Abstract
Collagen triple helix repeat containing-1 (CTHRC1), a secreted glycoprotein, is frequently upregulated in human cancers. However, the functional role of CTHRC1 in renal cell carcinoma (RCC) remains unclear. Thus, the aim of this study was to explore the role of CTHRC1 in RCC. Our results demonstrated that CTHRC1 was upregulated in RCC tissues and cell lines. Knockdown of CTHRC1 significantly inhibits the proliferation in RCCs. Furthermore, knockdown of CTHRC1 significantly inhibited the epithelial-to-mesenchymal transition (EMT) process in RCCs, as well as suppressed RCC cell migration and invasion. Mechanistically, knockdown of CTHRC1 inhibited the expression of β-catenin, c-Myc, and cyclin D1 in RCC cells. In conclusion, the results of the present study indicated that CTHRC1 downregulation inhibited proliferation, migration, EMT, and β-catenin expression in RCC cells. Therefore, CTHRC1 may be a potential therapeutic target for the treatment of RCC.
Key words: Collagen triple helix repeat containing-1 (CTHRC1), Renal cell carcinoma (RCC), Invasion, Wnt/β-catenin signaling
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of malignant tumor of the adult kidney in the world. The incidence of RCC is increasing steadily by a rate of approximately 2.5% each year (1). Although a multidisciplinary approach to treating RCC is evolving, the prognosis of RCC is still very poor (2–4). The prognosis of RCC is highly associated with the progression of localized primary tumors to advanced stages, which ultimately metastasize to multiple organs (5). Therefore, dissecting the molecular mechanism of RCC invasion and metastasis is crucial for developing novel and more effective therapeutic approaches.
Epithelial-to-mesenchymal transition (EMT) is a critical step in the development and progression of tumors. It is defined by the loss of epithelial characteristics and the acquisition of a motile, invasive, and migratory mesenchymal phenotype (6). Increasing lines of evidence suggest that EMT has an important role during RCC invasion and metastasis (7–9).
Collagen triple helix repeat containing-1 (CTHRC1), a secreted protein, was first identified in a screen for differentially expressed sequences in balloon-injured versus normal rat arteries (10). There is substantial evidence that CTHRC1 was implicated in vascular remodeling, bone formation, and developmental morphogenesis (11–13). In addition, CTHRC1 is highly expressed in most human solid tumors (14–16). Hou et al. reported that CTHRC1 expression was upregulated in paraffin-embedded epithelial ovarian cancer tissues, and ectopic transfection of CTHRC1 promoted the metastasis through induction of the EMT process in epithelial ovarian cancer (17). However, the functional role of CTHRC1 in RCC remains unclear. Thus, the aim of this study was to explore the role of CTHRC1 in RCC. Our results showed that CTHRC1 is highly expressed in human RCC tissues and plays a role in the progression and metastasis of RCC.
MATERIALS AND METHODS
Tissue Collection
Human RCC tissues and their corresponding adjacent nontumor renal tissues were obtained from the China–Japan Union Hospital of Jilin University (P.R. China). The samples were immediately stored in liquid nitrogen in preparation for use. Informed consent was obtained from all patients, and the work was approved by the Medical Ethics Committee of the China–Japan Union Hospital of Jilin University.
Cell Culture
Human RCC cell lines (786-O, Caki-1, A498, and UMRC-3) and immortalized proximal tubule epithelial cell line (HK-2) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and 1% (v/v) penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified atmosphere containing 5% CO2.
RNA Interference and Transfection
CTHRC1 siRNA was constructed by Genechem Company (Shanghai, P.R. China) as follows: 5′-GAAATGAATTCAACAATTA-3′. For in vitro transfection, 5 × 104 cells were seeded in each well of a 24-well plate, grown for 24 h to reach 30%–50% confluence, and then transfected with si-CTHRC1 or vector using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
RNA Extraction and Quantitative Real Time (qRT)-PCR
Total RNA was extracted from human RCC tissues and cells using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). First-strand cDNA was synthesized from 5 μg of total RNA using SuperScript II Reverse Transcriptase (Invitrogen). The following primers were used: CTHRC1, 5′-TGGTATTTCACATTCAATGGAGCTG-3′ (sense) and 5′-TGGGTAATCTGAACAAGTGCCAAC-3′ (antisense); β-actin, 5′-CCGTGAAAAGATGACCCAGATC-3′ (sense) and 5′-CACAGCCTGGATGGCTACGT-3′ (antisense). Relative gene expression was quantified by the 2−ΔΔCt method.
Protein Extraction and Western Blot Analysis
RCC cells were lysed in lysis buffer containing 1% NP40, 1 mM EDTA, 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, and complete protease inhibitors cocktail (Roche, Monza, Italy). Protein concentrations of the lysates were determined by the BCA kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein (30 μg of protein each lane) were separated by 10% polyacrylamide-SDS gel electrophoresis and transferred to nitrocellulose membranes (GE Healthcare, Milano, Italy) by electroblotting. Membranes were blocked with 10% defatted milk in PBS at 4°C overnight and then incubated with the primary antibodies overnight at 4°C. The antibodies used were as follows: anti-CTHRC1, anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-β-catenin, anti-c-Myc, anti-cyclin D1, and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequently, the blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) diluted (1:1,500) in the blocking buffer for 1 h at room temperature. Proteins were visualized using the enhanced ECL detection system (Thermo Fisher Scientific, Rockford, IL, USA).
Cell Proliferation Assay
For the MTT assay, RCC cells (1 × 105 cells per well) transfected with si-CTHRC1 or vector were seeded in a 96-well plate and then cultured at 24-h intervals for 4 days. The initial culture medium was replaced with fresh medium containing MTT (5 mg/ml; Sigma-Aldrich) and incubated for an additional 4 h. The supernatant was removed, and the crystals were dissolved in dimethyl sulfoxide (150 μl/well; Sigma-Aldrich). After shaking at room temperature for 10 min, the absorbance at 570 nm was measured with a microplate reader (Takara Biotechnology, Dalian, P.R. China).
Migration and Invasion Assays
Transwell migration assays were performed in modified Boyden chambers with 8-μm pore filter inserts in 24-well plates (BD Biosciences, Eugene, OR, USA). In brief, RCC cells (1 × 105 cells per well) transfected with si-CTHRC1 or vector were added to the upper chamber. The lower chamber was filled with DMEM containing 10% FBS. The cells were then incubated at 37°C in a humidified incubator in 5% CO2 for 24 h. Nonmigrating cells on the upper side of the filter were wiped off using a cotton swab. Migrating cells on the reverse side of the filter were fixed with 4% formaldehyde in PBS and were stained with 1% crystal violet in 20% methanol, and then counted in five random fields per well under a light microscope (magnification : 100×).
Cell invasion was carried out using Transwell chambers in the presence of Matrigel (BD Biosciences). The chambers were pretreated with a cold solution containing serum-free medium and Matrigel (diluted 1:4) to produce a biologically active matrix. Then the assay was performed as the migratory assay.
Statistical Analysis
Statistical analyses were done using SPSS 13.0 for Windows (SPSS, Chicago, IL, USA). The statistical significance of the difference was analyzed by ANOVA and post hoc Dunnett’s test. A value of p < 0.05 was considered statistically significant.
RESULTS
CTHRC1 Is Upregulated in Human RCC Tissues and Cell Lines
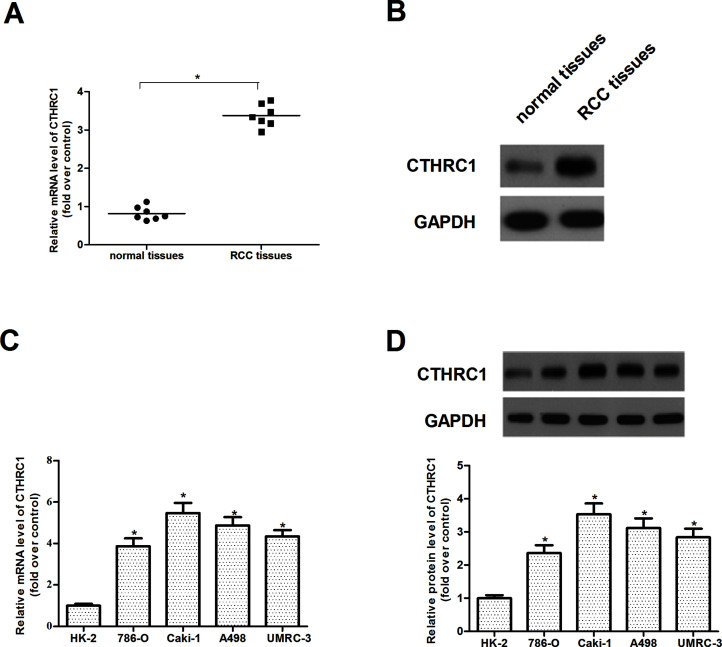
First, we used qRT-PCR to detect the CTHRC1 mRNA level in seven primary RCC tissues and seven nontumor normal tissues and found that the expression levels of CTHRC1 mRNA were significantly upregulated in RCC tissues compared to that in nontumor normal tissues (Fig. 1A). We further used Western blot to detect the expression of CTHRC1 and found a similar change with the CTHRC1 mRNA level (Fig. 1B). We observed that the expression levels of CTHRC1 for both mRNA (Fig. 1C) and protein (Fig. 1D) in four RCC cell lines (786-O, Caki-1, A498, and UMRC-3) were also increased compared to that in HK-2 cells.
Figure 1.
CTHRC1 is upregulated in human RCC tissues and cell lines. (A) qRT-PCR analysis of CTHRC1 mRNA expression in seven primary RCC tissues and seven nontumor normal tissues. (B) Western blot analysis of CTHRC1 protein expression in primary RCC tissues and nontumor normal tissues. GAPDH was used as a loading control. (C) qRT-PCR analysis shows that CTHRC1 mRNA expression is increased in RCC cell lines (786-O, Caki-1, A498, and UMRC-3) compared with the normal proximal tubule epithelial cell line (HK-2). (D) Western blot analysis shows that CTHRC1 protein expression is increased in RCC cell lines (786-O, Caki-1, A498, and UMRC-3) compared with the normal proximal tubule epithelial cell line (HK-2). All values shown are mean ± SD of three independent experiments. *p < 0.05 relative to relevant control.
CTHRC1 Downregulation Inhibits the Proliferation of RCC Cells
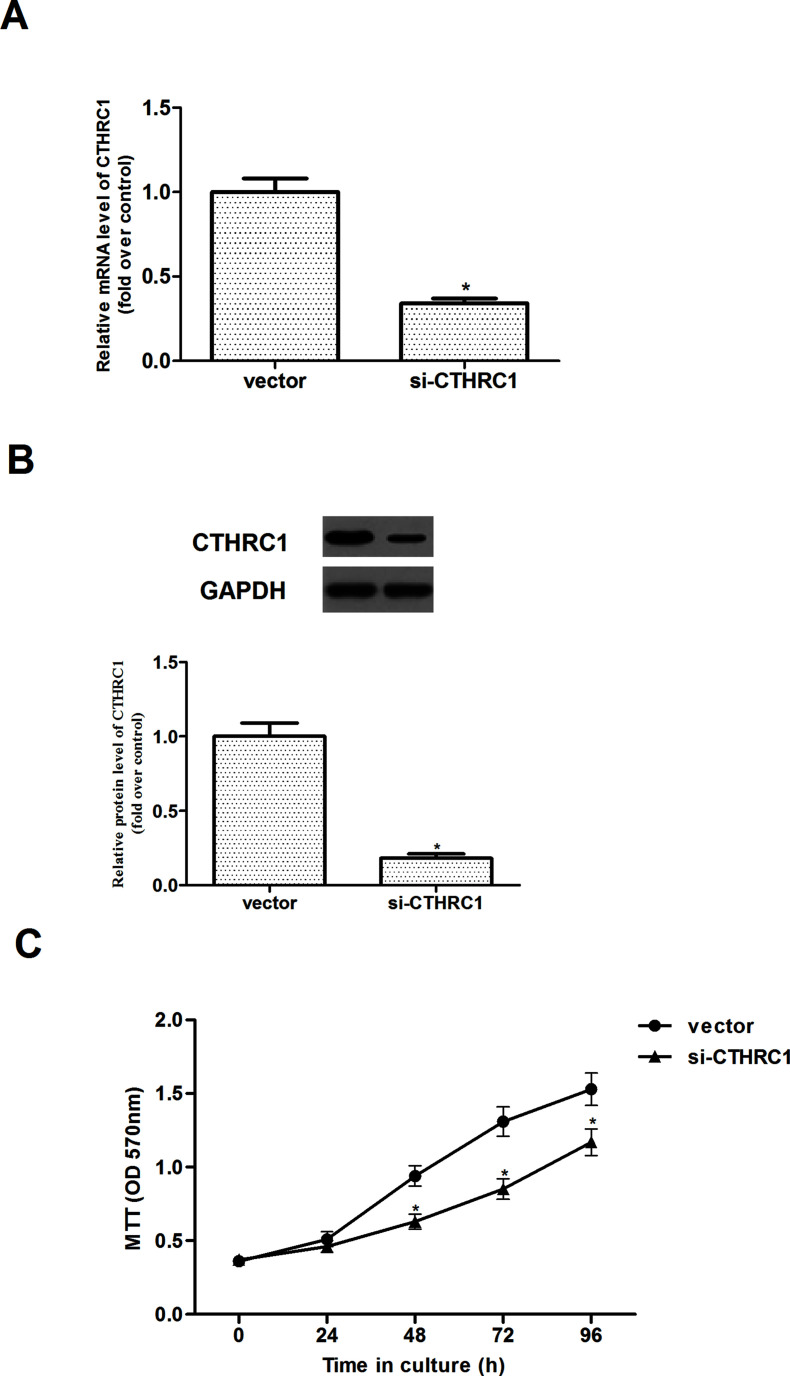
To generate stable CTHRC1 knockdown cells, Caki-1 cells were infected with si-CTHRC1. As shown in Figure 2A and B, transfection with the CTHRC1-specific siRNA, but not with vector, dramatically downregulated CTHRC1 expression in Caki-1 cells. Also, the effect of CTHRC1 on cell proliferation was observed. The results indicated that CTHRC1 downregulation significantly inhibited the proliferation of Caki-1 cells, in comparison to the vector (Fig. 2C).
Figure 2.
CTHRC1 downregulation inhibits the proliferation of RCC cells. (A, B) The expression levels of CTHRC1 expression for both mRNA and protein were detected in Caki-1 cells stably transfected with si-CTHRC1, respectively. (C) Effect of CTHRC1 knockdown on cell proliferation by MTT assay. All values shown are mean ± SD of three independent experiments. *p < 0.05 relative to relevant control.
CTHRC1 Downregulation Inhibits EMT in RCC Cells
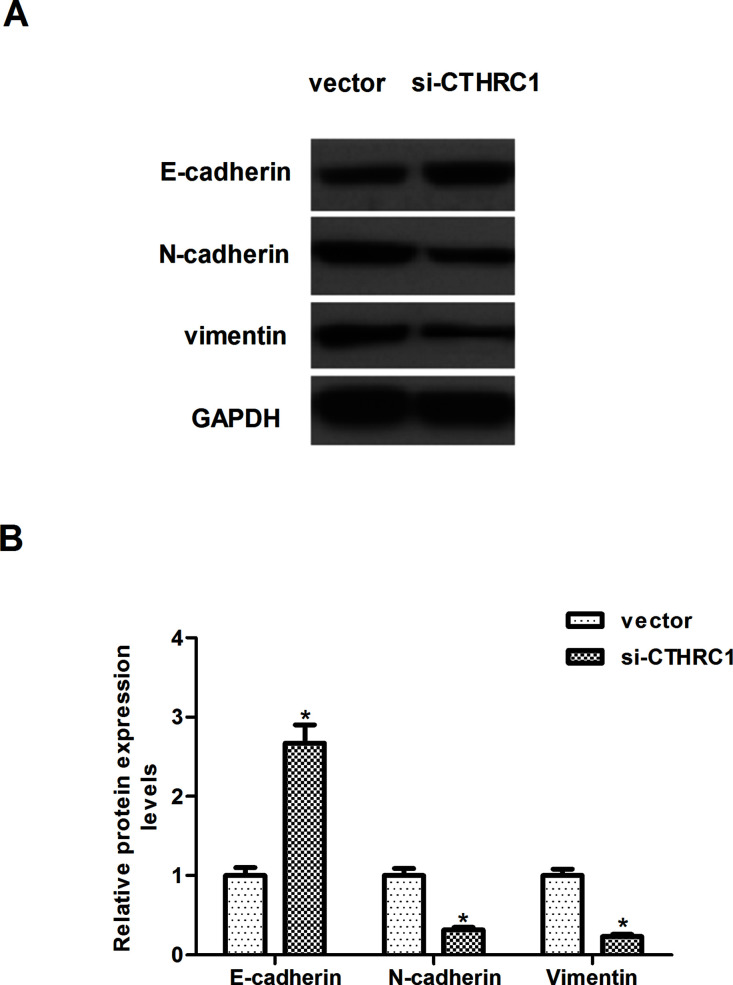
We then investigated the effect of CTHRC1 on EMT phenotype in RCC cells. Western blotting results showed knockdown of CTHRC1 markedly upregulated the expression of E-cadherin and downregulated the expression levels of N-cadherin and vimentin in Caki-1 cells, when compared with the cells transfected with vector (Fig. 3A). Quantification analysis of E-cadherin, N-cadherin, and vimentin is shown in Figure 3B.
Figure 3.
CTHRC1 downregulation inhibits EMT in RCC cells. Caki-1 cells were transfected with si-CTHRC1 or vector for 24 h. (A) Western blot analysis of E-cadherin, N-cadherin, and vimentin on the depletion of CTHRC1, respectively, in Caki-1 cells. GAPDH was used as a loading control. (B) Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. All values shown are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
CTHRC1 Downregulation Inhibits Migration and Invasion of RCC Cells
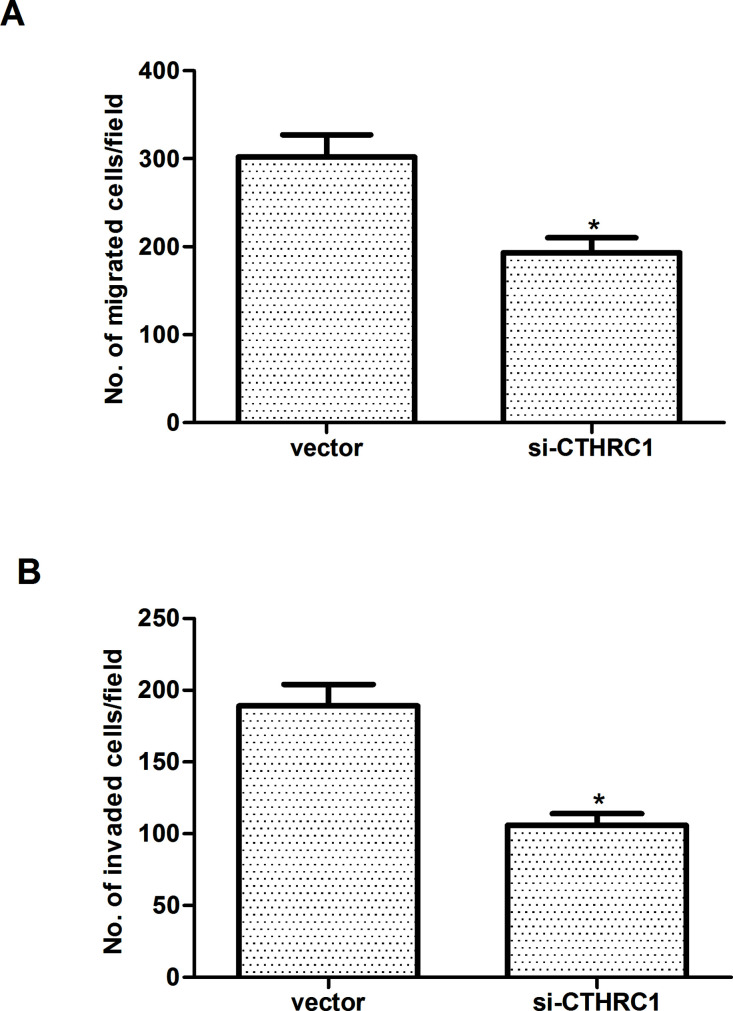
Previous studies have demonstrated that tumor cells show enhanced migration and invasion capabilities following EMT. Transwell migration assays indicated that the number of Caki-1 cells in the high-power lens of each field of vision in the si-CTRHC1-transfected group was less than that in the vector-transfected group (Fig. 4A). Invasion assay results demonstrated that the invasive capacity of Caki-1 cells was significantly suppressed by transfection with si-CTRHC1, when compared with the vector group (Fig. 4B).
Figure 4.
CTHRC1 downregulation inhibits migration and invasion of RCC cells. (A) Caki-1 cells were transfected with si-CTHRC1 or vector, and transfected cells were subject to Transwell cell migration assay and observed under a light microscope. (B) Knockdown of CTHRC1 suppressed the invasive capacity of Caki-1 cells. All values shown are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
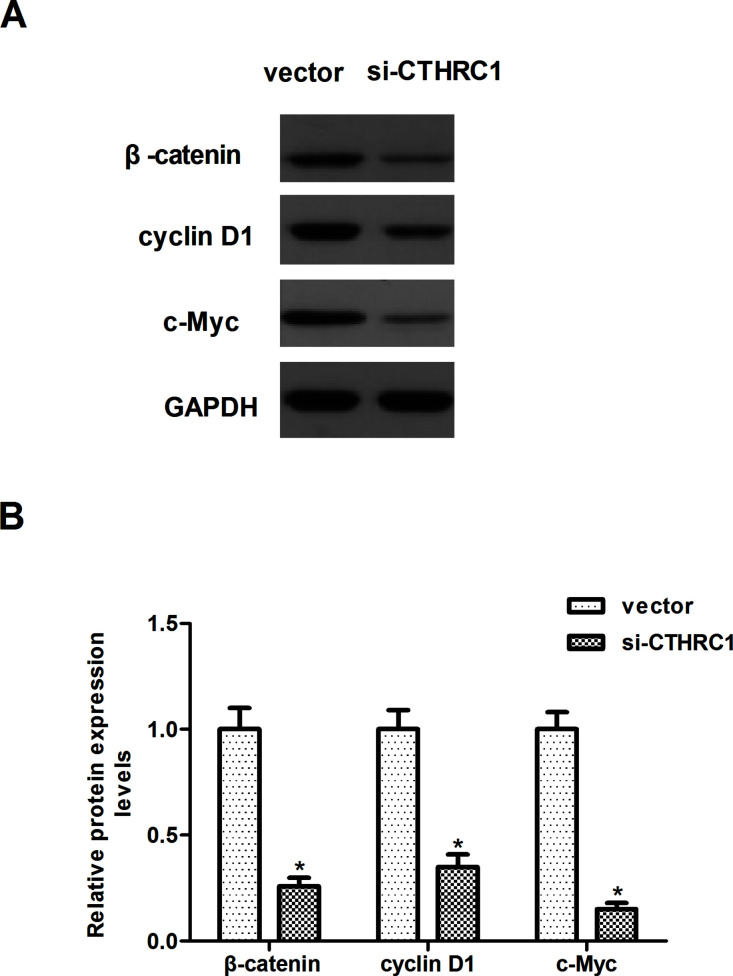
Knockdown of CTHRC1 Suppresses the Activation of Wnt/β-Catenin Signaling in RCC Cells
Recent studies have suggested that activation of Wnt/β-catenin signaling induces the EMT process. We were therefore interested in exploring the role of the Wnt/β-catenin signaling in CTHRC1-mediated EMT. As shown in Figure 5A, knockdown of CTHRC1 significantly decreased the expression of β-catenin, c-Myc, and cyclin D1 in Caki-1 cells, compared with the vector group. Quantification analysis of β-catenin, c-Myc, and cyclin D1 is shown in Figure 5B.
Figure 5.
Knockdown of CTHRC1 suppresses the activation of Wnt/β-catenin signaling in RCC cells. Caki-1 cells were transfected with si-CTHRC1 or vector for 24 h. (A) Western blot analysis of β-catenin, c-Myc, and cyclin D1 on the depletion of CTHRC1 in Caki-1 cells. GAPDH was used as a loading control. (B) Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. All values shown are mean ± SD of three independent experiments. *p < 0.05 versus vector group.
DISCUSSION
To our knowledge, this study is the first to describe the role of CTHRC1 in RCC. Our results demonstrated that CTHRC1 was upregulated in RCC tissues and cell lines. Knockdown of CTHRC1 significantly inhibits the proliferation in RCCs. Furthermore, knockdown of CTHRC1 significantly inhibited the EMT process in RCCs, as well as suppressed RCC cell migration and invasion. Mechanistically, knockdown of CTHRC1 inhibited the expression of β-catenin, c-Myc, and cyclin D1 in RCC cells.
CTHRC1 was reported to be amplified and overexpressed in many types of cancer. For example, Ke et al. confirmed that CTHRC1 was obviously overexpressed in human non-small cell lung cancer (NSCLC) tissues and NSCLC cell lines at both mRNA and protein levels; overexpression of CTHRC1 markedly promoted the proliferation in NSCLC cells, which was attenuated by the specific CTHRC1 siRNA (18). Another study showed that CTHRC1 was overexpressed in HCC tissues compared with the surrounding nontumor tissues, and CTHRC1 deletion caused reduced cell proliferation and motility in hepatocellular carcinoma (HCC) cells (19). Consistent with these discoveries, herein we observed that CTHRC1 expression is highly elevated in human RCC tissues and cell lines. Knockdown of CTHRC1 significantly inhibits the proliferation in RCCs. These results suggest that CTHRC1 may function as an oncogene in the development of RCC.
EMT is a vital reversible process by which fully differentiated cells lose their epithelial features and acquire a migratory mesenchymal phenotype. It contributes to the metastatic potential of RCC (20). Snail, a major regulator of EMT, is predominantly expressed in high-grade RCC (21). The current study showed that downregulation of CTHRC1 dramatically upregulated the expression of E-cadherin and downregulated the expression of N-cadherin and vimentin in RCC cells. Moreover, knockdown of CTHRC1 inhibited the migration and invasion of RCC cells. These results suggest that CTHRC1 downregulation in RCC cells might be an important suppressor to EMT progression, thus inhibiting the migration and invasion of RCC cells.
A growing body of evidence suggests that Wnt/β-catenin signaling pathway plays a crucial role in carcinogenesis and progression of several types of cancers, including RCC (22–24). Thus, influencing Wnt/β-catenin signaling pathway might represent a promising target in RCC treatment. The activation of the Wnt/β-catenin signaling pathway leads to the stabilization and accumulation of β-catenin in the cytoplasm, which translocates into the nucleus and induces the transcription of downstream target genes, such as c-Myc and cyclin D1 (25). Activation of these genes increased cell proliferation, reduced decreased cell–cell adhesion, and promoted EMT and cell migration (26–28). Previous studies reported that the expression of β-catenin is higher in RCC tissues than in normal kidney tissues, and β-catenin overexpression in RCC is related with the tumor stage and poor prognosis (29–31). Most recently, it was reported that CTHRC1 increased the invasive capabilities of EOC cells in vitro by activating the Wnt/β-catenin signaling pathway. Consistent with the results of a prior study, in the current study we observed that knockdown of CTHRC1 inhibited the expression of β-catenin, c-Myc, and cyclin D1 in RCC cells.
In conclusion, the results of the present study indicated that CTHRC1 downregulation inhibits proliferation, migration, EMT, and β-catenin expression in RCC cells. Therefore, CTHRC1 may be a potential therapeutic target for the treatment of RCC.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ljungberg B.; Campbell S. C.; Cho H. Y.; Jacqmin D.; Lee J. E.; Weikert S.; Kiemeney L. A. The epidemiology of renal cell carcinoma. Eur. Urol. 60:615–621; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Motzer R. J.; Hutson T. E.; Tomczak P.; Michaelson M. D.; Bukowski R. M.; Oudard S.; Negrier S.; Szczylik C.; Pili R.; Bjarnason G. A. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27:3584–3590; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Motzer R. J.; Bacik J.; Mariani T.; Russo P.; Mazumdar M.; Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non–clear-cell histology. J. Clin. Oncol. 20:2376–2381; 2002. [DOI] [PubMed] [Google Scholar]
- 4. Russo P. Renal cell carcinoma: Presentation, staging, and surgical treatment: Semin. Oncol. 27:160–176; 2000. [PubMed] [Google Scholar]
- 5. Motzer R. J.; Bacik J.; Schwartz L. H.; Reuter V.; Russo P.; Marion S.; Mazumdar M. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J. Clin. Oncol. 22:454–463; 2004. [DOI] [PubMed] [Google Scholar]
- 6. Huber M. A.; Kraut N.; Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558; 2005. [DOI] [PubMed] [Google Scholar]
- 7. Chuang M. J.; Sun K. H.; Tang S. J.; Deng M. W.; Wu Y. H.; Sung J. S.; Cha T. L.; Sun G. H. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 99:905–913; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harada K. i.; Miyake H.; Kusuda Y.; Fujisawa M. Expression of epithelial–mesenchymal transition markers in renal cell carcinoma: Impact on prognostic outcomes in patients undergoing radical nephrectomy. BJU Int. 110:E1131–E1137; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Yoshino H.; Enokida H.; Itesako T.; Tatarano S.; Kinoshita T.; Fuse M.; Kojima S.; Nakagawa M.; Seki N. Epithelial–mesenchymal transition-related microRNA-200s regulate molecular targets and pathways in renal cell carcinoma. J. Hum. Genet. 58:508–516; 2013. [DOI] [PubMed] [Google Scholar]
- 10. Pyagay P.; Heroult M.; Wang Q.; Lehnert W.; Belden J.; Liaw L.; Friesel R. E.; Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ. Res. 96:261–268; 2005. [DOI] [PubMed] [Google Scholar]
- 11. LeClair R.; Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor β signaling. Trends Cardiovas. Med. 17:202–205; 2007. [DOI] [PubMed] [Google Scholar]
- 12. Takeshita S.; Fumoto T.; Matsuoka K.; Park K.-A.; Aburatani H.; Kato S.; Ito M.; Ikeda K. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J. Clin. Invest. 123:3914–3924; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto S.; Nishimura O.; Misaki K.; Nishita M.; Minami Y.; Yonemura S.; Tarui H.; Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the wnt-receptor complex. Dev. Cell 15:23–36; 2008. [DOI] [PubMed] [Google Scholar]
- 14. Ma M.-Z.; Zhuang C.; Yang X.-M.; Zhang Z.-Z.; Ma H.; Zhang W.-M.; You H.; Qin W.; Gu J.; Yang S. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 16:265–278; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang P.; Wang Y. C.; Chen X. Y.; Shen Z. Y.; Cao H.; Zhang Y. J.; Yu J.; Zhu J. D.; Lu Y. Y.; Fang J. Y. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-β1 and may be associated with metastasis in human gastric cancer. Cancer Sci. 103:1327–1333; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh J.-T. K.; Kim B. Y.; Lee S.-J.; Choe Y.-K.; Hyeok D.; Kim S.-H. K.; Chae S. W.; Kim K. D.; Lee H. G. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget 5:519–29; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou M.; Cheng Z.; Shen H.; He S.; Li Y.; Pan Y.; Feng C.; Chen X.; Zhang Y.; Lin M. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget 6:35813–35829; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ke Z.; He W.; Lai Y.; Guo X.; Chen S.; Li S.; Wang Y.; Wang L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget 5:9410–9424; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tameda M.; Sugimoto K.; Shiraki K.; Yamamoto N.; Okamoto R.; Usui M.; Ito M.; Takei Y.; Nobori T.; Kojima T. Collagen triple helix repeat containing 1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation and motility. Int. J. Oncol. 45:541–548; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piva F.; Giulietti M.; Santoni M.; Occhipinti G.; Scarpelli M.; Lopez-Beltran A.; Cheng L.; Principato G.; Montironi R. Epithelial to mesenchymal transition in renal cell carcinoma: Implications for cancer therapy. Mol. Diagn.Ther. 20:111–117; 2016. [DOI] [PubMed] [Google Scholar]
- 21. Mikami S.; Oya M.; Mizuno R.; Kosaka T.; Katsube K.-i.; Okada Y. Invasion and metastasis of renal cell carcinoma. Med. Mol. Morphol. 47:63–67; 2014. [DOI] [PubMed] [Google Scholar]
- 22. Hsu R.-J.; Ho J.-Y.; Cha T.-L.; Yu D.-S.; Wu C.-L.; Huang W.-P.; Chu P.; Chen Y.-H.; Chen J.-T.; Yu C.-P. Wnt10a plays an oncogenic role in renal cell carcinoma by activating Wnt/β-catenin pathway. PLoS One 7:e47649; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banumathy G.; Cairns P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 10:658–664; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ueno K.; Hirata H.; Majid S.; Tabatabai Z. L.; Hinoda Y.; Dahiya R. IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int. J. Cancer 129:2360–2369; 2011. [DOI] [PubMed] [Google Scholar]
- 25. Clevers H. Wnt/β-catenin signaling in development and disease. Cell 127:469–480; 2006. [DOI] [PubMed] [Google Scholar]
- 26. Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17:45–51; 2007. [DOI] [PubMed] [Google Scholar]
- 27. Cappellen D.; Schlange T.; Bauer M.; Maurer F.; Hynes N. E. Novel c-myc target genes mediate differential effects on cell proliferation and migration. EMBO Rep. 8:70–76; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mejlvang J.; Kriajevska M.; Vandewalle C.; Chernova T.; Sayan A. E.; Berx G.; Mellon J. K.; Tulchinsky E. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol. Biol. Cell 18:4615–4624; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bilim V.; Kawasaki T.; Katagiri A.; Wakatsuki S.-j.; Takahashi K.; Tomita Y. Altered expression of β-catenin in renal cell cancer and transitional cell cancer with the absence of β-catenin gene mutations. Clin. Cancer Res. 6:460–466; 2000. [PubMed] [Google Scholar]
- 30. Kim Y. S.; Kang Y. K.; Kim J. B.; Han S.; Kim K. I.; Paik S. R. B-catenin expression and mutational analysis in renal cell carcinomas. Pathol. Int. 50:725–730; 2000. [DOI] [PubMed] [Google Scholar]
- 31. Bruder E.; Moch H.; Ehrlich D.; Leuschner I.; Harms D.; Argani P.; Briner J.; Graf N.; Selle B.; Rufle A. Wnt signaling pathway analysis in renal cell carcinoma in young patients. Mod. Pathol. 20:1217–1229; 2007. [DOI] [PubMed] [Google Scholar]