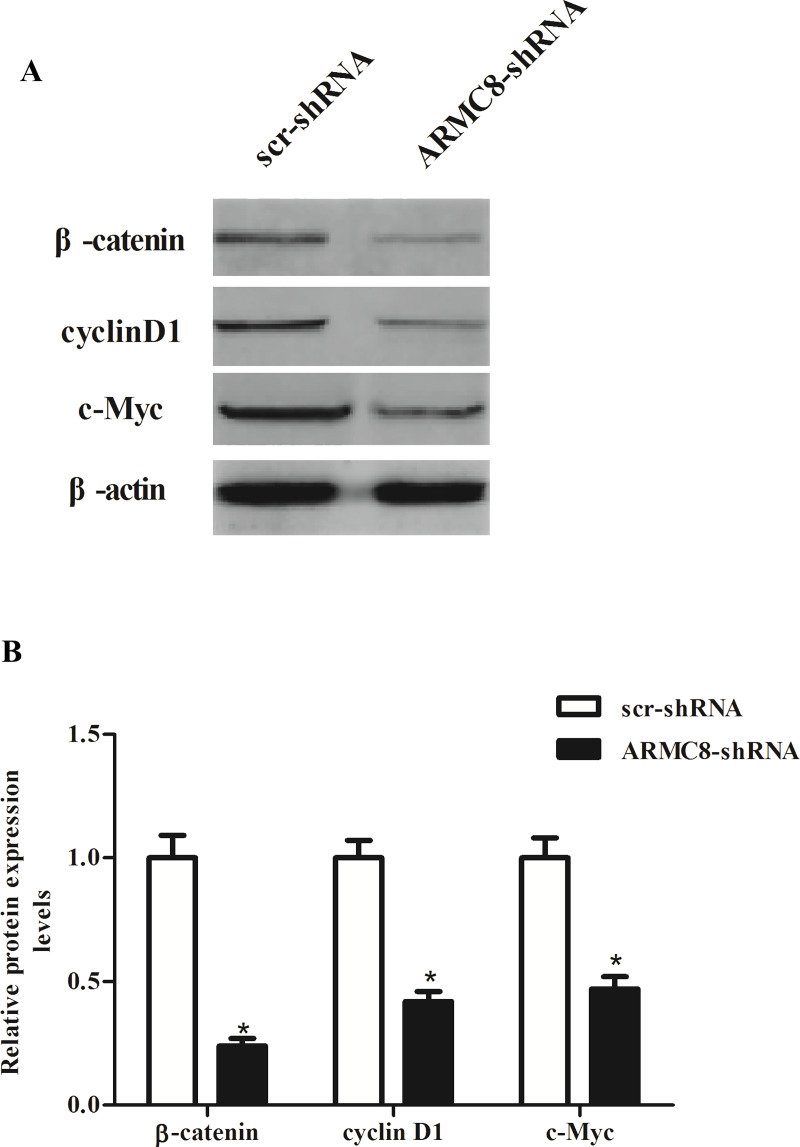
ARMC8 Silencing Inhibits the Activation of Wnt/β-Catenin Signaling Pathway in Osteosarcoma Cells
To gain insights into the downstream signaling pathways modulated by ARMC8 silencing in tumor inhibition, we investigated the effect of ARMC8 silencing on the activation of Wnt/β-catenin signaling pathway in osteosarcoma cells. The results of the Western blot analysis indicated that knockdown of ARMC8 obviously inhibited the expression of β-catenin, c-Myc, and cyclin D1 in MG-63 cells, compared with scr-shRNA-transfected cells (Fig. 6A). Quantification analysis of β-catenin, c-Myc, and cyclin D1 is shown in Figure 6B.
Figure 6.
ARMC8 silencing inhibits the activation of Wnt/β-catenin signaling pathway in OS cells. (A) MG-63 cells were transfected with ARMC8-shRNA or scr-shRNA for 24 h. The protein expression of β-catenin, cyclin D1, and c-Myc was detected by Western blot. (B) Relative expression of β-catenin, cyclin D1, and c-Myc was quantified using Image-Pro Plus 6.0 software after normalization with β-actin. Data represent mean ± SD from three independent experiments. *p < 0.05.
DISCUSSION
To the best of our knowledge, the present study represents the first demonstration that ARMC8 was overexpressed in osteosarcoma cell lines. Knockdown of ARMC8 significantly inhibited osteosarcoma cell proliferation in vitro and markedly inhibited xenograft tumor growth in vivo. ARMC8 silencing also suppressed the EMT phenotype, as well as inhibited the migration and invasion in osteosarcoma cells. Furthermore, knockdown of ARMC8 obviously inhibited the expression of β-catenin, c-Myc, and cyclin D1 in MG-63 cells.
Recently, research reported that the expression of ARMC8 was upregulated in ovarian cancer and the borderline ovarian tumor tissues, in comparison with the benign ovarian tumors and the normal ovarian tissues (11). Fan et al. confirmed that ARMC8 expression in breast carcinoma including breast carcinoma in situ, infiltrating ductal carcinoma, and infiltrating lobular carcinoma was higher than that in normal breast epithelia, usual ductal hyperplasia, benign breast tumors, and breast epithelia with dysplasia (12). Consistent with the above data, in this study we found that ARMC8 was overexpressed in osteosarcoma cell lines. All these data suggest that ARMC8 may act as an oncogenic factor in osteosarcoma.
Previous studies demonstrated that ARMC8 was implicated in the growth of malignant tumors (9,10,13). For example, Xie et al. confirmed that ARMC8α downregulation by siRNA knockdown inhibited growth and colony formation in A549 lung cancer cells, while ARMC8α overexpression promoted growth and colony formation in H1299 lung cancer cells (10). In agreement with the prior study, herein we observed that knockdown of ARMC8 significantly inhibited osteosarcoma cell proliferation in vitro and markedly inhibited xenograft tumor growth in vivo. Collectively, these data obtained from both in vitro and in vivo experiments strongly suggest that ARMC8 may play an important role in the development and progression of human osteosarcoma.
EMT is known to be a central mechanism responsible for invasiveness and metastasis of various cancers (14–16). A particular characteristic of EMT is downregulation of the epithelial marker E-cadherin and increase in mesenchymal markers such as N-cadherin, vimentin, and fibronectin (17). Furthermore, previous studies indicated that ARMC8 plays an important role in tumor cell migration and invasion (9–11). We observed that ARMC8 silencing also suppressed the EMT phenotype, as well as inhibiting the migration and invasion in osteosarcoma cells. These data suggest that ARMC8 positively regulates EMT, consequently markedly inducing osteosarcoma cell migration and invasion in vitro.
The Wnt/β-catenin signaling pathway plays a pivotal role in regulating osteosarcoma cell growth, EMT, invasion, and metastasis (18–21). Its aberrant activation is a key event in the pathogenesis and progression of human osteosarcoma. β-Catenin is a main downstream effector of the canonical Wnt signaling pathway and induces the transcription of several target genes, such as cyclin D1 and c-Myc, which are involved in cell survival, proliferation, and metastasis (22). Most interestingly, in a recent study ARMC8α knockdown downregulated canonical Wnt signaling pathway activity and the expression of cyclin D1 and matrix metalloproteinase-7 (MMP-7) in non-small cell lung cancer cells (10). Similarly, in this study we found that ARMC8 obviously inhibited the expression of β-catenin, c-Myc, and cyclin D1 in MG-63 cells. These results suggest that ARMC8 silencing inhibits proliferation and invasion of osteosarcoma cells through suppression of the Wnt/β-catenin signaling pathway.
In conclusion, this report demonstrates that ARMC8 silencing inhibits proliferation and invasion in osteosarcoma cells. Therefore, ARMC8 may play an important role in the development and progression of human osteosarcoma and may represent a novel therapeutic target in the treatment of osteosarcoma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ward E.; DeSantis C.; Robbins A.; Kohler B.; Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 64:83–103; 2014. [DOI] [PubMed] [Google Scholar]
- 2. Edward E. D.; Rosdiana N.; Farhat O. R.; Lubis B. Prevalence and risk factors of hearing loss in children with solid tumors treated with platinum-based chemotherapy. Paediatr. Indones. 55:121; 2015. [Google Scholar]
- 3. Fiori R.; Vivo D. D.; Scarano A. L.; D’Onofrio S.; Calabria E.; Giovanni S. Local invasion of jaw osteosarcoma. Int. J. Case Rep. Images 6:224–227; 2015. [Google Scholar]
- 4. Geller D. S.; Revskaya E.; Khan M.; Morris J.; Gorlick R.; Dadachova E. Targeted therapy of osteosarcoma with radiolabeled monoclonal antibody to an insulin-like growth factor-2 receptor. Cancer Res. 75:1798–1798; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaya M. Prognostic factors for osteosarcoma patients. In Ueda T.; Kawai A., eds. Osteosarcoma. New York: Springer; 2016:73–79. [Google Scholar]
- 6. Wafai Z. W.; Manzoor S.; Koul A. M.; Basit Amin Q. U.; Ain H. Q.; Tyub S.; Lone G. N.; Qadri R. A. Comparative proteomics and global genome-wide expression data implicate role of ARMC8 in lung cancer. Asian Pac. J. Cancer Prev. 16:3691–3696; 2015. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki T.; Ueda A.; Kobayashi N.; Yang J.; Tomaru K.; Yamamoto M.; Takeno M.; Ishigatsubo Y. Proteasome-dependent degradation of α-catenin is regulated by interaction with ARMC8α. Biochem. J. 411:581–591; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi N.; Yang J.; Ueda A.; Suzuki T.; Tomaru K.; Takeno M.; Okuda K.; Ishigatsubo Y. RanBPM, muskelin, p48EMLP, p44CTLH, and the armadillo-repeat proteins ARMC8α and ARMC8β are components of the CTLH complex. Gene 396:236–247; 2007. [DOI] [PubMed] [Google Scholar]
- 9. Jiang G.; Zhang Y.; Zhang X.; Fan C.; Wang L.; Xu H.; Yu J.; Wang E. ARMC8 indicates aggressive colon cancers and promotes invasiveness and migration of colon cancer cells. Tumor Biol. 36:9005–9013; 2015. [DOI] [PubMed] [Google Scholar]
- 10. Xie C.; Jiang G.; Fan C.; Zhang X.; Zhang Y.; Miao Y.; Lin X.; Wu J.; Wang L.; Liu Y. ARMC8α promotes proliferation and invasion of non-small cell lung cancer cells by activating the canonical Wnt signaling pathway. Tumor Biol. 35:8903–8911; 2014. [DOI] [PubMed] [Google Scholar]
- 11. Jiang G.; Yang D.; Wang L.; Zhang X.; Xu H.; Miao Y.; Wang E.; Zhang Y. A novel biomarker ARMC8 promotes the malignant progression of ovarian cancer. Hum. Pathol. 46:1471–1479; 2015. [DOI] [PubMed] [Google Scholar]
- 12. Fan C.; Zhao Y.; Mao X.; Miao Y.; Lin X.; Jiang G.; Zhang X.; Han Q.; Luan L.; Wang E. ARMC8 expression was elevated during atypia-to-carcinoma progression and associated with cancer development of breast carcinoma. Tumor Biol. 35:11337–11343; 2014. [DOI] [PubMed] [Google Scholar]
- 13. Zhou D.; Zhang W.; Wang Y.; Chen L.; Luan J. ARMc8: A potential diagnostic and therapeutic target for cancers. Hum. Pathol. 3001–3006; 2016. [DOI] [PubMed] [Google Scholar]
- 14. Yilmaz M.; Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 28:15–33; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Sánchez-Tilló E.; Liu Y.; de Barrios O.; Siles L.; Fanlo L.; Cuatrecasas M.; Darling D. S.; Dean D. C.; Castells A.; Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell. Mol. Life Sci. 69:3429–3456; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jing Y.; Han Z.; Zhang S.; Liu Y.; Wei L. Epithelial-mesenchymal transition in tumor microenvironment. Cell Biosci. 1:1; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L. K.; Jiang X. Y.; Zhou X. X.; Wang D. M.; Song X. L.; Jiang H. B. Upregulation of vimentin and aberrant expression of E-cadherin/β-catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod. Pathol. 23:213–224; 2010. [DOI] [PubMed] [Google Scholar]
- 18. Hoang B. H.; Kubo T.; Healey J. H.; Yang R.; Nathan S. S.; Kolb E. A.; Mazza B.; Meyers P. A.; Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-β-catenin pathway. Cancer Res. 64:2734–2739; 2004. [DOI] [PubMed] [Google Scholar]
- 19. Cai Y.; Mohseny A. B.; Karperien M.; Hogendoorn P. C.; Zhou G.; Cleton-Jansen A. M. Inactive Wnt/β-catenin pathway in conventional high-grade osteosarcoma. J. Pathol. 220:24–33; 2010. [DOI] [PubMed] [Google Scholar]
- 20. Leow P. C.; Tian Q.; Ong Z. Y.; Yang Z.; Ee P. L. R. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/β-catenin antagonists against human osteosarcoma cells. Invest. New Drugs 28:766–782; 2010. [DOI] [PubMed] [Google Scholar]
- 21. Li R.; Wang L. Fibulin-4 is a novel Wnt/β-catenin pathway activator in human osteosarcoma. Biochem Biophys. Res. Commun. 474:730–735; 2016. [DOI] [PubMed] [Google Scholar]
- 22. Clevers H. Wnt/β-catenin signaling in development and disease. Cell 127:469–480; 2006. [DOI] [PubMed] [Google Scholar]