Abstract
Background
In infants admitted to an ICU with respiratory failure, there is an association between the ratio of CD8+ to CD4+ T cells within the upper respiratory tract and disease severity. Whether this ratio is associated with respiratory disease severity within children presenting to a pediatric emergency department is not known.
Methods
We studied a convenience sample of 63 children presenting to a pediatric emergency department with respiratory symptoms. T cell subsets in the nasal mucosa were analyzed by flow cytometry. We compared CD4+ and CD8+ T cells subsets in these samples and analyzed the proportion of these subsets that expressed markers associated with tissue residency.
Results
We were able to identify major subsets of CD8 and CD4 T cells within the nasal mucosa using flocked swabs. We found no difference in the ratio CD8+ to CD4+ T cells in children with upper or lower respiratory illness. A positive association between tissue-resident memory T cell frequency and patient age was identified.
Conclusions
In our patient populations, the CD8+:CD4+ ratio was not associated with disease severity. The majority of T cells collected on nasal swabs are antigen experienced, and there is an association between the frequency of tissue-resident T cells and age.
Impact
Immune cell populations from the nasal mucosa can be captured using flocked nasal swabs and analyzed by flow cytometry.
Nasal CD8+:CD4+ ratio does not predict respiratory illness severity in children presenting to the emergency department.
The frequency of CD8+ and CD4+ resident memory T cells within the nasal mucosa increases with age.
Introduction
Viral respiratory disease is one of the most common diagnoses in the pediatric emergency department (ED). While significant complications of respiratory tract infections include wheezing, hypoxia, and respiratory failure, these likely result from both direct viral-mediated pathology and the resulting immune response to viral infection.1 A recent multicenter Canadian study of pediatric asthma exacerbations described 61.7% of patients with ≥1 pathogen identified and an increased treatment failure with non-rhinovirus infection (13% absolute risk difference).2 The cellular immune response including the role of T cell-mediated immunity in the development of viral airways disease has been implicated in the pathogenesis of asthma, respiratory syncytial virus, and influenza.3–7 Cytotoxic CD8+ T cells have an essential role in providing immunity to viruses at mucosal surfaces, and in addition to providing help to CD8+ T cells, cytokines secreted by CD4+ T cells, including interferon-γ, interleukin (IL)-4, IL-5, IL-13, and IL-17, have been hypothesized to have potentially pathogenic functions in asthma.8–11 Given the potential role of both CD8+ and CD4+ cells in the development of lower airway pathology during viral infection, there has been interest in profiling their relative abundance in respiratory disease and determining whether this correlates with disease severity.11 A prior study demonstrated an association between an increased ratio of CD8+ to CD4+ T cells within the upper respiratory tract and lower airway disease severity among a cohort of infants admitted to an intensive care unit with respiratory failure of presumed viral etiology.12 However, it has not been determined whether elevation in the CD8+:CD4+ ratio is associated with disease severity in patients presenting to the emergency room with symptoms consistent with viral respiratory infection.
We hypothesize that understanding the distribution and function of T cell populations in the nose in response to respiratory virus infection may reveal immunological pathways that are associated with disease severity as well as clues to underlying pathogenesis. If true, sampling and analyzing nasal T cells could represent a novel approach to understanding disease mechanisms and potentially offer a basis for rationally stratifying patients for the purpose of therapeutic interventions.
Methods
Subjects
We recruited a cross-sectional convenience sample of children aged 6 months to 18 years presenting to the ED at Boston Children’s Hospital during summer 2018 through spring 2019. This study was approved by the Institutional Review Board at Boston Children’s Hospital and required informed consent. Potentially eligible children were identified by research coordinators and clinicians in the ED and approached for the purpose of obtaining consent. Patients were excluded if they had an episode of epistaxis or otolaryngological surgery in the past week, had atypical facial/nasal anatomy or concurrent suspicion for a nasal foreign body, treated with immune suppressants (other than steroids), or had a history of significant immunodeficiency (SCID, XLA, Hyper IgM Syndrome, etc.). We also excluded children enrolled on a prior ED visit. Cases were comprised of children presenting to the ED with a respiratory complaint. These cases were stratified into two groups: (1) those with signs of upper respiratory illness (URI) defined as cough, congestion, or rhinorrhea alone but no signs of lower respiratory tract involvement and (2) those with signs of lower respiratory illness (LRI) defined as hypoxemia ≤92% at triage or wheezing with increased work of breathing.13–15 Controls were patients presenting with an extremity injury without respiratory symptoms.
Measurements
Nasal samples were obtained by the one of the authors (P.R.C.) or a trained research coordinator using FLOQSwabs (Copan flocked swabs) following the manufacturer’s instructions. Swabs were placed in freezing media (2 ml of 10% dimethyl sulfoxide in fetal bovine serum (FBS)) and stored in a −20 °C freezer located in the ED and then transferred to a −80 °C laboratory freezer within 24 h.
Clinical information was obtained using a real-time survey instrument and a retrospective review of the electronic medical record. Clinical variables obtained included: duration of current illness, history of wheezing, treatment with steroids or antibiotics prior to obtaining swab, presenting respiratory rate, temperature and oxygen saturation, wheezing on exam, disposition (admission or discharge), self-reported influenza vaccination, readmission in ensuing 5 days, and history of antibiotic use. Chest X-ray result was defined as the reading radiologist’s impression that was consistent with a bacterial pneumonia, a viral pattern or otherwise read as “negative” or “normal.” Similar to the stratification used in pediatric pneumonia studies, we added viral pattern interpretation from an attending radiologist interpretation of the chest radiograph.16–18 Clinical data were stored in an encrypted, password protected, RedCapTM database and linked to biospecimens using unique, de-identified patient identifiers.19
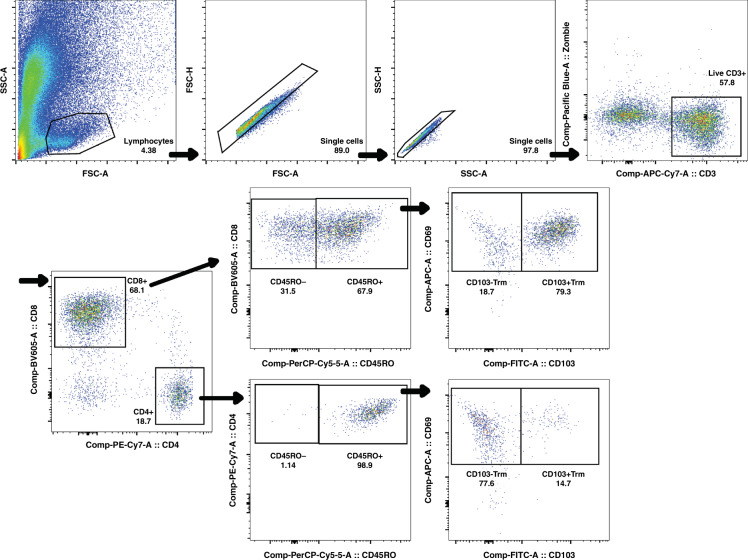
Frozen nasal samples were thawed in a 37 °C water bath. To thawed or freshly acquired nasal samples, dithiothreitol (Sigma Aldrich, St. Louis, MO) was added to a final concentration of 1.5 mM and incubated at 37 °C for 20 min with gentle agitation. Samples were then pelleted by centrifugation at 4 °C and washed twice in cold phosphate-buffered saline (PBS). Samples were resuspended in cold PBS containing Zombie VioletTM fixable viability stain (BioLegend, San Diego, CA) (1:500 ratio) with Fc block (BioLegend) and stained with fluorescently labeled antibodies as indicated for 20 min at 4 °C in the dark. Samples were then washed with 1 ml FACS buffer (PBS with 2% FBS and 0.1% sodium azide), and resuspended in FACS buffer prior to flow cytometric analysis. The following reagents and anti-human antibodies were used for flow cytometry: anti-CD3 (clone HIT3a, BioLegend), anti-CD4 (clone RPA-T4, BioLegend), anti-CD8 (clone SK1, BioLegend), anti-CD45RO (UCHL1, BioLegend), anti-CD103 (Ber-ACT8, BioLegend), and anti-CD69 (FN50, eBioscience). The gating strategy employed is shown in Fig. 1.
Fig. 1. Gating strategy for total CD4+ and CD8+ T cells as well as TRM on nasal swabs.
Please see “Results” section for details.
Data analysis
A power calculation was performed using estimated means and standard deviations derived from a prior data set evaluating the ratio of CD8+ to CD4+ cells in nasal washes.12 Using this data and effect size, we estimated that detecting a change in the mean CD8+:CD4+ ratio from 1 to 2.6 with a power of 90% and an alpha level of 0.05 would require 47 patients in both the URI and LRI groups, assuming a 1:1 enrollment ratio (total 94). T cell counts and the ratio of CD8+ to CD4+ to T cells was compared between the URI and LRI groups using non-parametric tests (Kruskal–Wallis and two-sample Wilcoxon rank sum). A priori subgroup analyses included comparisons between patients who were or were not wheezing. A post hoc comparison was performed between children who did or did not have chest radiograph, as well as a comparison based on radiographic findings. Further post hoc analysis included the correlation of resident memory T cells (TRM) by age.
Results
We approached 127 subjects for enrollment in the ED and 4 withdrew their consent. Of those who consented, we obtained nasal swabs from 123 with 10 samples used for laboratory protocol optimization and pilot data. Nasal samples obtained from 113 patients were analyzed using our final gating strategy. Cells identified within the lymphocyte gate by forward and side scatter were further gated on single cells by forward and side scatter (area and height). Live CD3+ cells within this gate were identified as negative for Zombie Violet. Cells within this live CD3+ gate were segregated based on staining with CD4 and CD8 and evaluated for the presence of the memory marker CD45RO. Expression of the TRM marker CD69 (also a marker of activated T cells) as well as CD103 (a marker of intraepithelial location) on CD45RO+ cells was evaluated.
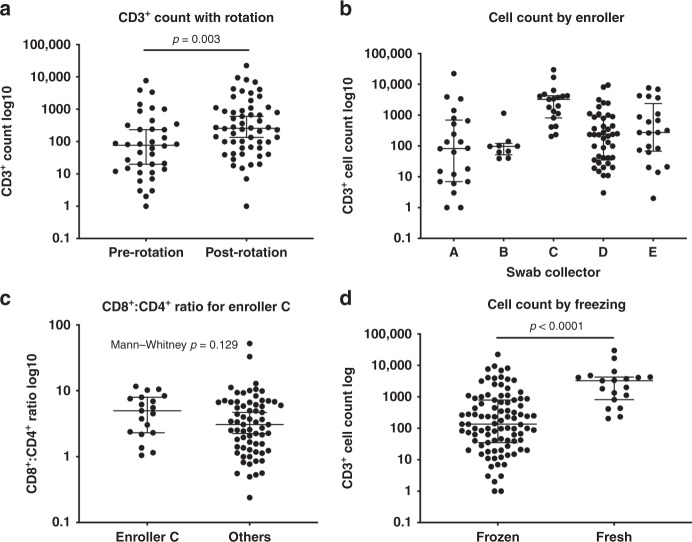
Live CD3+ T cells were identified on virtually all swabs (median 92, interquartile range 21–828). However, after the first several months of the study, it was recognized that research assistants were not routinely rotating the nasal swab following insertion as recommended by the manufacturer. In response to this, research assistants were encouraged to rotate the swab following insertion, and we believe that this was consistently applied after this point. Interestingly, there was a statistically significant increase in CD3+ cell count per swab obtained after initiating routine rotation of the swab (Fig. 2a), which may suggest that rotation increases yield of CD3+ T cells. We also noted a significantly higher CD3+ lymphocyte yield when enroller C performed the sample collection, p < 0.0001 (Fig. 2b), although there was no significant difference in the CD8+:CD4+ ratio in samples collected by enroller C compared to other enrollers (Fig. 2c). Interestingly, a higher percentage of samples collected by enroller C were processed immediately in the absence of freezing, and we observed a higher yield of CD3+ lymphocytes in samples that were processed in the absence of freezing (p < 0.0001) (Fig. 2d), raising the possibility that difference between collectors may be the result of sample processing rather than the technique of the individual collector. There was also a small increase in CD8+:CD4+ ratio of the fresh samples when compared to frozen (p = 0.0280 Mann–Whitney), suggesting that freezing could mildly depress the CD8+:CD4+ ratio. However, as most fresh samples were collected from control patients by enroller C it is difficult to know whether the decrease in CD8+:CD4+ ratio found in frozen sample is the direct result of freezing prior to analysis, differences in collection technique, and/or biological differences between patients and controls. Taken together, these results indicate that nasal CD3+ T cells can be collected from patients presenting to a pediatric emergency room by performing a commonly employed collection technique with a commercially available nasal swab and, whether fresh or frozen, successfully analyzed by flow cytometry.
Fig. 2. Identification of Live CD3+ T cells on nasal swabs.
a Total CD3+ count per swab: Mann–Whitney test showing difference before and after swab rotation, p = 0.003. b Total CD3+ lymphocyte count obtained by enrolling provider or assistant. Kruskal–Wallis quality of populations rank test, p < 0.001. c CD8+:CD4+ ratio by enroller: CD3+ count per swab obtained by enroller. d Cell count per swab showing fewer cells with freezing protocol, Mann–Whitney test, p < 0.001.
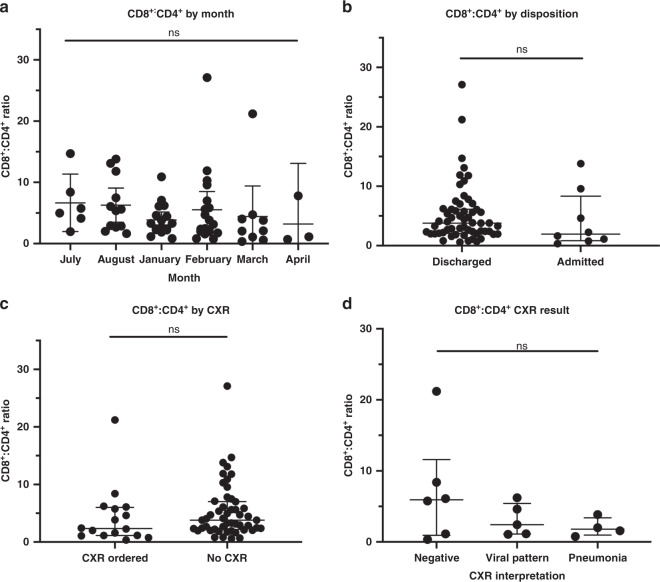
Our primary outcome for this study was the CD8+:CD4+ T cell ratio on nasal swabs obtained from children with acute respiratory illness. Given that the CD8+:CD4+ ratio is highly variable in subjects with low numbers of total CD3+ T cells, subjects were excluded from this data analysis if fewer than 25 CD8+ CD3+ lymphocytes were identified by flow cytometry in the cell populations isolated from their swab. This resulted in 63 subjects being included in further analyses (Fig. 3). Population characteristics between the controls, URI, and LRI subgroups within these 63 subjects are summarized in Table 1. The median number of CD8+ and CD4+ T cells in this data set was 443 and 81, respectively. We did not find a significant difference in the CD8+:CD4+ T cell ratio between patients with and without signs of lower respiratory tract disease (Fig. 4). The CD8+:CD4+ ratio did not vary significantly by month of sample acquisition (Fig. 5a). As another means of assessing potential association between disease severity and the CD8+:CD4+ ratio, we compared this ratio between patients who were discharged from the emergency room and those who were admitted to the hospital (including two to critical care; Fig. 5b). Consistent with a lack of effect of disease severity on the CD8+:CD4+ ratio, we were unable to observe a significant difference between these groups. We also did not observe a significant difference in CD8+:CD4+ T cell ratio between children who were selected by the clinician to have a chest radiograph performed (Fig. 5c). On analysis of the subgroup of children who were selected to have a chest radiograph performed, we did not detect a difference based on the radiologist’s interpretation of the X-ray (normal, viral airways disease, or consistent with pneumonia; Fig. 5d).
Fig. 3. Enrollment flow diagram.
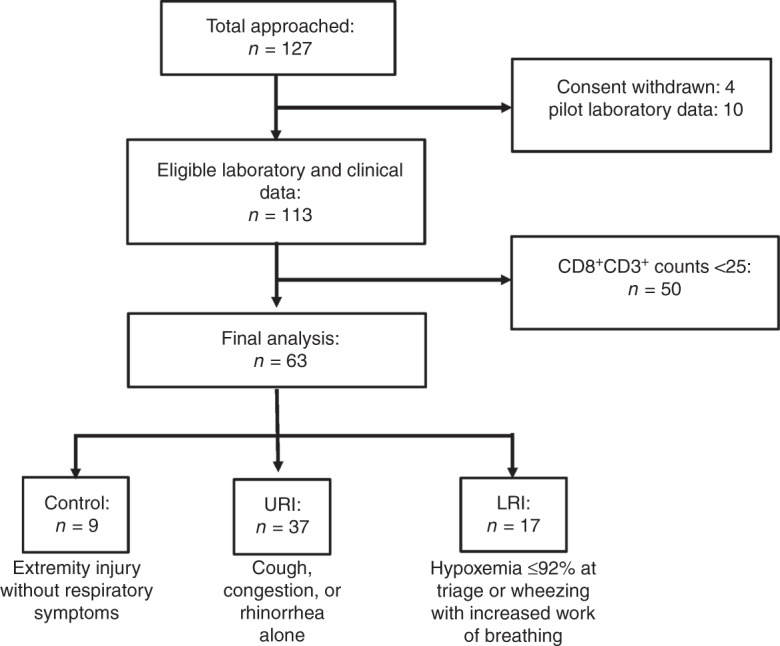
Control: patients presenting with an injury without respiratory symptoms. URI (upper respiratory infection): cough, congestion, or rhinorrhea alone. LRI (lower respiratory infection): hypoxemia ≤92% at triage or wheezing with increased work of breathing.
Table 1.
Demographic and clinical characteristics of patients aged 6 months to 18 years evaluated in the emergency department (ED) who had samples with ≥25 CD3+ CD8+ lymphocytes.
| Demographic characteristics | Control, n = 9 | URI, n = 37 | LRI, n = 17 |
|---|---|---|---|
| Age in yearsa | 10 [8, 15] | 6 [2, 9] | 2 [1, 4] |
| Sex (female) | 4 (44) | 25 (68) | 9 (53) |
| Raceb | |||
| White | 3 (50) | 14 (38) | 7 (41) |
| Black | 2 (33) | 12 (32) | 3 (17) |
| Other | 1 (17) | 10 (27) | 6 (35) |
| Ethnicity | |||
| Not Latino | 6 (67) | 27 (73) | 11 (65) |
| Latino | 3 (33) | 10 (27) | 6 (35) |
| History of wheezingc | 3 (33) | 19 (51) | 11 (65) |
| Fever | |||
| No fever | 9 (100) | 14 (38) | 7 (41) |
| 1 day | 0 | 5 (14) | 3 (18) |
| 2 days | 0 | 6 (16) | 5 (29) |
| ≥3 days | 0 | 7 (19) | 2 (12) |
| Duration of illnessa | |||
| N/A | 9 (100) | 1 (3) | 0 |
| ≤2 days | 0 | 5 (14) | 4 (24) |
| ≥3 days | 0 | 31 (84) | 13 (76) |
| Clinical characteristics | |||
| Wheezing on auscultationa | 0 (0) | 2 (5) | 12 (71) |
| Dispositiona | |||
| Discharged home | 8 (89) | 35 (95) | 12 (71) |
| Admission to floor | 1 (11) | 1 (3) | 4 (24) |
| Admission to ICP/ICU | 0 | 1 (3) | 1 (6) |
| Re-presentation within 5 days | 0 (0) | 3 (8) | 1 (6) |
Values in table represent median percentage [interquartile range] or frequency (proportion). Control: patients presenting with an injury without respiratory symptoms. URI (upper respiratory infection): cough, congestion, or rhinorrhea alone. LRI (lower respiratory infection): hypoxemia ≤92% at triage or wheezing with increased work of breathing.
ICP intermediate care placement, ICU intensive care unit, N/A not available
aKruskal–Wallis equality of populations rank test with p < 0.05.
bMissing data for race. Group size available for this analysis was: Control 6, URI 36, LRI 16.
cWheezing requiring inhaler/nebulizer.
Fig. 4. CD8+:CD4+ ratio in the control, URI, and LRI groups.
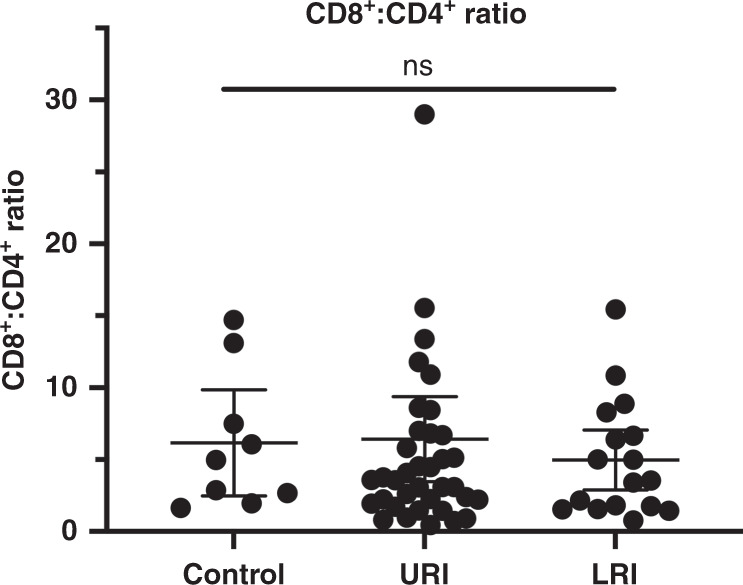
Control median 4.98 (IQR: 2.32–10.3), URI median 3.58 (IQR: 2.07–10.30), and LRI median 3.54 (IQR:1.66–7.48).
Fig. 5. Lack of association between the CD8+:CD4+ ratio and disease severity.
a CD8+:CD4+ ratio by acquisition month. July median 5.37 (IQR: 3.58–9.98), August median 5.50 (IQR: 2.73–10.72), January median 3.30 (IQR: 2.24–5.36), February median 3.14 (IQR: 1.93–7.00), March median 2.09 (IQR: 0.83–4.38). April median 1.12 (IQR: 0.69–7.8). b CD8+:CD4+ ratio according to patient disposition. (Discharged: n = 58, Inpatient: n = 5, ICU/ICP: n = 2). Discharged: patients discharged home from the emergency department. Admitted: admission to hospital under general pediatric inpatient service with two admitted to critical care. Total lymphocyte CD8+:CD4+ ratio according to performance of a chest radiograph (c) and result (d).
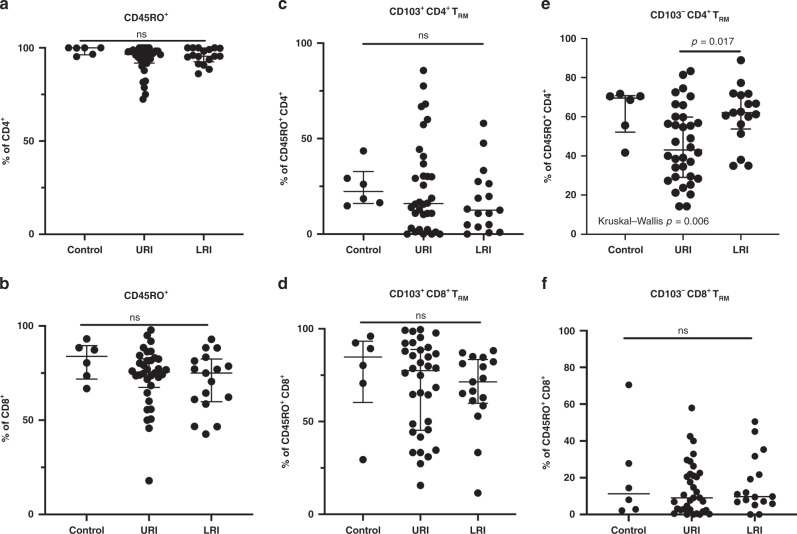
It has previously been demonstrated that the majority of T cells at mucosal surfaces including the upper airways are antigen experienced, and a significant proportion of these antigen-experienced T cells including those in the airways exhibit markers of tissue residency such CD69+ (also a marker for T cell activation). A significant proportion of mucosal CD8+ TRM also express CD103, a marker associated with intraepithelial location, while expression of CD103 is less prevalent on mucosal CD4+ TRM.20 Indeed, we found that a large proportion of both CD4+ and CD8+ T cells isolated on nasal swabs were antigen experienced based on the expression of CD45RO, and that the majority of these antigen-experienced T cells expressed CD69 consistent with tissue residency. However, we did not identify association between the proportion of memory CD4+ or CD8+ and disease severity (Fig. 6a, b) nor did we find association between the percentage of these memory CD4+ or CD8+ T cell that were CD103+ TRM and disease severity (Fig. 6c, d). Interestingly, while the percentage of memory CD8+ T cells that were CD103− TRM was generally low, we found that a significantly higher percentage of memory CD4+ T cells were CD103− CD4+ TRM in children with LRI as compared to children with URI (p = 0.017; Fig. 6e, f).
Fig. 6. Analysis of total memory and resident memory T cells by clinical group.
a Percentage of total CD4+ cells that are CD45RO+. No difference between groups on non-parametric analysis (Kruskal–Wallis). b Percentage of total CD8+ cells that are CD45RO+. No difference between groups on non-parametric analysis (Kruskal–Wallis). c Percentage of CD103+CD69+ cells (TRM) of CD45RO+ CD4+ cells, No difference between groups on nonparametric analysis (Kruskal–Wallis). d Percentage of CD103+CD69+ cells (TRM) of CD45RO+ CD8+ cells. No difference between groups on non-parametric analysis (Kruskal–Wallis). e Percentage of CD103−CD69+ cells (TRM) of CD45RO+ CD4+ cells. Kruskal–Wallis difference between groups p = 0.006 with only group difference LRI vs URI p = 0.017. f Percentage of CD103−CD69+ cells (TRM) of CD45RO+ CD8+ cells. No difference between groups on non-parametric analysis (Kruskal–Wallis).
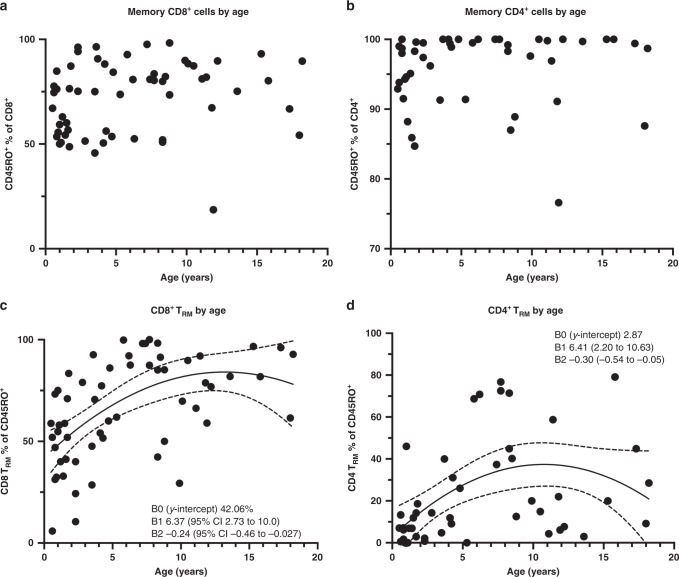
It has previously been suggested that the percentage of CD8+ TRM is higher in upper airway samples obtained from patients aged >2 months compared to those aged <2 months.12 Therefore, we evaluated the association of both CD4+ and CD8+ memory cell subsets with age. Whereas we did not observe an association between age and the percentage of either CD8+ or CD4+ that were CD45RO+ memory cells, remarkably the percentage both CD8+ and CD4+ memory cells that were CD103+ TRM demonstrated a quadratic relationship with age (Fig. 7). This analysis is consistent with prior reports of rapid rises in the percentage of upper airway T early in life12 and further suggests the possibility of a decline later in adolescence although this analysis is limited by fewer adolescent samples.
Fig. 7. Analysis of total memory and resident memory T cells as a function of age.
a Percentage of total CD8+ cells that are CD45RO+. No correlation with age is noted. b Percentage of total CD4+ cells that are CD45RO+. No correlation with age is noted. c Percentage of CD103+CD69+ cells (TRM) of CD45RO+ CD8+ cells; B1 is positive indicating a positive correlation that then curves downward (B2). Confidence intervals indicate that both trends are significant. B0 is the y-intercept. d Percentage of CD103+CD69+ cells (TRM) of CD45RO+ CD4+ cells; B1 is positive indicating a positive correlation that then curves downward (B2). Confidence intervals indicate that both trends are significant.
Discussion
In this study, we describe a process for acquiring and analyzing nasal T cells from children with acute respiratory illness that may provide an important tool for evaluating disease pathogenesis. Rotation of the nasal swab may be an important factor in obtaining sufficient quantity of cells for analysis, as CD3+ cell yields were significantly higher after research coordinators uniformly rotated the swab during collection. Further, there is some indication that freezing swabs prior to analysis may reduce cell yield and potentially subtly decrease the CD8+:CD4+ T cell ratio within the CD3+ population, but this issue requires further study. Thus, conclusions presented here are dependent on our methodologies described, including sample acquisition, freezing, and processing. However, our results demonstrate that collecting T cells on nasal swabs from pediatric patients presenting to the ED and successful immunophenotyping of these T cells is quite feasible.
While we did not identify an increasing CD8+:CD4+ ratio in children with signs of lower respiratory tract involvement, our patient population is significantly older and less ill than a previously evaluated patient population,12 which included children with respiratory failure or meeting pediatric cute respiratory distress syndrome (ARDS) criteria and had a median age of under a year old compared to 4.3 years in the current study. Interestingly, we did find that median CD8+:CD4+ ratio for children with LRI was 3.30, which is similar to the previously described median ratio of 2.58 for children meeting pediatric ARDS criteria.21 This suggests that, although there were technical differences in the collection protocols between studies, the overall magnitude of the CD8+:CD4+ ratio appears consistent, and therefore our inability to find a difference between patients with URI and LRI may represent a true biological phenomenon, rather than a technical difference between this study and the prior study.
Prior studies have compared T cell subsets within the respiratory tract and circulation. These include those of Hogan et al., who found a substantial population of antigen-specific CD8+ T cells in the lung that persist for months after infection,22,23 and Teijaro et al., who found that CD4+ cells also remain in the lungs to provide in situ protection to respiratory viral challenge, in contrast to recirculated splenic CD4+ T cells that did not mediate protection to viral infection.24 Across a wide range of individuals, Thome et al. described mucosal CD4+ and CD8+ T cell subset maintenance and compartmentalization that did not vary with age, was established early, and was stably maintained through life.25 Connors et al. demonstrated that T cell CD8+ and CD4+ subsets are comparable in the upper and lower respiratory tract among infants (R2 = 0.63).12 However, to our knowledge there is little prior data directly evaluating the frequency of CD4+ and CD8+ T cells in the nasal mucosa of older children with symptoms of acute respiratory viral infection. Given our finding that the baseline ratio of CD8+ to CD4+ cells in the nose are similar to those reported by Connors et al. and stable throughout the respiratory tract, this suggests that there are likely respiratory tract-specific factors that govern the recruitment and accumulation of T cell subsets. Understanding these factors will be an interesting topic of future investigation.
Interestingly, the vast majority of T cells isolated on nasal swabs were antigen experienced and many expressed markers associated with tissue residency. While we did not observe an association between either the percentage of CD4+ and CD8+ T cells that were memory cells and disease severity, we found higher percentages of CD103− CD4+ in patients with LRI compared to URI raising the possibility that specific CD4+ subsets are increased during LRI, although this will clearly require validation in larger cohorts. We also observed a significant positive correlation of both CD4+ and CD8+ (CD69+ CD103+) TRM lymphocytes with patient age. This builds on the initial data from Connors et al. who described a positive linear correlation between both CD4 and CD8 TRM cells with age, in the first 4 years of life,12 and is also consistent with a prior study that describes an increasing prevalence of mucosal CD103+ CD8+ TRM with increasing age.8 Our data represent more patients over a greater age span and we have thus identified a fitting quadratic model that describes a steep increase in CD4+ and CD8+ TRM in the early years with a small relative attrition in TRM percentage during adolescence although limited by the low number of adolescent samples. During infancy and early years thereafter, children are subjected to frequent viral respiratory infections26,27 whose resolution is closely related to viral-specific CD8+ T cell effector and memory function in the lung.28,29 As our patient population was older than those previously described, it is possible that the increase in frequency of TRM observed during childhood may represent the cumulative effects of both primary and repeat infection with common respiratory viruses. Indeed, the observation of increasing proportion of TRM cells with age may suggest an accumulation of viral-specific mucosal memory with increasing viral exposures, although this issue remains to be explored in additional detail.
The quadratic model for association between percentage TRM and age allows us to estimate the percentage of TRM present at birth by assessing where curves cross the y-axis. Curves cross the y-intercept above 0 (CD8+: 42.06, CD4+: 2.87), which strongly suggests the presence of CD8+ TRM, in particular at birth, and also suggests additional non-viral-mediated processes for the development of respiratory TRM given the presumed lack of viral exposure at this time. These observations are consistent with recent results demonstrating the presence of mucosal TRM first at 16 weeks of gestational age and increasing over time.30 This raises an important question of whether TRM that develop during fetal life are able to contribute to antiviral protection after birth. If so, this would significantly alter our current conception of immune responses in the neonate and thus is an important topic for future investigation.
Limitations
The results of our analysis will need to be interpreted in the context of its limitations. This was a non-randomized and non-blinded study to describe nasal T cell populations in children presenting to the ED. Our overall population is heterogeneous, which may hide differences between subgroups. Sample acquisition in the ED may favor children with less severe illness, given the natural consent process. This study was also performed at one site, which may limit its generalizability. Our encountered methodological challenges prompting the optimization of sample acquisition during the recruitment process also limit the potential impact of our findings. Although we identified a statistically significant attrition of TRM percentage in adolescence, we had fewer patients and this observation requires further study. Also, we were not able to reach our target enrollment, which reduced our power to detect our a priori hypothesized difference. However, given that we found lower CD8+:CD4+ ratios in the lower respiratory disease group (i.e., an effect in the opposite direction of the hypothesized difference), it is highly unlikely that further recruitment would have altered the findings to be consistent with the initial hypothesis.
Acknowledgements
This study was funded by the Michael Shannon grant from the Division of Emergency Medicine at Boston Children Hospital.
Author contributions
All authors contributed substantially to the conception and design, acquisition, analysis, and interpretation of data; drafting and revising the article critically for important intellectual content; and final approval of the version to be published.
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was required for this study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Troy NM, Bosco A. Respiratory viral infections and host responses; insights from genomics. Respir. Res. 2016;17:156. doi: 10.1186/s12931-016-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merckx, J. et al. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics142, e20174105 (2018). [DOI] [PubMed]
- 3.Pizzolla, A. et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017). [DOI] [PubMed]
- 4.Kinnear E, et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:249–256. doi: 10.1038/mi.2017.46. [DOI] [PubMed] [Google Scholar]
- 5.Turner, D. L. et al. Biased generation and in situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J. Immunol. 200, 1561–1569 (2018). [DOI] [PMC free article] [PubMed]
- 6.Slutter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017). [DOI] [PMC free article] [PubMed]
- 7.Jozwik A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thome JJ, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med. 2016;22:72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisniewski JA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J. Allergy Clin. Immunol. 2018;141:2048.e13–2060.e13. doi: 10.1016/j.jaci.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin. Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors TJ, et al. Developmental regulation of effector and resident memory T cell generation during pediatric viral respiratory tract infection. J. Immunol. 2018;201:432–439. doi: 10.4049/jimmunol.1800396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusel MMH, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur. Respir. J. 2012;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 14.Teo SM, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusel MMH, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr. Infect. Dis. J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 16.Nelson KA, Morrow C, Wingerter SL, Bachur RG, Neuman MI. Impact of chest radiography on antibiotic treatment for children with suspected pneumonia. Pediatr. Emerg. Care. 2016;32:514–519. doi: 10.1097/PEC.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 17.Neuman MI, Monuteaux MC, Scully KJ, Bachur RG. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011;128:246–253. doi: 10.1542/peds.2010-3367. [DOI] [PubMed] [Google Scholar]
- 18.Lipsett SC, Monuteaux MC, Bachur RG, Finn N, Neuman MI. Negative chest radiography and risk of pneumonia. Pediatrics. 2018;142:e20180236. doi: 10.1542/peds.2018-0236. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zens KD, Farber DL. Memory CD4 T cells in influenza. Curr. Top. Microbiol. Immunol. 2015;386:399–421. doi: 10.1007/82_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 2015;16:S23–S40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 22.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 23.Hogan RJ, et al. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J. Immunol. 2002;169:4976–4981. doi: 10.4049/jimmunol.169.9.4976. [DOI] [PubMed] [Google Scholar]
- 24.Teijaro JR, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J. Infect. Dis. 2013;207:982–989. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remakus S, Sigal LJ. Memory CD8+ T cell protection. Adv. Exp. Med. Biol. 2013;785:77–86. doi: 10.1007/978-1-4614-6217-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Heidema J, et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. 2007;179:8410–8417. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 30.Stras SF, et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell. 2019;51:357.e5–373.e5. doi: 10.1016/j.devcel.2019.09.008. [DOI] [PubMed] [Google Scholar]