Abstract
Bone morphogenetic protein-7 (BMP-7) inhibited the pathogenesis of renal injury in response to a variety of stimuli. However, little is known about the molecular regulation and mechanism of endogenous BMP-7 and its renoprotective functions. This study examined the regulation of BMP-7 and its role in the fibronectin secretion and apoptosis of NRK-52E cells resulting from transforming growth factor-β1 (TGF-β1) in vitro. Results showed that TGF-β1 promoted factor-associated suicide (FAS), FAS ligand (FASL), fibronectin (FN), and miRNA-21 expression, while it downregulated phospho-Smad1 (pSmad1), pSmad5, and pSmad8 expressions in NRK-52E cells. In contrast, BMP-7 alleviated TGF-β1-induced cell apoptosis, inhibited TGF-β1-induced higher expression of miRNA-21 and FN, and enhanced TGF-β1-attenuated phosphorylation of Smad1, Smad5, and Smad8. Furthermore, a chemical inhibitor of miRNA-21 also negatively affected TGF-β1-induced apoptosis and FN secretion. On the other hand, overexpression of miRNA-21 counteracted the inhibitory effect of BMP-7 on TGF-β1-induced FN secretion and apoptosis. However, BMP-7 showed no effects on TGF-β1-induced FN secretion and apoptosis following knockdown of miRNA-21. Taken together, these findings demonstrated that BMP-7 might inhibit TGF-β1-induced FN secretion and apoptosis by the suppression of miRNA-21 in NRK-52E cells.
Key words: Bone morphogenetic protein-7 (BMP-7), Transforming growth factor-β1 (TGF-β1), Fibronectin (FN) secretion, Apoptosis, miRNA-21, Smad
INTRODUCTION
Bone morphogenetic proteins (BMPs), the members of the transforming growth factor-β (TGF-β) superfamily, play a major role in development, differentiation, and apoptosis of tissues (1,2). Among these members, bone morphogenetic protein-7 (BMP-7) has been shown to regulate fibrosis especially in kidney and liver diseases. Previous studies suggested that BMP-7 inhibited corneal fibrosis (3), oral submucous fibrosis (4), and pulmonary fibrosis (5). Furthermore, BMP-7 was correlated with development of hepatic fibrosis (6,7) and even prevented the level of hepatic fibrosis in rats and mice (8,9). Recent studies have shown that BMP-7 exerted beneficial effects on acute and chronic kidney injuries induced by different pathological conditions. For instance, BMP-7 protected human renal tubular epithelial cells (HK-2) against aristolochic acid (AA)-induced injury in vitro (10) and inhibited the transdifferentiation of these kind of cells (11). TGF-β was thought to be proinvasive by inducing epithelial-to-mesenchymal transition (EMT). BMP-7 could inhibit TGF-β-induced EMT and tumor invasion in normal breast and kidney epithelial cells (12) and in breast and prostate tumors (13). However, the effect and mechanism of BMP-7 on TGF-β1-induced cell apoptosis in vitro are unknown.
MicroRNAs (miRNAs) are short, noncoding RNAs that posttranscriptionally regulate the expression of multiple genes. In particular, miRNA-21 is overexpressed in various human cancers and plays an important role in cancer development, progression, and metastasis (14). miRNA-21 is primarily enriched in myofibroblasts in the fibrotic lungs and colorectal cancer tissues (15). The observation of Yang et al. showed that miRNA-21 negatively regulated the sensitivity of prostate cancer cells to chemotherapeutic drugs (16). The role of miRNA-21 in organ fibrosis and the antifibrotic action of miRNA-21 inhibitors were further confirmed by a later study in bleomycin-induced pulmonary fibrosis model, suggesting a central role for miRNA-21 in the pathogenesis of lung fibrosis (17). The authors found that miRNA-21 was upregulated by TGF-β, which in turn inhibited Smad7 leading to amplification of TGF-β signaling and finally resulting in a fibrotic response in human primary fibroblasts. Patrick et al. proved that miRNA-21 inhibition affected the therapeutic benefit in pathological cardiac remodeling in mice exposed to transverse aortic constriction (TAC) for 6 weeks (18).
In the present study, we investigated the effect and mechanism of BMP-7 on TGF-β1-induced FN secretion and apoptosis in NRK-52E cells and strongly suggested that BMP-7 was a potent inhibitor of TGF-β1-induced FN secretion and apoptosis through the suppression of miRNA-21 in NRK-52E cells and might be a promising agent for TGF-β1-induced kidney damage.
MATERIALS AND METHODS
Cell Culture
The rat renal proximal tubular cell line NRK-52E purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) was cultured in low-glucose Dulbecco’s modified Eagle medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum, 100 IU/ml penicillin, and 10 mg/ml streptomycin. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was changed every 3 days, and cells were subcultured before forming confluent monolayer.
Treatments
The experiments were randomly divided into eight groups: Control, TGF-β1 treatment, BMP-7 treatment, BMP-7 plus TGF-β1 treatment, miRNA-21 mimic plus TGF-β1 treatment, miRNA-21 siRNA plus TGF-β1 treatment, miRNA-21 mimic plus TGF-β1 and BMP-7 treatment, and miRNA-21 siRNA plus TGF-β1 and BMP-7 treatment. TGF-β1 was purchased from PeproTech (Rocky Hill City, NJ, USA), BMP-7 was provided by Creative Biomolecules, Inc. (Hopkinton, MA, USA), and miRNA-21 mimic (sense: UAGCUUAUCAGACUGAUGUUGA; antisense: AACAUCAGUCUGAUAAGCUAUU) and miRNA-21 siRNA were purchased from Genepharma (Shanghai, China).
Flow Cytometry
A total of 2.0 × 104 cells were seeded onto a 96-well plate and 24 h later treated for 8 h with TGF-β1 (5 ng/ml), BMP-7 (100 ng/ml), BMP-7 (100 ng/ml) plus TGF-β1(5 ng/ml) treatment, and cell apoptotic rate was identified by flow cytometry according to the manufacturer instructions. Cells were collected after trypsinization and then fixed in 70% ethanol. After ethanol was removed, the samples were stained with 50 µg/ml FITC, Annexin V, and PI (BD Biosciences, San Jose, CA, USA), respectively. Cell apoptotic rate was assayed using a FACScan flow cytometry apparatus (BD Biosciences).
ELISA
The levels of factor-associated suicide (FAS), FAS ligand (FASL), and fibronectin (FN) in cell culture were analyzed using the method of ELISA (enzyme-linked immunosorbent assay). The assay kits of FN were provided by Beijing North Institute of Biological Technology (Beijing, China).
Western Blot Analysis
The examination of the protein expression levels of pSmad1, pSmad5, and pSmad8 was performed separately using Western blot analysis. Total protein was extracted, and the protein concentration was measured. Briefly, a total of 10 µg of protein was separated by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane using wet transfer apparatus (Bio-Rad, Hercules, CA, USA). The membranes were then blocked with 5% skimmed milk and incubated overnight at 4°C with the primary antibodies, followed by incubation with the secondary antibodies labeled with HRP. Next, the protein bands were visualized using an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA), and the protein levels were detected using the chemiluminescence reader, ImageQuant™ LAS4000 (GE Healthcare, Pittsburgh, PA, USA). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Band density was quantitated using ImageJ software.
Gene Expression Analysis
Expression of miRNA-21 in cells was determined at indicated times by RNA preparation and quantitative reverse transcription polymerase chain reaction (RT-PCR). Briefly, total cellular RNA was isolated from cells on six-well plates using TRIzol reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). According to the manufacturers’ instructions, real-time RT-PCR analysis was carried out using the QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA, USA) under the ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The reaction run at one cycle of 54°C for 1 min and 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 57°C for 30 s, and 72°C for 45 s. β-Actin expression was used as an internal control. Specific primer sequences were synthesized in BIOSUNE Biological Technology Corp (Shanghai, China), and the sequences of the primers were as follows: miRNA-21, sense: 5′-TAGCTTATCAGACTGATG-3′, antisense: 5′-GCTGTCAACGATACGCTACGTAACG-3′; β-actin, sense: 5′-GACATGCCGCCTGGAGAAAC-3′, antisense: 5′-AGCCCAGGATGCCCTTTAGT-3′.
Small Interference RNA (siRNA)
miRNA-21-specific siRNA was as follows: UCAACAUCAGUCUGAUAAGCUA; miRNA-21 siRNA control: CAGUACUUUUGUGUAGUACAA. For transient transfections, cells in the exponential growth phase were grown to 65% confluence and then transfected with 3 µg of miRNA-21-specific siRNA construct or nontargeting siRNA using HiPerFect (Qiagen) according to the manufacturer’s protocols. After cells were cultured in medium for 48 h, the efficiency of miRNA-21 siRNA was determined by RT-PCR. Furthermore, cells treated with TGF-β1 in the exponential growth phase were grown to 65% confluence and transfected with miRNA-21 siRNA or mimic for 48 h; cell FN secretion and apoptosis were investigated.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Student’s two-tailed t-test was used to determine the statistical differences between the treatment and control groups. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
BMP-7 Attenuated TGF-β1-Induced FN Secretion and Apoptosis
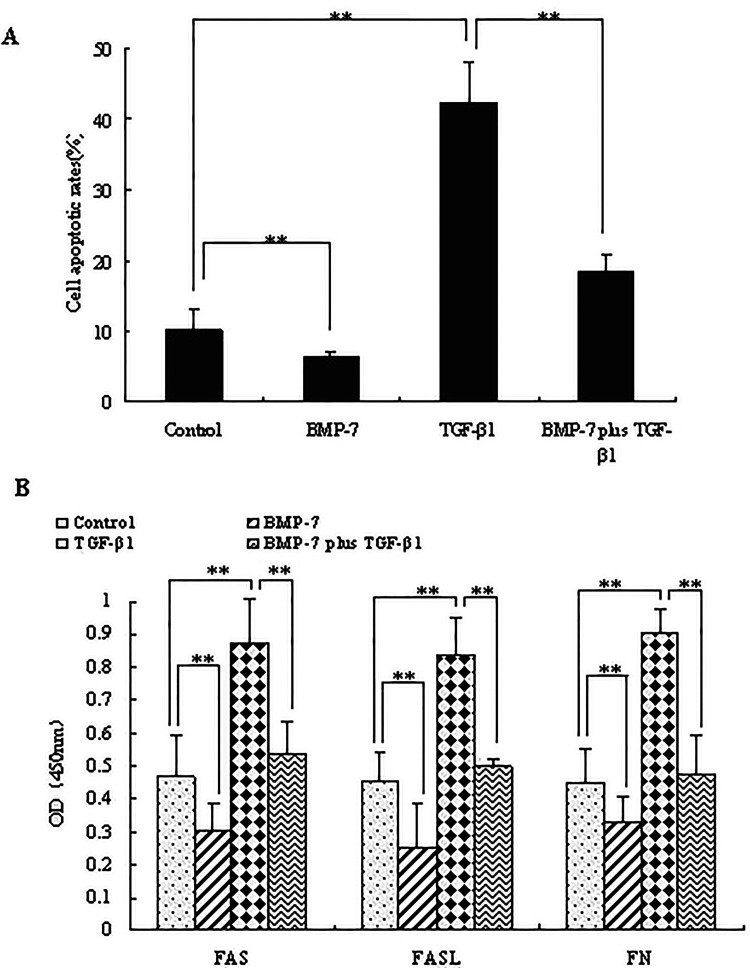
To investigate whether BMP-7 has an inhibitory effect on TGF-β1, the effects of BMP-7 on FN secretion and apoptosis of NRK-52E cells were examined following TGF-β1 treatment. It was suggested that, compared with the control (10.22 ± 3.04%), the apoptotic rate of cells treated with TGF-β1 (42.18 ± 6.05%) showed a significant increase (p < 0.05), while it was decreased in the BMP-7-treated group (6.24 ± 0.96%) (p < 0.05). Obviously, the apoptotic rate of cells treated with both BMP-7 and TGF-β1 was 18.58 ± 2.11%, showing a significant decrease in comparison with TGF-β1-treated group (p < 0.05) (Fig. 1A). Additionally, the protein levels of apoptotic markers FAS and FASL, and fibrotic marker FN (Fig. 1B) were investigated. It was found that, in the presence of TGF-β1, protein expressions of FAS, FASL, and FN were obviously increased (p < 0.05). BMP-7 strongly inhibited the secretion of FAS, FASL, and FN (p < 0.05), while it had inhibitory effects on TGF-β1-induced cell apoptosis and FN secretion (p < 0.05). Taken together, it was indicated that BMP-7 attenuated TGF-β1-induced FN secretion and apoptosis.
Figure 1.
Effects of BMP-7 on TGF-β1-induced FN secretion and apoptosis. NRK-52E cells were treated with TGF-β1 (5 ng/ml), BMP-7 (100 ng/ml), BMP-7 (100 ng/ml) plus TGF-β1 (5 ng/ml) for 8 h. (A) Cell apoptotic rate was identified by flow cytometry. (B) FAS, FASL, and FN expression in cell culture were analyzed using the method of ELISA. Data are expressed as mean ± SD of three independent experiments in triplicate.
BMP-7 Activated TGF-β1-Suppressed Smad Signaling
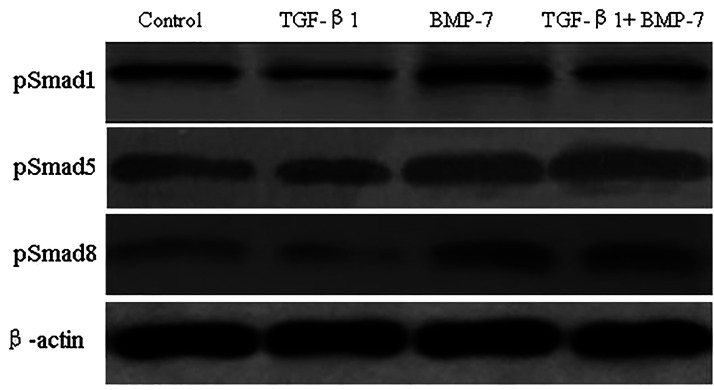
To investigate the molecular mechanism by which BMP-7 inhibited TGF-β-induced invasion, we analyzed the effects of BMP-7 on Smad signaling after cells were treated with TGF-β1. First, we analyzed whether TGF-β1 had an inhibitory effect on the phosphorylation of Smad1, Smad5, and Smad8 and found that TGF-β1 suppressed protein expression of pSmad1, pSmad5, and pSmad8. Second, we proved that BMP-7 promoted TGF-β-induced lower phosphorylation of Smad1, Smad5, and Smad8 (Fig. 2). Thus, BMP-7 activated TGF-β-suppressed Smad signaling in NRK-52E cells.
Figure 2.
Effects of BMP-7 on TGF-β1-suppressed Smad signaling. NRK-52E cells were treated with TGF-β1 (5 ng/ml), BMP-7 (100 ng/ml), BMP-7 (100 ng/ml) plus TGF-β1 (5 ng/ml) for 24 h, and Western blot analysis was used to measure the protein levels of pSmad1, pSmad5, and pSmad8. Blots were incubated with pSmad1, pSmad5, and pSmad8 antibodies.
BMP-7 Reduced TGF-β-Induced Higher Expression of miRNA-21
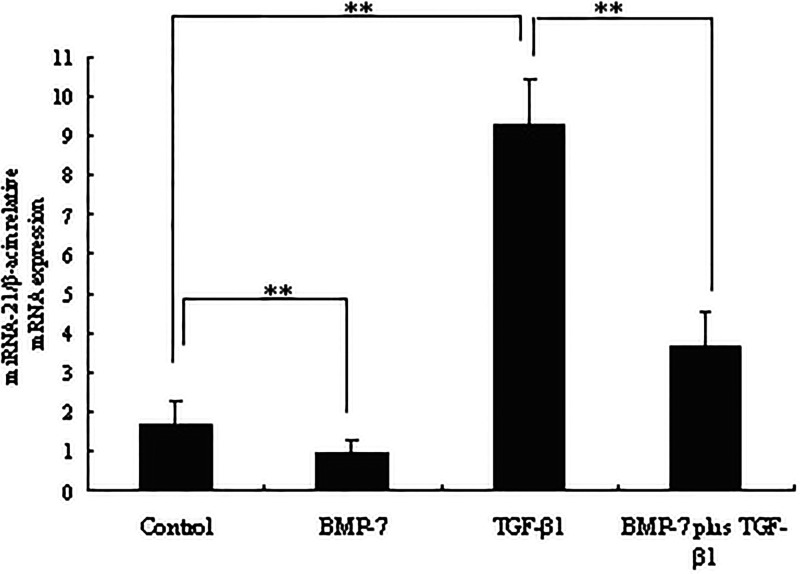
The influence of BMP-7 on the expression of miRNA-21 induced by TGF-β1 was next examined. Importantly, as shown in Figure 3, compared to the non-TGF-β1 treatment group, TGF-β1 potently induced higher miRNA-21 expression in NRK-52E cells (p < 0.05). However, BMP-7 obviously inhibited the TGF-β-induced higher expression of miRNA-21 (p < 0.05). It was suggested that BMP-7 specifically inhibited TGF-β-induced miRNA-21 expression.
Figure 3.
Effects of BMP-7 on TGF-β1-induced higher expression of miRNA-21. NRK-52E cells were treated with TGF-β1 (5 ng/ml), BMP-7 (100 ng/ml), BMP-7 (100 ng/ml) plus TGF-β1 (5 ng/ml) for 8 h. Total cellular RNA was isolated from cells using TRIzol reagent, and miRNA-21 expression was determined by RT-PCR. Data are expressed as mean ± SD of three independent experiments in triplicate.
miRNA-21 Was a Crucial Mediator of TGF-β-Induced FN Secretion and Apoptosis
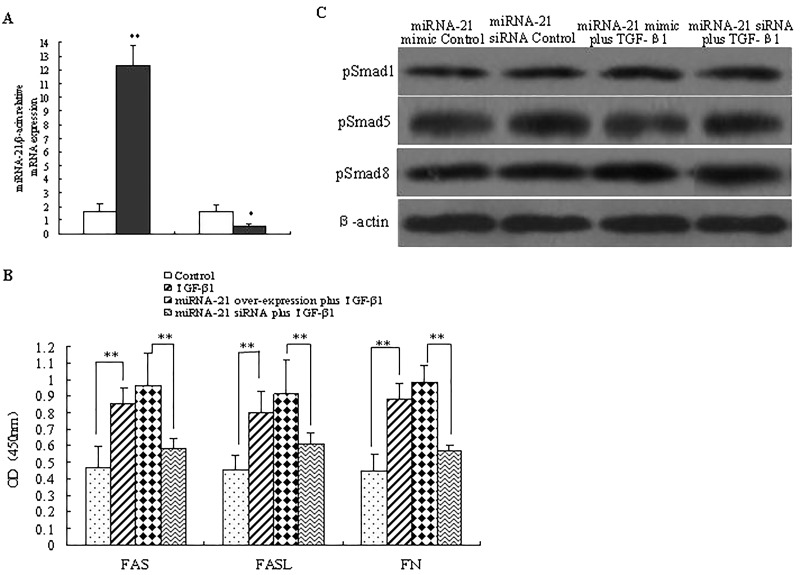
To find out whether miRNA-21 participated in cellular processes, TGF-β1-induced FN secretion, apoptosis, and Smad signal transduction in vitro were determined by transient transfection of miRNA-21 mimic or siRNA into NRK-52E cells. As shown in Figure 4A, NRK-52E cells transducted with miRNA-21 mimic exhibited higher levels of miRNA-21 compared to the control cells (p < 0.05), while miRNA-21 expression was significantly downregulated in miRNA-21 siRNA-treated cells (p < 0.05), indicating the good efficiency of overexpression and knockdown of miRNA-21 in NRK-52E cells. The expression levels of FAS, FASL, and FN induced by TGF-β1 were significantly enhanced by miRNA-21 mimic treatment, but inhibited by miRNA-21 siRNA treatment (p < 0.05), indicating miRNA-21 was a crucial mediator of TGF-β-induced FN secretion and apoptosis (Fig. 4B). However, the phosphorylation of Smad1, Smad5, and Smad8 in TGF-β1-treated cells showed little significant difference between miRNA-21 knockdown cells and miRNA-21 overexpression cells. The results above indicated that miRNA-21 was a crucial mediator of TGF-β-induced FN secretion and apoptosis in NRK-52E cells.
Figure 4.
Effects of miRNA-21 on TGF-β-induced FN secretion, apoptosis, and Smad signaling. (A) NRK-52E cells were transfected with miRNA-21 mimic or siRNA for 24 h, and the efficiency of overexpression and knockdown was measured by RT-PCR method. Data are expressed as mean ± SD of three independent experiments in triplicate. (B) Cells were treated with TGF-β1 (5 ng/ml), miRNA-21 mimic plus TGF-β1 (5 ng/ml), miRNA-21 siRNA plus TGF-β1 (5 ng/ml) for 8 h. Expression of FAS, FASL, and FN in cell culture was analyzed using ELISA method. Data are expressed as mean ± SD of three independent experiments in triplicate. (C) NRK-52E cells were treated with miRNA-21 siRNA or miRNA-21 mimic for 48 h, the protein levels of pSmad1, pSmad5, and pSmad8. Blots were incubated with pSmad1, pSmad5 and pSmad8 antibodies were measured using Western blot analysis.
BMP-7 Inhibited TGF-β-Induced FN Secretion and Apoptosis by miRNA-21
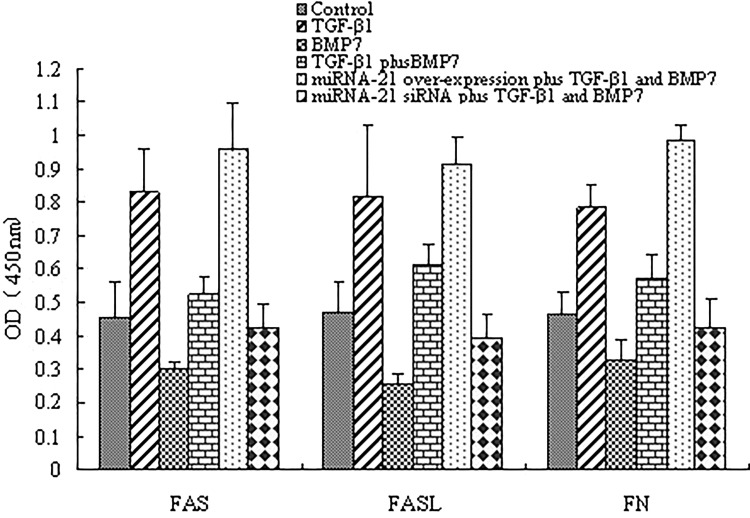
To demonstrate if miRNA-21 is important for TGF-β-induced FN secretion and apoptosis from BMP-7 inhibition, miRNA-21 mimic or siRNA that overexpressed or downregulated miRNA-21 was transfected in NRK-52E cells. As demonstrated in Figure 5, BMP-7 inhibited TGF-β-induced FN secretion and apoptosis, overexpression of miRNA-21 aggravated TGF-β-induced higher expression of FAS, FASL and FN, and completely counteracted the inhibitory effect of BMP-7 (p < 0.05). However, when miRNA-21 expression was suppressed using siRNA method, BMP-7 showed little effects on TGF-β-induced higher expression of FAS, FASL, and FN (p < 0.05). Taken together, these data suggested that BMP-7 inhibited TGF-β-induced FN secretion and apoptosis by inhibiting miRNA-21 expression.
Figure 5.
BMP-7 inhibited TGF-β-induced FN secretion and apoptosis by miRNA-21. NRK-52E cells were treated with TGF-β1 (5 ng/ml), BMP-7 (100 ng/ml), BMP-7 (100 ng/ml) plus TGF-β1 (5 ng/ml), miRNA-21 mimic plus TGF-β1 (5 ng/ml) + BMP-7 (100 ng/ml), miRNA-21 siRNA plus TGF-β1(5 ng/ml) + BMP-7 (100 ng/ml) for 8 h, and FAS, FASL, and FN expressions were analyzed by ELISA. Data are expressed as mean ± SD of three independent experiments in triplicate.
DISCUSSION
Among BMP family members, BMP-7 has shown to be a multifunctional cytokine with a wide range of effects on cell growth, differentiation, and apoptosis (19,20) and thus plays a crucial role in the development of many organs. Previous study suggested that BMP-7 was beneficial at limiting fibrosis of the kidney when the BMP-7 was administered during the progression of fibrotic disease, indicating that BMP-7 administration might be an effective treatment to restore or preserve renal histology and renal function in the experimental model of renal disease (12). In the present study, we investigated the effects and mechanism of BMP-7 in TGF-β1-induced FN secretion and apoptosis, and found that BMP-7 could inhibit TGF-β1-induced FN secretion and apoptosis by suppressing miRNA-21 expression.
TGF-β and BMP-7 are two key members in the TGF-β superfamily and play important but diverse roles in chronic kidney diseases (CKDs) characterized by progressive tubulointerstitial fibrosis. BMP-7 has been shown to play a pivotal role in eye development during embryogenesis (21), and exogenous BMP-7 administration could attenuate fibrosis (22). Furthermore, BMP-7 could be used as an EMT inhibitor to suppress the progression of cholangiocarcinoma (CCA) and might be a therapeutic approach to improve efficiency for CCA treatment (13). Mostly important, the activation of BMP-7 signaling has been reported to oppose TGF-β biological activity (20), and administration of recombinant BMP-7 could reverse TGF-β-derived fibrosis in organs such as the kidney and cardiac tissue (22). The certain levels of BMP-7 could prevent and inhibit mRNA expression of TGF-β1 and its type II receptor, thus inhibiting the transdifferentiation of renal tubular epithelial cells (11). In addition, BMP-7 partially inhibited TGF-β1-induced an increase in MMP-2 and MMP-13 release, and TGF-β1-induced a myofibroblast-like transformation (23). This study also suggested that BMP-7 could attenuate TGF-β-induced FN secretion and apoptosis in cultured NRK-52E cells.
Both TGF-β and BMP-7 share similar downstream Smad signaling pathways, but counterregulate each other to maintain the balance of their biological activities. During renal injury in CKDs, TGF-β signaling was upregulated by inducing TGF-β1 and activating Smad3, whereas BMP-7 and its downstream Smad1/5/8 were downregulated. In the context of renal fibrosis, Smad3 is pathogenic, whereas Smad2 and Smad7 are renoprotective. Overexpression of renal Smad7 restored the balance of TGF-β/Smad signaling and had therapeutic effects on CKDs. Furthermore, activation of TGF-β/Smad signaling inhibited renal BMP-7 expression and BMP/Smad signaling. On the other hand, overexpression of renal BMP-7 was capable of inhibiting TGF-β/Smad3 signaling and protected the kidney from TGF-β-mediated renal injury, suggesting the therapeutic potential by targeting TGF-β/Smad signaling or restoring BMP-7 in CKDs (24). Another study showed that BMP-7 gene delivery in rabbit cornea modulated wound healing and inhibited fibrosis in vivo by counterbalancing TGF-β1-mediated profibrotic Smad signaling (3). In this study, using in vitro cultured NRK-52E cells as models, it was demonstrated that BMP-7 could activate TGF-β-suppressed Smad signaling.
The small regulatory RNA miRNA-21 plays a crucial role in a plethora of biological functions and diseases including development, cancer, cardiovascular diseases, and inflammation. miRNA-21 has been found to be upregulated in many pathological conditions including cancer and cardiovascular diseases (25). Inhibition of miRNA-21 has been shown to provide significant benefits during cancer or heart failure in mice models (26). Recent studies also suggested that Smad3 mediated renal fibrosis by upregulating miRNA-21 and miRNA-192, but downregulating miRNA-29 and miRNA-200 families. Therefore, restoring miRNA-29/miRNA-200 or suppressing miRNA-21/miRNA-192 was able to treat progressive renal fibrosis. Many researchers have made their efforts to find out the mechanic functions of BMP-7 in renal fibrosis. In vitro, BMP-7 inhibited TGF-β-induced invasion of the metastatic breast cancer cell line MCF10CA1a through inhibition of integrin β3 expression (27) and downregulated TGF-β-induced CCA cell migration through inhibition of TGF-β-mediated Twist and N-cadherin expressions (28). Manson et al. examined transcriptional regulation of BMP-7 and its role in the pathogenesis of renal injury resulting from urinary tract dysfunction and found that BMP-7 potentiated TGF-β-mediated renal fibrosis in obstructive uropathy (29). BMP-7 antagonized the profibrotic effects of TGF-β1 through inhibition of de novo expression of α-smooth muscle actin (α-SMA) (30). This study further proved that miRNA-21 was an important mediator of TGF-β-induced FN secretion and Smad signaling, and BMP-7 could attenuate TGF-β1-induced FN secretion and apoptosis in NRK-52E cells through the suppression of miRNA-21.
In conclusion, the current understanding of the crucial roles and mechanisms of TGF-β1 and BMP-7 in FN secretion and apoptosis of NRK-52E cells implied that targeting miRNA-21 or restoring BMP-7 signaling might represent novel and effective treatment to renal injury.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Zhejiang Province (No. LQ13H050002).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Yeh L. C. In vitro and in vivo studies on the effects of bone morphogenetic protein-7 on human kidney and lung tumor cells. Int. J. Biomed. Sci. 6(3):176–181; 2010. [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng L.; Liu J. M.; Wang J. X.; Li M. Z.; Lian W. G.; Xie P.; Liu S. F. Effect of bone morphogenetic protein 7 on differentiation of adipose derived mesenchymal stem cells into brown adipocytes in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 36(6):654–659; 2014. [DOI] [PubMed] [Google Scholar]
- 3. Tandon A.; Sharma A.; Rodier J. T.; Klibanov A. M.; Rieger F. G.; Mohan R. R. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS One 8(6):e66434; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khan I.; Agarwal P.; Thangjam G. S.; Radhesh R.; Rao S. G.; Kondaiah P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors 29(4):119–127; 2011. [DOI] [PubMed] [Google Scholar]
- 5. Yang G.; Zhu Z.; Wang Y.; Gao A.; Niu P.; Tian L. Bone morphogenetic protein-7 inhibits silica-induced pulmonary fibrosis in rats. Toxicol. Lett. 220(2):103–108; 2013. [DOI] [PubMed] [Google Scholar]
- 6. Aktug Demir N.; Kolgelier S.; Inkaya A. C.; Sumer S.; Demir L. S.; Pehlivan F. S.; Arslan M.; Arpaci A. Are bone morphogenetic protein-7 (BMP-7) serum levels correlated with development of hepatic fibrosis? J. Infect. Dev. Ctries. 8(5):605–610; 2014. [DOI] [PubMed] [Google Scholar]
- 7. Bi W. R.; Xu G. T.; Lv L. X.; Yang C. Q. The ratio of transforming growth factor-β1/bone morphogenetic protein-7 in the progression of the epithelial-mesenchymal transition contributes to rat liver fibrosis. Genet. Mol. Res. 13(1):1005–1014; 2014. [DOI] [PubMed] [Google Scholar]
- 8. Chen B. L.; Peng J.; Li Q. F.; Yang M.; Wang Y.; Chen W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-β/Smad signaling. World J. Gastroenterol. 19(9):1405–1415; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang S. L.; Yang C. Q.; Qi X. L.; Yuan M.; Chang Y. Z.; Yang L.; Gao H. J. Inhibitory effect of bone morphogenetic protein-7 on hepatic fibrosis in rats. Int. J. Clin. Exp. Pathol. 6(5):897–903; 2013. [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z.; Zhao J.; Zhang J.; Wei J.; Zhang J.; Huang Y. Protective effect of BMP-7 against aristolochic acid-induced renal tubular epithelial cell injury. Toxicol. Lett. 198(3):348–357; 2010. [DOI] [PubMed] [Google Scholar]
- 11. Tan X. Y.; Zheng F. L.; Yang J. H.; Duan L.; Li Y.; Zhou Q. G. Effect of BMP-7 on the transdifferentiation of cultured human tubular epithelial cell induced by TGF-beta1. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 26(3):274–278; 2004. [PubMed] [Google Scholar]
- 12. Morrissey J.; Hruska K.; Guo G.; Wang S.; Chen Q.; Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J. Am. Soc. Nephrol. 13(Suppl. 1):S14–21; 2002. [PubMed] [Google Scholar]
- 13. Duangkumpha K.; Techasen A.; Loilome W.; Namwat N.; Thanan R.; Khuntikeo N.; Yongvanit P. BMP-7 blocks the effects of TGF-β-induced EMT in cholangiocarcinoma. Tumour Biol. 35(10):9667–9676; 2014. [DOI] [PubMed] [Google Scholar]
- 14. Calin G. A.; Croce C. M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 6:857–866; 2006. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen B. S.; Jorgensen S.; Fog J. U.; Sokilde R.; Christensen I. J.; Hansen U.; Brünner N.; Baker A.; Møller S.; Nielsen H. J. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin. Exp. Metastasis 28:27–38; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang C. H.; Yue J.; Fan M.; Pfeffer L. M. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res. 70:8108–8116; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu G.; Friggeri A.; Yang Y.; Milosevic J.; Ding Q.; Thannickal V. J.; Kaminski N.; Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207:1589–1597; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patrick D. M.; Montgomery R. L.; Qi X.; Obad S.; Kauppinen S.; Hill J. A.; van Rooij E.; Olson E. N. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J. Clin. Invest. 120:3912–3916; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buijs J. T.; Petersen M.; van der Horst G.; van der Pluijm G. Bone morphogenetic proteins and its receptors; therapeutic targets in cancer progression and bone metastasis? Curr. Pharm. Des. 16:1291–1300; 2010. [DOI] [PubMed] [Google Scholar]
- 20. Kim M.; Choe S. BMPs and their clinical potentials. BMB Rep. 44:619–634; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zouvelou V.; Passa O.; Segklia K.; Tsalavos S.; Valenzuela D. M.; Economides A. N.; Graf D. Generation and functional characterization of mice with a conditional BMP7 allele. Int. J. Dev. Biol. 53:597–603; 2009. [DOI] [PubMed] [Google Scholar]
- 22. Zeisberg M.; Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr. Nephrol. 23:1395–1398; 2008. [DOI] [PubMed] [Google Scholar]
- 23. Pegorier S.; Campbell G. A.; Kay A. B.; Lloyd C. M. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF). Respir. Res. 11:85; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meng X. M.; Chung A. C.; Lan H. Y. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin. Sci (Lond) 124(4):243–254; 2013. [DOI] [PubMed] [Google Scholar]
- 25. Jazbutyte V.; Thum T. MicroRNA-21: From cancer to cardiovascular disease. Curr. Drug Targets 11:926–935; 2010. [DOI] [PubMed] [Google Scholar]
- 26. Kumarswamy R.; Volkmann I.; Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 8(5):706–713; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naber H. P.; Wiercinska E.; Pardali E.; van Laar T.; Nirmala E.; Sundqvist A.; van Dam H.; van der Horst G.; van der Pluijm G.; Heckmann B.; Danen E. H.; Ten Dijke P. BMP-7 inhibits TGF-β-induced invasion of breast cancer cells through inhibition of integrin β(3) expression. Cell Oncol. (Dordr). 35(1):19–28; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bin S.; Li H. D.; Xu Y. B.; Qi S. H.; Li T. Z.; Liu X. S.; Tang J. M.; Xie J. L. BMP-7 attenuates TGF-β1-induced fibroblast-like differentiation of rat dermal papilla cells. Wound Repair Regen. 21(2):275–281; 2013. [DOI] [PubMed] [Google Scholar]
- 29. Manson S. R.; Song J. B.; Hruska K. A.; Austin P. F. HDAC dependent transcriptional repression of Bmp-7 potentiates TGF-β mediated renal fibrosis in obstructive uropathy. J. Urol. 191(1):242–252; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veerasamy M.; Nguyen T. Q.; Motazed R.; Pearson A. L.; Goldschmeding R.; Dockrell M. E. Differential regulation of E-cadherin and alpha-smooth muscle actin by BMP 7 in human renal proximal tubule epithelial cells and its implication in renal fibrosis. Am. J. Physiol. Renal Physiol. 297(5):F1238–1248; 2009. [DOI] [PubMed] [Google Scholar]