Abstract
Hematopoietic pre-B-cell leukemia transcription factor (PBX)-interacting protein (HPIP/PBXIP1) is a corepressor for the transcription factor PBX. Previous studies showed that HPIP is frequently overexpressed in many tumors. However, the role of HPIP in head-and-neck squamous cell carcinoma (HNSCC) has not yet been determined. Thus, we decided to investigate the effects and mechanisms of HPIP in HNSCC. Our results demonstrated that HPIP is highly expressed in human HNSCC cell lines and provides the first evidence that knockdown of HPIP obviously inhibits proliferation and migration/invasion in HNSCC cells in vitro, as well as inhibits tumor growth in vivo. Furthermore, knockdown of HPIP significantly inhibits the expression of p-PI3K and p-Akt in human HNSCC cells. In conclusion, our study demonstrated that knockdown of HPIP significantly inhibits the proliferation and migration/invasion of HNSCC cells by suppressing the PI3K/Akt signaling pathway. Therefore, HPIP may be a novel potential therapeutic target for the treatment of HNSCC.
Key words: Hematopoietic pre-B-cell leukemia transcription factor (PBX)-interacting protein (HPIP), Head-and-neck squamous cell carcinoma (HNSCC), Proliferation, Invasion
INTRODUCTION
Head-and-neck squamous cell carcinoma (HNSCC) refers to a large heterogeneous group of carcinomas of the face, nasopharynx, oral cavity, oropharynx, hypopharynx, and larynx (1). It is the sixth most common cancer with about 500,000 new HNSCC cases being reported annually in the world. Furthermore, the prevalence of HNSCC is increasing (2,3). Currently, treatments for HNSCC are mainly surgery, radiotherapy, and adjuvant chemotherapy (4,5). Despite the advances in combined therapy over the past several decades, the overall 5-year survival rate for patients with HNSCC is only 30–35% (6). This is most likely due to the aggressive metastasis or postsurgical recurrence of HNSCC. Therefore, elucidation of the molecular mechanisms underlying HNSCC tumorigenesis and progression is critical to individual treatment of HNSCC.
Hematopoietic pre-B-cell leukemia transcription factor (PBX)-interacting protein (HPIP/PBXIP1) is a corepressor for the transcription factor PBX (7). It has been demonstrated that HPIP is expressed in early hematopoietic precursors and plays an important role in erythroid differentiation (8). Overexpression of HPIP in human CD34+ cells enhances hematopoietic colony formation in vitro, whereas HPIP knockdown leads to a reduction in the number of such colonies (9). Moreover, it was reported that HPIP is frequently overexpressed in many tumors, including gastric cancer, colorectal cancer, breast infiltrative ductal carcinoma, and astrocytoma. HPIP regulates tumor cell proliferation and invasion (10–12). However, the role of HPIP in HNSCC has not yet been determined. Thus, we decided to investigate the effects and mechanisms of HPIP in HNSCC.
MATERIALS AND METHODS
Cell Culture
Four human HNSCC cell lines (OSC-20, SNU-1076, HSC-3, and Ca9-22) and normal human esophageal squamous cell line (HEEC) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All of the cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Cells were cultured in a 5% CO2 humidified atmosphere at 37°C.
RNA Interference and Transfection
Small interference RNA (siRNA) targeting HPIP and its negative control (mock) used in knockdown experiments were synthesized by RiBo Biotech (GuangZhou RiBo Biotech). Briefly, OSC-20 and HSC-3 cells were transfected with siRNA-HPIP or mock using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), respectively, according to the manufacturer’s instructions.
RNA Preparation and RT-PCR
RNA was isolated using RNAspin Mini kit (GE Healthcare, Milwaukee, WI, USA) according to the manufacturer’s instructions. RT-PCR was conducted using 5 µg of total cellular RNA and the Superscript cDNA Pre-amplification System (Clontech, Palo Alto, CA, USA) in a Perkin-Elmer amplification cycler (Weiterstadt, Germany). The primers for RT-PCR were the following: HPIP forward 5′-GTCCCCTCGAGGAGTTGTGT-3′ and reverse 5′-ATCTTCCATCATCTGAGGGC-3′; β-actin forward 5′-TCA TGA AGT GTG ACG TTG ACA TCC GT-3′ and reverse 5′-CCT AGA AGC ATT TGC GGT GCA CGA TG-3′. Data were analyzed using the formula: R = 2−(ΔCT sample−ΔCT control).
Western Blot Analysis
The proteins were extracted using RIPA lysis buffer (Beyotime, Nantong, China), and the protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). Fifty micrograms of protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred onto polyvinylidene difluoride membranes (Immobilon P; Millipore, Bedford, MA, USA). Blots were then incubated with HPIP, PI3K, p-PI3K, Akt, and p-Akt antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4°C. HRP-conjugated goat anti-mouse IgG was used as the secondary antibody. The target protein was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Cell Proliferation Assay
Cell proliferation was measured by a cell counting kit-8 assay (CCK-8; Dojindo, Kumamoto, Japan). In brief, OSC-20 and HSC-3 cells (1 × 104 cells/well) were seeded in 96-well plates and transfected with siRNA-HPIP or mock, respectively. After transfection for 24, 48, 72, and 96 h, the CCK-8 reagents were added and incubated with the cells for 1 h. The absorbance at 450 nm was measured by an enzyme-linked immunosorbent assay plate reader.
In Vitro Migration and Invasion Assays
Cell invasion assays were performed using Transwell™ chambers (Costar, Boston, MA, USA). Briefly, cells were transfected with siRNA-HPIP or mock at a density of 5 × 104 cells/well, plated in the top chamber of the insert precoated with Matrigel (8-mm pore size; BD Biosciences). A medium containing 10% FBS as a chemoattractant was added to the lower chamber. After 24 h of incubation, cells on the upper surface of the filters were scraped off with swabs. Invaded cells to the lower surface of the filters were washed, fixed, stained with Giemsa, and counted under a microscope (magnification 100×). To assess migration, cell migration assays were performed under the same conditions as the Transwell invasion assays without Matrigel-coated Transwell chambers.
Xenograft Tumor Assay
For tumor cell implantation, HSC-3 cells (1 × 106 cells/mouse) expressing siRNA-HPIP or negative control were injected subcutaneously into the left flank of nude mice (n = 5 per group). Tumors were examined every week; length, width, and thickness measurements were obtained with calipers, and tumor volumes were calculated. At 5 weeks after injection, the animals were euthanized, and the tumors were weighed. This study was performed with approval from the Animal Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (China).
Statistical Analysis
Data were expressed as the mean ± SD. Statistical significance was analyzed with one-way factorial ANOVA or Student’s two-tailed t-test. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 15.0 software.
RESULTS
HPIP Is Upregulated in Human HNSCC Cell Lines
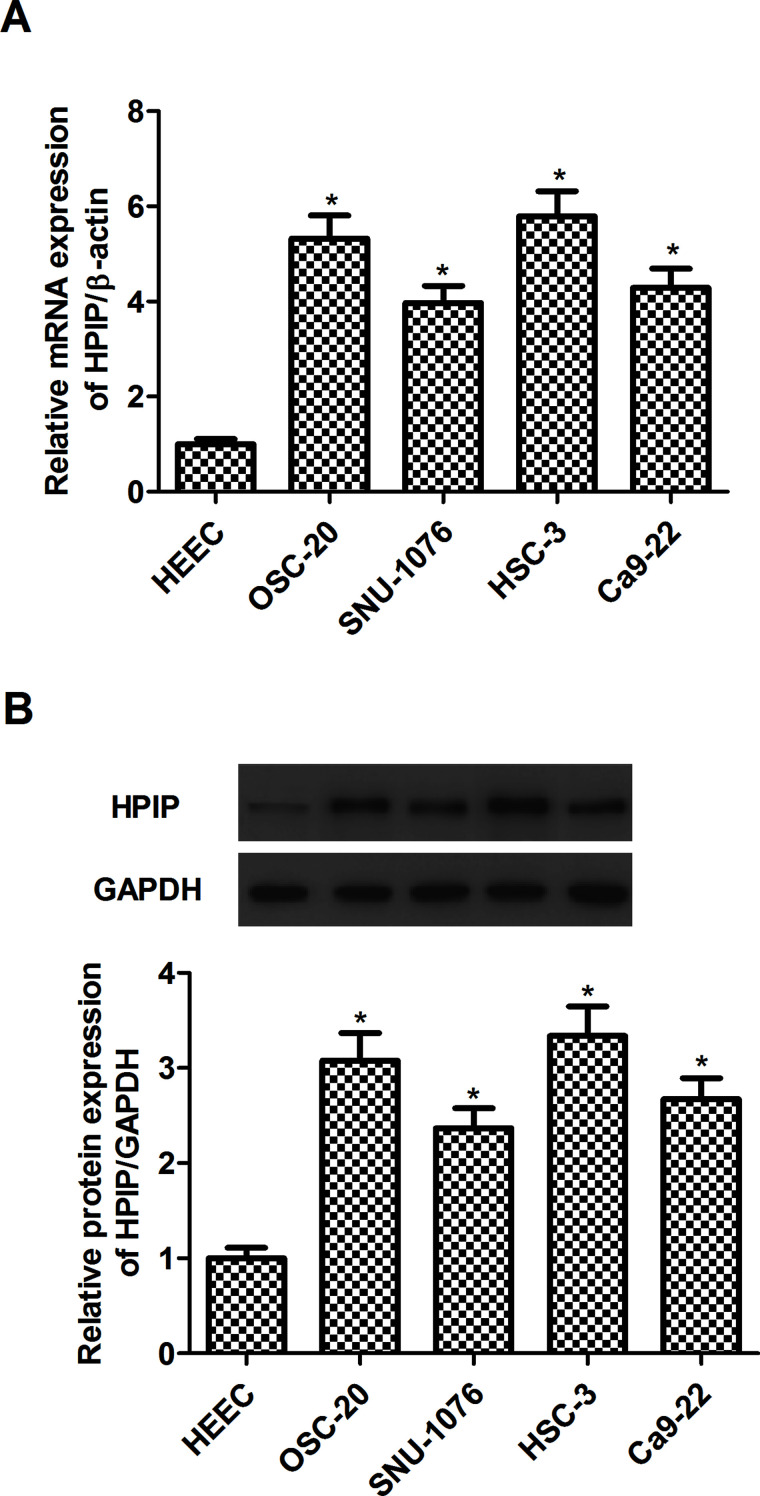
First we evaluated the expression of HPIP in human HNSCC cell lines (OSC-20, SNU-1076, HSC-3, and Ca9-22) by RT-qPCR and Western blot. The results of RT-qPCR analysis indicated that the expression of HPIP gene was significantly upregulated in four human HNSCC cell lines, compared with the HEEC group (Fig. 1A). Similarly, Western blot analysis demonstrated that HPIP protein was also increased in human HNSCC cell lines (Fig. 1B).
Figure 1.
HPIP is upregulated in human HNSCC cell lines. (A) RT-qPCR analysis of HPIP expression in HEEC and four human HNSCC cell lines. (B) Western blot analysis of HPIP expression in HEEC and four human HNSCC cell lines. GAPDH was used as a loading control. The results are expressed as mean ± SD and n = 3 per group. *p < 0.05 versus HEEC group.
HPIP Silencing Inhibits HNSCC Cell Proliferation
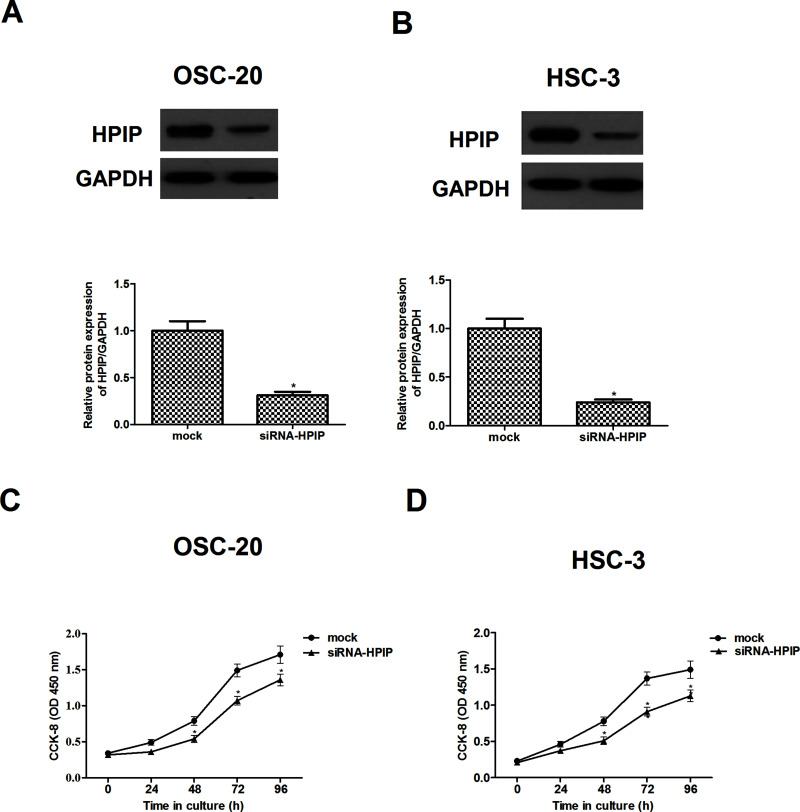
To further investigate the biological role of HPIP in the progression of HNSCC, HPIP was knocked down with specific siRNA vector against HPIP in OSC-20 and HSC-3 cell lines whose expression of HPIP was higher among the four HNSCC cell lines involved. As indicated in Figure 2A and B, knockdown of HPIP significantly decreased the expression of HPIP protein in OSC-20 HSC-3 cells, respectively. Next we examined the effects of HPIP on the proliferation of HNSCC cells. The results of CCK-8 assay showed that compared to the mock group, knockdown of HPIP greatly inhibited the proliferation in OSC-20 (Fig. 2C) and HSC-3 cells (Fig. 2D), exhibiting a time-dependent manner.
Figure 2.
HPIP silencing inhibits HNSCC cell proliferation. Western blot analysis of HPIP expression in HPIP-silenced OSC-20 (A) and HSC-3 cells (B). GAPDH was used as a loading control. CCK-8 assays indicated that the proliferation was decreased in HPIP-silenced OSC-20 (C) and HSC-3 cells (D), respectively. The results are expressed as mean ± SD and n = 3 per group. *p < 0.05 versus mock group.
HPIP Silencing Inhibits HNSCC Cell Invasion and Migration
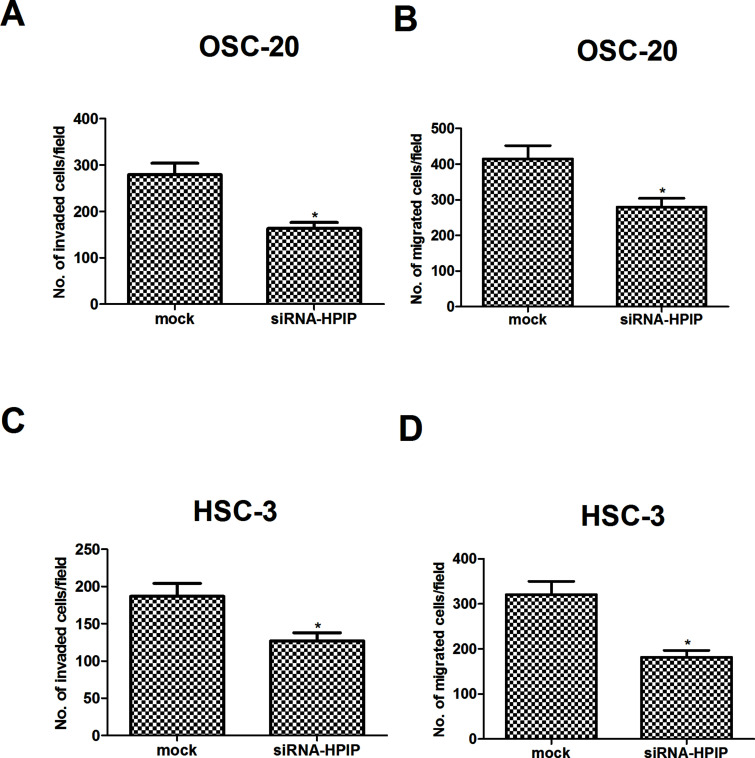
To explore the effect of HPIP gene downregulation on tumor cell invasion and migration, we performed Transwell assays in cultured NSCLC cells. Invasion assay using Matrigel-coated Transwell invasion chambers revealed that downregulation of HPIP inhibits the invasive activity of OSC-20 cells, compared with the mock group (Fig. 3A). In addition, OSC-20 cells transfected with siRNA-HPIP had a slower migrating rate compared with the mock cells (Fig. 3B). Similarly, knockdown of HPIP also obviously inhibited the invasion and migration of HSC-3 cells, respectively (Fig. 3C and D).
Figure 3.
HPIP silencing inhibits HNSCC cell invasion and migration. Cell invasion was assessed by the Matrigel invasion chamber in HPIP-silenced OSC-20 (A) and HSC-3 cells (C), respectively. Cell migration was assessed by the Transwell chamber in HPIP-silenced OSC-20 (B) and HSC-3 cells (D), respectively. The results are expressed as mean ± SD and n = 3 per group. *p < 0.05 versus mock group.
HPIP Silencing Inhibits the Activation of PI3K/Akt Signaling Pathway
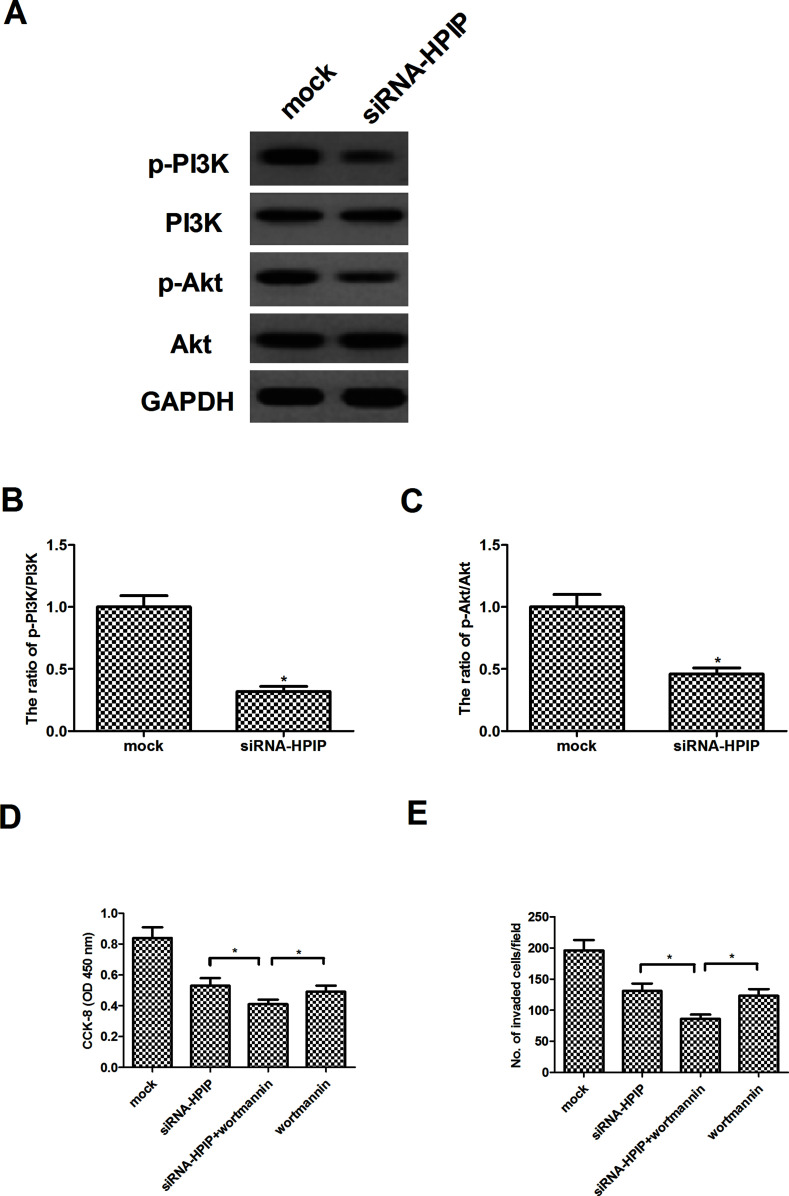
PI3K/Akt signaling pathway plays an important role in the development of tumors. Therefore, we detected the protein levels of PI3K, p-PI3K, Akt, and p-Akt in HSC-3 cells after knockdown of HPIP by siRNA. Western blot analysis demonstrated that no significant difference was observed in the expression of PI3K and Akt after siRNA-HPIP. However, knockdown of HPIP greatly decreased the phosphorylation of p-PI3K and p-Akt in HSC-3 cells (Fig. 4A). Quantification of p-PI3K/PI3K and p-Akt/Akt is also shown in Figure 4B and C, respectively. Furthermore, we examined the effects of Akt inhibitor (wortmannin) on HPIP-mediated HNSCC cell proliferation and invasiveness. As shown in Figure 4D, the cell proliferation analysis results demonstrated a dramatic enhancement of siRNA-HPIP-inhibited HSC-3 cell proliferation by wortmannin. Similarly, wortmannin also remarkably enhanced the inhibitory effect of siRNA-HPIP on cell invasion in HSC-3 cells (Fig. 4E).
Figure 4.
HPIP silencing inhibits the activation of PI3K/Akt signaling pathway in HNSCC cells. HSC-3 cells were transfected with siRNA-HPIP or mock for 24 h. (A) The levels of phosphorylated PI3K, total PI3K, phosphorylated Akt, and total Akt were detected by Western blot analysis. Quantification of (B) p-PI3K/PI3K and (C) p-Akt/Akt. *p < 0.05 versus mock group. (D) HSC-3 cells were transfected with siRNA-HPIP or mock in the presence or absence of the wortmannin (100 nM) for 24 h. Cell proliferation was detected by the CCK-8 assay. (E) Cell invasion was evaluated by the Transwell invasion chamber assay. The results are expressed as mean ± SD and n = 3 per group. *p < 0.05.
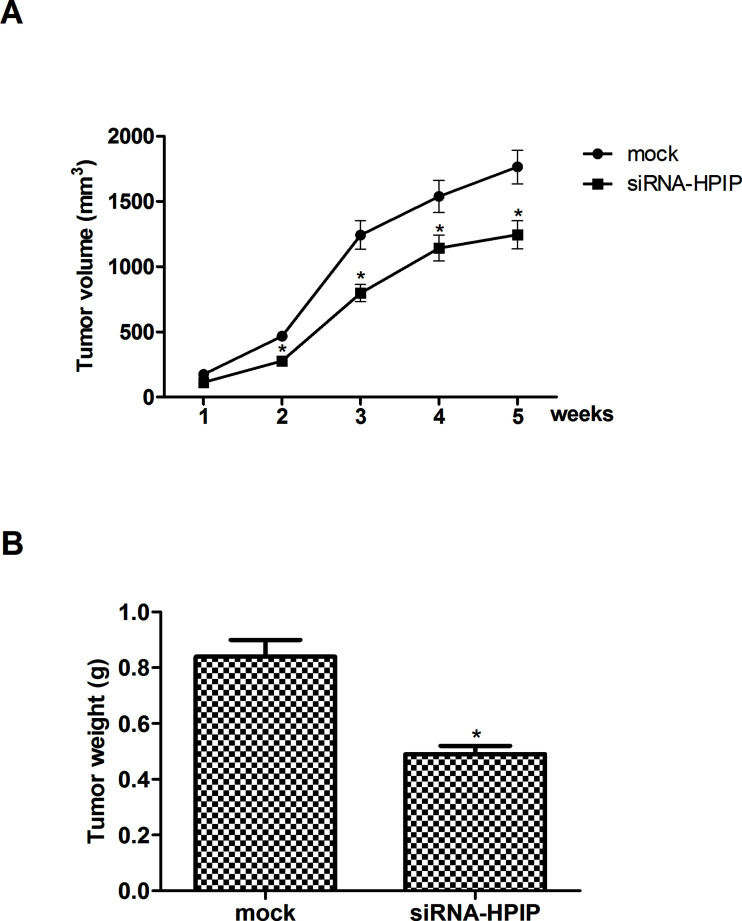
HPIP Silencing Inhibits Tumor Growth in an Allograft Murine Model
For further investigation of role of HPIP in the progression of HNSCC, we injected HSC-3 cells transfected with siRNA-HPIP into the left flank of nude mice. The results showed that tumors formed by HPIP-silenced cells were smaller, in both size (Fig. 5A) and weight (Fig. 5B), than the tumors formed from mock cells.
Figure 5.
HPIP silencing inhibits tumor growth in an allograft murine model. HSC-3 cells (1 × 106 cells/mouse) expressing siRNA-HPIP or negative control were injected subcutaneously into the left flank of nude mice. (A) The tumor volumes were calculated in each group every 1 week from week 1 to week 5. (B) Knockdown of HPIP significantly inhibited tumor weight of BALB/c nude mice. The results are expressed as mean ± SD and n = 3 per group. *p < 0.05 versus mock group.
DISCUSSION
Tumorigenesis is a complex multistep process and is associated with various alterations in genes or proteins related to regulation of cell proliferation, invasion, and death (13–15). Therefore, identification of the genes that lead to tumorigenesis is critical for providing potential therapeutic targets. To the best of our knowledge, this is the first study to investigate the expression and role of HPIP in human HNSCC. Our results demonstrated that HPIP is highly expressed in human HNSCC cell lines and provides the first evidence that knockdown of HPIP obviously inhibits proliferation, migration, and invasion in HNSCC cells in vitro, as well as inhibits tumor growth in vivo. Furthermore, knockdown of HPIP inhibits the expression of p-PI3K and p-Akt in human HNSCC cells.
Previous studies have demonstrated that upregulated expression of HPIP was implicated in tumorigenesis. A previous study by Wang et al. reported that the expression of HPIP is upregulated in thyroid carcinoma cell lines (16). It also has been shown that HIPIP is highly expressed in non-small cell lung cancer cell lines (17). Consistent with these results, in this study we found that HPIP is highly expressed in human HNSCC cell lines. Several reports have also provided evidence that HPIP is involved in the proliferation of malignant phenotypes of cancer cells and the development of cancer (10,17,18). Similarly, herein we observed that silencing HPIP reduced the tumorigenicity of HNSCC cells both in vitro and in vivo, implicating that HPIP may function as an oncogenic protein in the development and progression of HNSCC.
Cancer cell migration and invasion are the critical steps for cancer progression and metastasis (5,19). Feng et al. confirmed that overexpression of HPIP increased migration and invasion ability in gastric cancer cells, while HPIP knockdown inhibited gastric cancer cell migration and invasion (18). Another study reported that HPIP promotes colorectal cancer cell migration and invasion and regulates epithelial–mesenchymal transition (EMT) (11). In agreement with these reports, our data showed that knockdown of HPIP obviously inhibited the migration and invasion of HNSCC cells, suggesting that HPIP plays an important role in promoting HNSCC metastasis.
Several lines of evidence have suggested that the PI3K/Akt signaling pathway plays an important role in regulating the proliferation, migration, and invasion of HNSCC cells (20–22). PI3K has been shown to be highly activated in a majority of HNSCC samples. Activated PI3K promoted HNSCC cell growth and survival (23–25), whereas a selective PI3K inhibitor attenuated Akt phosphorylation, cell growth, and angiogenesis factor expression in HNSCC cells (4,26). Akt, a serine/threonine kinase, is a central mediator of the PI3K pathway, and activation of Akt further phosphorylates various proteins that regulate multiple cellular responses, including cell proliferation, metastasis, and apoptosis. Targeting Akt by MK-2206 was found to potently attenuate growth and migration of HNSCC lines in vitro, and tumorigenesis and metastasis in an orthotopic HNSCC model in vivo (27). Therefore, the suppression of PI3K/Akt signaling may be a suitable approach for reducing tumor aggressiveness in HNSCC. In this study, we found that knockdown of HPIP significantly inhibits the phosphorylation of PI3K and Akt in HSC-3 cells. These results strongly suggest that knockdown of HPIP inhibits the proliferation and migration/invasion of HNSCC cells by suppressing the PI3K/Akt signaling pathway.
In conclusion, our study demonstrated that knockdown of HPIP significantly inhibits the proliferation, migration, and invasion of HNSCC cells by suppressing the PI3K/Akt signaling pathway. Our findings may be helpful for the development of novel potential therapeutic target for the treatment of HNSCC.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Mao L.; Hong W. K.; Papadimitrakopoulou V. A. Focus on head and neck cancer. Cancer Cell 5:311–316; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Sankaranarayanan R.; Masuyer E.; Swaminathan R.; Ferlay J.; Whelan S. Head and neck cancer: A global perspective on epidemiology and prognosis. Anticancer Res. 18:4779–4786; 1997. [PubMed] [Google Scholar]
- 3. Forastiere A.; Koch W.; Trotti A.; Sidransky D. Head and neck cancer. N. Engl. J. Med. 345:1890–1900; 2001. [DOI] [PubMed] [Google Scholar]
- 4. Koyfam S. A.; Joshi N.; Vidimos A. Adjuvant radiotherapy in high-risk cutaneous squamous cell cancer of the head and neck in immunosuppressed patients. JAAD Case Rep. 1:S5–S7; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marur S.; Forastiere A. A. Head and neck squanous cell carcinoma: Update on epidemiology, diagnosis and treatment. Mayo Clin. Proc. 91:386–396; 2016. [DOI] [PubMed] [Google Scholar]
- 6. Bauman J. E.; Michel L. S.; Chung C. H. New promising molecular targets in head and neck squamous cell carcinoma. Curr. Opin. Oncol. 24:235–242; 2012. [DOI] [PubMed] [Google Scholar]
- 7. Abramovich C.; Chavez E. A.; Lansdorp P. M.; Humphries R. K. Functional characterization of multiple domains involved in the subcellular localization of the hematopoietic Pbx interacting protein (HPIP). Oncogene 21:6766–6771; 2002. [DOI] [PubMed] [Google Scholar]
- 8. Abramovich C.; Shen W. F.; Pineault N.; Imren S.; Montpetit B.; Largman C.; Humphries R. K. Functional cloning and characterization of a novel nonhomeodomain protein that inhibits the binding of PBX1-HOX complexes to DNA. J. Biol. Chem. 275:26172–26177; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Manavathi B.; Lo D.; Bugide S.; Dey O.; Imren S.; Weiss M. J.; Humphries R. K. Functional regulation of pre-B-cell leukemia homeobox interacting protein 1 (PBXIP1/HPIP) in erythroid differentiation. J. Biol. Chem. 287:5600–5614; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X.; Jiang C.; Wang S.; Tai Y.; Wang T.; Kang L.; Fan Z.; Li S.; Li L.; Fu J. HPIP is upregulated in liver cancer and promotes hepatoma cell proliferation via activation of G2/M transition. IUBMB Life. 65:873–882; 2013. [DOI] [PubMed] [Google Scholar]
- 11. Feng Y.; Xu X.; Zhang Y.; Ding J.; Wang Y.; Zhang X.; Wu Z.; Kang L.; Liang Y.; Zhou L. HPIP is upregulated in colorectal cancer and regulates colorectal cancer cell proliferation, apoptosis and invasion. Sci. Rep. 5:9429; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X.; Fan Z.; Kang L.; Han J.; Jiang C.; Zheng X.; Zhu Z.; Jiao H.; Lin J.; Jiang K. Hepatitis B virus x protein represses miRNA-148a to enhance tumorigenesis. J. Clin. Invest. 123:630–645; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baylin S. B.; Herman J. G. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 16:168–174; 2000. [DOI] [PubMed] [Google Scholar]
- 14. Cross M.; Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell 64:271–280; 1991. [DOI] [PubMed] [Google Scholar]
- 15. Hwang H.; Mendell J. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 94:776–780; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang S. C.; Chai D. S.; Chen C. B.; Wang Z. Y.; Wang L. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/AKT signaling pathway. Biomed. Pharmacother. 75:33–39; 2015. [DOI] [PubMed] [Google Scholar]
- 17. Pan J.; Qin Y.; Zhang M. HPIP promotes non-small cell lung cancer cell proliferation, migration and invasion through regulation of the sonic hedgehog signaling pathway. Biomed. Pharmacother. 77:176–181; 2016. [DOI] [PubMed] [Google Scholar]
- 18. Feng Y.; Li L.; Zhang X.; Zhang Y.; Liang Y.; Lv J.; Fan Z.; Guo J.; Hong T.; Ji B. Hematopoietic pre-B cell leukemia transcription factor interacting protein is overexpressed in gastric cancer and promotes gastric cancer cell proliferation, migration, and invasion. Cancer Sci. 106:1313–1322; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedl P.; Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 3:362–374; 2003. [DOI] [PubMed] [Google Scholar]
- 20. Vander Broek R.; Mohan S.; Eytan D.; Chen Z.; Van Waes C. The PI3K/AKT/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 21:815–825; 2015. [DOI] [PubMed] [Google Scholar]
- 21. Wongkajornsilp A.; Rhys-Evans P. H.; Eccles S. A. Signaling pathways required for matrix metalloproteinase-9 induction by betacellulin in head-and-neck squamous carcinoma cells. Int. J. Cancer 111:174–183; 2004. [DOI] [PubMed] [Google Scholar]
- 22. Bian Y.; Terse A.; Du J.; Hall B.; Molinolo A.; Zhang P.; Chen W.; Flanders K. C.; Gutkind J. S.; Wakefield L. M. Progressive tumor formation in mice with conditional deletion of TGF-β signaling in head and neck epithelia is associated with activation of the PI3K/AKT pathway. Cancer Res. 69:5918–5926; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothenberg S. M.; Ellisen L. W. The molecular pathogenesis of head and neck squamous cell carcinoma. J. Clin. Invest. 122:1951–1957; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murugan A. K.; Hong N. T.; Fukui Y.; Munirajan A. K.; Tsuchida N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int. J. Oncol. 32:101–111; 2008. [PubMed] [Google Scholar]
- 25. Morris L. G.; Taylor B. S.; Bivona T. G.; Gong Y.; Eng S.; Brennan C. W.; Kaufman A.; Kastenhuber E. R.; Banuchi V. E.; Singh B. Genomic dissection of the epidermal growth factor receptor (EGFR)/PI3K pathway reveals frequent deletion of the EGFR phosphatase PTPRS in head and neck cancers. Proc. Natl. Acad. Sci. USA 108:19024–19029; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bancroft C. C.; Chen Z.; Yeh J.; Sunwoo J. B.; Yeh N. T.; Jackson S.; Jackson C.; Van Waes C. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int. J. Cancer 99:538–548; 2002. [DOI] [PubMed] [Google Scholar]
- 27. Knowles J. A.; Golden B.; Yan L.; Carroll W. R.; Helman E. E.; Rosenthal E. L. Disruption of the akt pathway inhibits metastasis in an orthotopic model of head and neck squamous cell carcinoma. Laryngoscope 121:2359–2365; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]