Abstract
Background: This study aimed to evaluate thrombotic microangiopathy’s (TMA) incidence, risk factors, and impact on outcomes and resource use in hospitalized patients with systemic lupus erythematosus (SLE). Methods: We used the National Inpatient Sample to construct a cohort of hospitalized patients with SLE from 2003–2014. We compared clinical characteristics, in-hospital treatments, outcomes, and resource use between SLE patients with and without TMA. Results: Of 35,745 hospital admissions for SLE, TMA concurrently presented or developed in 188 (0.5%) admissions. Multivariable analysis showed that age ≥ 40 years and Hispanics were significantly associated with decreased risk of TMA, whereas Asian/Pacific Islanders and history of chronic kidney disease were significantly associated with increased risk of TMA. TMA patients required more kidney biopsy, plasmapheresis, mechanical ventilation, and renal replacement therapy. TMA was significantly associated with increased risk of in-hospital mortality and acute conditions including hemoptysis, glomerulonephritis, encephalitis/myelitis/encephalopathy, hemolytic anemia, pneumonia, urinary tract infection, sepsis, ischemic stroke, seizure, and acute kidney injury. The length of hospital stays and hospitalization cost was also significantly higher in SLE with TMA patients. Conclusion: TMA infrequently occurred in less than 1% of patients admitted for SLE, but it was significantly associated with higher morbidity, mortality, and resource use.
Keywords: systemic lupus erythematosus, SLE, hospitalization, outcomes, risk factor, mortality
1. Introduction
Thrombotic microangiopathy (TMA) is a clinical syndrome described by the clinical presentation of thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and evidence of organ injury [1]. Pathologically, TMA consists of microvascular thromboses in the vessel walls of arterioles and capillaries [2]. TMA can lead to significant morbidity and mortality. Renal involvement with acute kidney injury (AKI) that can progress to need for renal replacement therapy is one such common complication of TMA [1]. TMA can be hereditary or acquired. Acquired causes of TMA can be classified into ADAMTS13 deficiency-mediated, Shiga toxin-mediated, drug-mediated, and complement-mediated TMA [2]. Several systemic diseases have also been associated with TMA. Thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) are the most commonly described hematological diseases associated with TMA [2]. Autoimmune diseases, such as systemic lupus erythematosus (SLE), scleroderma renal crisis, catastrophic antiphospholipid syndrome, or malignant hypertension can also cause TMA [2,3,4,5,6].
The concurrent presence of TMA in SLE patients has been reported in small series to range from approximately 1% to 24% [2,3,4,5,6,7,8,9]. Current evidence suggests that SLE-associated TMA may be driven by antiphospholipid syndrome, HELLP syndrome during pregnancy, TMA associated glomerulonephritis, or complement-mediated TMA [6,10,11]. Mortality in SLE patients who develop TMA has been reported to be as high as 31.9%, despite plasma exchange [12]. While SLE patients with TMA have a high mortality risk, data from large contemporary studies on hospitalized SLE patients with TMA were limited.
Thus, we conducted this study to evaluate TMA’s incidence, risk factors, and impact on outcomes and resource use in hospitalized SLE patients in the United States
2. Materials and Methods
2.1. Data Source
This cohort study utilized the National Inpatient Sample (NIS) database, the largest all-payer inpatient database in the United States. The Healthcare Cost and Utilization Project (HCUP) under the sponsorship of the Agency for Healthcare Research and Quality (AHRQ) manages and maintains the NIS database. The NIS database contains hospitalization data from a 20% stratified sample of hospitals in the United States. The NIS database does not examine individual patients, but rather single inpatient admissions. The data includes patient demographics, primary diagnosis, up to 24 secondary diagnoses, and procedural codes. Institutional review board approval was exempted because the NIS is a publicly available de-identified database.
2.2. Study Population
Using the NIS data from 2003 to 2014, we constructed a retrospective cohort of hospitalized patients with a primary discharge diagnosis of SLE based on International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code of 710.0. We categorized SLE patients based on the presence of TMA during hospitalization. We identified TMA using ICD-9 diagnosis code of 446.6.
2.3. Data Collection
We identified clinical characteristics, treatments, and outcomes using ICD-9 codes, shown in Table S1. Clinical characteristics consisted of age, sex, race, hypertension, dyslipidemia, chronic kidney disease, and cirrhosis. Procedures and treatments consisted of kidney biopsy, plasmapheresis, invasive mechanical ventilation, and renal replacement therapy. Complications and outcomes consisted of hemoptysis, pleural effusion/pleuritis, glomerulonephritis, encephalitis/myelitis/encephalopathy, hemolytic anemia, thrombocytopenia, pneumonia, urinary tract infection, sepsis, ischemic stroke, seizure, acute kidney injury, and in-hospital mortality. Resource use consisted of length of hospital stay and hospitalization cost.
2.4. Statistical Analysis
The difference in clinical characteristics, treatments, outcomes, and resource use between SLE patients with and without TMA was tested using student’s t-test for continuous variables and Chi-squared test for categorical variables. Multivariable logistic regression with backward stepwise selection was performed to evaluate independent risk factors for TMA. The associations of TMA with treatments and outcomes were evaluated using logistic regression analysis. The associations of TMA with resource use were evaluated using linear regression analysis. Analyses were adjusted for baseline clinical characteristics. Analysis results were statistically significant when two-tailed p-value < 0.05. SPSS statistical software (version 22.0, IBM Corporation, Armonk, NY, USA) was used for all analyses.
3. Result
3.1. TMA Incidence and Risk Factors in Hospitalized SLE Patients
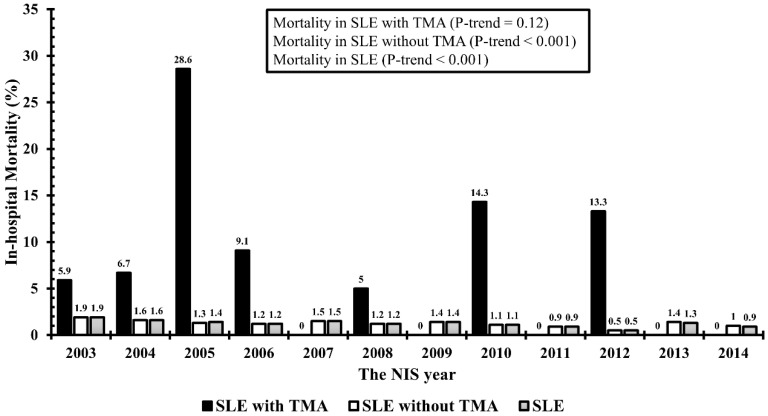
Of 35,745 hospital admissions for SLE, 188 (0.5%) had a concurrent diagnosis of TMA during their hospitalization. Table 1 compares clinical characteristics, in-hospital treatments, outcomes, and resource use between SLE patients with and without TMA. SLE patients with TMA had significant higher in-hospital mortality than those without TMA (Figure 1). Multivariable analysis showed that age ≥ 40 years and Hispanics were significantly associated with decreased risk of TMA, whereas Asian/Pacific Islanders and history of chronic kidney disease were significantly associated with increased risk of TMA (Table 2).
Table 1.
Clinical characteristics, in-hospital treatments, complications, outcomes, and resource utilization in SLE patients with and without TMA.
| Total | SLE with TMA | SLE without TMA | p-Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| N (%) | 35,745 | 188 (0.5) | 35,557 (99.5) | |
| Age (years), mean (SD) | 35.8 (15.9) | 31.7 (14.8) | 35.8 (15.9) | <0.001 |
| <20 | 5595 (15.7) | 37 (19.7) | 5558 (15.6) | 0.02 |
| 20–29 | 8942 (25.0) | 60 (31.9) | 8882 (25.0) | |
| 30–39 | 7646 (21.4) | 41 (21.8) | 7605 (21.4) | |
| 40–49 | 6458 (18.1) | 25 (13.3) | 6433 (18.1) | |
| ≥50 | 7087 (19.8) | 25 (13.3) | 7062 (19.9) | |
| Male | 4732 (13.3) | 29 (15.4) | 4703 (13.2) | 0.38 |
| Race | ||||
| Caucasian | 8577 (24.0) | 47 (25.0) | 8530 (24.0) | <0.001 |
| African American | 13,156 (36.8) | 66 (35.1) | 13,090 (36.8) | |
| Hispanic | 6103 (17.1) | 17 (9.0) | 6086 (17.1) | |
| Asian or Pacific Islander | 1570 (4.4) | 22 (11.7) | 1548 (4.4) | |
| Other | 6339 (17.7) | 36 (19.1) | 6303 (17.7) | |
| Hypertension | 17,174 (48.0) | 103 (54.8) | 17,071 (48.0) | 0.06 |
| Dyslipidemia | 2965 (8.3) | 13 (6.9) | 2952 (8.3) | 0.49 |
| Chronic kidney disease | 5989 (16.8) | 53 (28.2) | 5936 (16.7) | <0.001 |
| Cirrhosis | 1275 (3.6) | 11 (5.9) | 1264 (3.6) | 0.09 |
| Management | ||||
| Kidney biopsy | 414 (1.2) | 40 (21.3) | 4401 (12.4) | <0.001 |
| plasmapheresis | 444 (1.2) | 82 (43.6) | 362 (1.0) | <0.001 |
| Invasive mechanical ventilation | 897 (2.5) | 32 (17.0) | 865 (2.4) | <0.001 |
| Renal replacement therapy | 4002 (11.2) | 68 (36.2) | 3934 (11.1) | <0.001 |
| Complication and outcomes | ||||
| Hemoptysis | 688 (1.9) | 12 (6.4) | 676 (1.9) | <0.001 |
| Pleural effusion/pleuritis | 3354 (9.4) | 17 (9.0) | 3337 (9.4) | 0.87 |
| Glomerulonephritis | 18,201 (50.9) | 159 (84.6) | 18,042 (50.7) | <0.001 |
| Encephalitis/myelitis/encephalopathy | 2351 (6.6) | 33 (17.6) | 2318 (6.5) | <0.001 |
| Antiphospholipid syndrome | 1882 (5.3) | 22 (11.7) | 1860 (5.2) | <0.001 |
| Hemolytic anemia | 6341 (17.7) | 65 (34.6) | 6276 (17.7) | <0.001 |
| Thrombocytopenia | 3474 (9.7) | 21 (11.2) | 3453 (9.7) | 0.50 |
| Pneumonia | 2775 (7.8) | 32 (17.0) | 2743 (7.7) | <0.001 |
| Urinary tract infection | 3689 (10.3) | 27 (14.4) | 3662 (10.3) | 0.07 |
| Sepsis | 1143 (3.2) | 18 (9.6) | 1125 (3.2) | <0.001 |
| Ischemic stroke | 727 (2.0) | 21 (11.2) | 706 (2.0) | <0.001 |
| Seizure | 2990 (8.4) | 33 (17.6) | 2957 (8.3) | <0.001 |
| Acute kidney injury | 5404 (15.1) | 81 (43.1) | 5323 (15.0) | <0.001 |
| In-hospital mortality | 455 (1.3) | 12 (6.4) | 443 (1.2) | <0.001 |
| Resource utilization | ||||
| Length of hospital stay (days), mean (SD) | 6.6 (8.6) | 19.2 (17.2) | 6.5 (8.5) | <0.001 |
| Hospitalization cost ($), mean (SD) | 43,825.3 (82769.4) | 196,213.5 (210558.5) | 43,021.0 (80,814.5) | <0.001 |
Figure 1.
In-hospital mortality among SLE patients with TMA vs without TMA.
Table 2.
Factors associated with TMA in SLE patients.
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Crude Odds Ratio (95%CI) | p-Value | Adjusted Odds Ratio (95%CI) | p-Value | |
| Age (years) | ||||
| <20 | 1 (reference) | 1 (reference) | ||
| 20–29 | 1.02 (0.67–1.53) | 0.94 | 1.00 (0.66–1.51) | 0.99 |
| 30–39 | 0.81 (0.52–1.27) | 0.35 | 0.80 (0.51–1.26) | 0.34 |
| 40–49 | 0.58 (0.35–0.97) | 0.04 | 0.58 (0.35–0.97) | 0.04 |
| ≥50 | 0.53 (0.32–0.88) | 0.02 | 0.49 (0.29–0.82) | 0.007 |
| Male | 1.20 (0.80–1.78) | 0.38 | ||
| Race | ||||
| Caucasian | 1 (reference) | 1 (reference) | ||
| African American | 0.92 (0.63–1.33) | 0.64 | 0.75 (0.51–1.10) | 0.13 |
| Hispanic | 0.51 (0.29–0.88) | 0.02 | 0.39 (0.22–0.69) | 0.001 |
| Asian or Pacific Islander | 2.58 (1.55–4.29) | <0.001 | 1.99 (1.18–3.35) | 0.009 |
| Other | 1.04 (0.67–1.60) | 0.87 | 0.89 (0.57–1.38) | 0.61 |
| Hypertension | 1.31 (0.98–1.75) | 0.06 | ||
| Dyslipidemia | 0.82 (0.47–1.44) | 0.49 | ||
| Chronic kidney disease | 1.96 (1.42–2.70) | <0.001 | 2.04 (1.48–2.82) | <0.001 |
| Cirrhosis | 0.59 (0.32–1.09) | 0.09 | ||
3.2. Association of TMA in SLE patients with In-Hospital Treatments, Outcomes, and Resource Use
Kidney biopsy was performed in 21.3% of TMA compared to 12.4% of non-TMA SLE patients. Plasmapheresis was performed in 43.6% in TMA compared to 1.0% of non-TMA SLE patients. Renal replacement therapy was performed in 36.2% in TMA compared to 11.1% of non-TMA SLE patients. After adjusting for baseline characteristics, TMA was significantly associated with higher requirements of kidney biopsy (OR 1.64; p = 0.007), plasmapheresis (OR 67.44; p < 0.001), invasive mechanical ventilation (OR 7.27; p < 0.001), and renal replacement therapy (OR 5.01; p < 0.001) (Table 3).
Table 3.
The association between TMA in SLE patients and in-hospital treatment, complications, outcomes, resource utilization.
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Crude Odds Ratio (95% CI) | p-Value | Adjusted Odds Ratio* (95% CI) | p-Value | |
| Treatments | ||||
| Kidney biopsy | 1.91 (1.35–2.72) | <0.001 | 1.64 (1.14–2.34) | 0.007 |
| Therapeutic plasmapheresis | 75.21 (55.37–102.16) | <0.001 | 67.44 (49.12–92.60) | <0.001 |
| Invasive mechanical ventilation | 8.23 (5.59–12.11) | <0.001 | 7.27 (4.90–10.79) | <0.001 |
| Renal replacement therapy | 4.56 (3.38–6.15) | <0.001 | 5.01 (3.43–7.31) | <0.001 |
| Complications and outcomes | ||||
| Hemoptysis | 3.52 (1.95–6.35) | <0.001 | 3.27 (1.80–5.91) | <0.001 |
| Pleural effusion/pleuritis | 0.96 (0.58–1.58) | 0.87 | 0.92 (0.56–1.52) | 0.75 |
| Glomerulonephritis | 5.32 (3.58–7.91) | <0.001 | 5.25 (3.39–8.14) | <0.001 |
| Encephalitis/myelitis/encephalopathy | 3.05 (2.09–4.46) | <0.001 | 2.92 (1.99–4.26) | <0.001 |
| Antiphospholipid syndrome | 2.40 (1.54–3.76) | <0.001 | 2.31 (1.47–3.63) | <0.001 |
| Hemolytic anemia | 2.47 (1.82–3.33) | <0.001 | 2.13 (1.55–2.92) | <0.001 |
| Thrombocytopenia | 1.17 (0.74–1.84) | 0.50 | 1.00 (0.63–1.58) | 1.00 |
| Pneumonia | 2.45 (1.67–3.60) | <0.001 | 2.49 (1.69–3.66) | <0.001 |
| Urinary tract infection | 1.46 (0.97–2.20) | 0.07 | 1.53 (1.01–2.31) | 0.04 |
| Sepsis | 3.24 (1.99–5.29) | <0.001 | 2.99 (1.82–4.90) | <0.001 |
| Ischemic stroke | 6.21 (3.92–9.83) | <0.001 | 6.30 (3.96–10.02) | <0.001 |
| Seizure | 2.35 (1.61–3.42) | <0.001 | 2.16 (1.48–3.17) | <0.001 |
| Acute kidney injury | 4.30 (3.22–5.75) | <0.001 | 3.92 (2.90–5.30) | <0.001 |
| In-hospital mortality | 5.40 (2.99–9.77) | <0.001 | 5.54 (3.03–10.15) | <0.001 |
| Coefficient (95% CI) | p-value | Adjusted coefficient * (95% CI) | p-value | |
| Resource utilization | ||||
| Length of hospital stay (days) | 12.7 (11.5–13.9) | <0.001 | 12.4 (11.2–13.6) | <0.001 |
| Hospitalization cost ($) | 153,192.5 (141,308.8–165,076.2) | <0.001 | 148,862.0 (137,111.8–160,612.0) | <0.001 |
* Adjusted for age, sex, race, hypertension, dyslipidemia, chronic kidney disease, and cirrhosis.
In adjusted analyses, TMA in SLE patients was significantly associated with increased risk of several complications, including hemoptysis (OR 3.27; p < 0.001), glomerulonephritis (OR 5.25; p < 0.001), encephalitis/myelitis/encephalopathy (OR 2.92; p < 0.001), hemolytic anemia (OR 2.13; p < 0.001), pneumonia (OR 2.49; p < 0.001), urinary tract infection (OR 1.53; p = 0.04), sepsis (OR 2.99; p < 0.001), ischemic stroke (OR 6.30; p < 0.001), seizure (OR 2.16; p < 0.001), and acute kidney injury (OR 3.92; p < 0.001). TMA was significantly associated with a higher risk of in-hospital mortality compared to non-TMA SLE patients (OR 5.54; p < 0.001).
TMA in SLE patients was significantly associated with both a longer mean length of hospital stay with a difference of 12.4 days (p < 0.001) and higher mean hospitalization cost with a difference of $148,862 (p < 0.001).
4. Discussion
Multiple studies looked at the association of TMA with SLE, with reported prevalence of TMA among patient with SLE varying between 1% and 24% [2,3,4,5,6,7,8,9]. However, from the previous studies, the largest study included 285 patients [2,3,4,5,6,7,8,9]. It has been hypothesized that the difference in numbers was due to some diagnoses being made based on histopathology only, not taking in consideration clinical evaluation [6]. In this study, we reported an incidence of concurrent TMA of 0.5% among hospitalized patients with SLE. To date, our study is the largest cohort to describe the incidence of TMA in SLE patients. More importantly, we also described (1) the risk factors (Asian/Pacific Islanders and history of chronic kidney disease) and (2) the protective factors (age ≥ 40 years, and Hispanic race) for TMA in SLE patients for the first time.
A large-population study by Gomez Puerta was the first study to observed lower mortality rates among Hispanic patients with SLE compared to black, white and Native American, while most previous studies reported higher mortality among Blacks and Hispanics [13]. This study describes a term “Hispanic paradox” among SLE patients, claiming that after adjusting for age and annual income, Hispanic population with SLE had a lower mortality even though this population has a higher burden of SLE [13]. Our study revealed that Hispanic race is indeed associated with decreased risk of developing TMA in SLE patients. TMA, in its worst form, can lead to thrombi formation in glomeruli, arterioles and capillaries, causing decline in renal function [7]. Likewise, SLE exhibits renal manifestation in 50% of patients [14]. Studies by Chen at al. [9] and Letchumanan et al. [15] also revealed that patients with baseline renal impairment had lower survival rates than those with normal baseline renal function. Our study identified presence of CKD as a significant factor associated with TMA in SLE patients. Among other factors such as hypertension, dyslipidemia, and cirrhosis, only CKD showed to be statistically significant risk factor. Baseline CKD is a major risk factor for development of TMA in SLE. Besides CKD, Asian/Pacific islander race was also associated with higher risk of developing TMA in SLE. There are not enough studies at this time to elaborate on this association, however it was previously reported that mortality from SLE has been three to six times higher in Asians than Whites [16,17].
Previously, Barrera-Vargas et al. [18] attempted to identify possible risk factors contributing to TMA in SLE patients, and further define TMA’s impact on clinical outcomes. This study showed lymphopenia and anti-Ro positivity as independent risk factors for developing renal TMA. It also showed no significant difference in outcomes for patients with similar eGFR with or without TMA, and no significant effect on renal survival in TMA patients [18]. A study out of Northern Taiwan including 2461 SLE patients highlighted infection as a potential major trigger for TMA occurrence [9]. Our study findings identified other potential factors significantly associated with increased risk of developing TMA among hospitalized SLE patients. Increased risk was associated with Asian/Pacific islander race and history of CKD, while decreased risk with age ≥ 40 years and Hispanic race.
Prior studies have also investigated the impact of TMA on morbidity and mortality for SLE patients. Chen et al. previously reported that age > 40 years, acute kidney injury, presence of infection, TMA features and low complement C3 < 60 mg/dL were risk factors for increased mortality among 25 SLE patients with TMA [9]. In addition, Peigne et al. demonstrated that cardiac involvement in SLE-associated TMA patients was associated with increased mortality while plasma exchange was a significant protective factor [19]. The clinical outcomes of patients with SLE-associated TMA may be dependent on ADAMTS13 activity. Some studies have identified that a subgroup of SLE-associated TMA patients with acquired ADAMTS13 deficiency had a relatively benign outcome, even though these patients presented with extreme thrombocytopenia and CNS symptoms [20,21]. In line with our study, SLE-associated TMA was linked to complications in almost every organ with increased risk of hemoptysis, pneumonia, neurologic manifestations such as encephalitis/myelitis/encephalopathy, ischemic stroke, seizure, hemolytic anemia, UTI, sepsis, glomerulonephritis and AKI. We also identified that TMA was associated with higher requirements for kidney biopsy, plasmapheresis, invasive mechanical ventilation and renal replacement therapy. With the identified increased morbidity and mortality of SLE-associated TMA, it was not surprising that TMA was also associated with significantly increased length of hospital stay and cost. The length of hospital stays and cost in SLE-associated TMA patients was increased by 12.4 days and $148,862 in comparison to their non-TMA counterparts, respectively.
Our study has several limitations. First, although the NIS database is a large hospitalization database in the U.S., due to limitations of the database, it is impossible to determine the onset of the SLE diagnosis prior to hospitalization and the disease activity. Second, the diagnosis of TMA was identified by ICD-9 diagnosis code. Only 21.3% of SLE patients with TMA had a kidney biopsy in our study representing that majority of TMA was diagnosed by clinical diagnosis. In addition, laboratory data and kidney biopsy results were not reported by the NIS. It would be clinically relevant to know the activities of SLE by laboratory parameters, and if renal involvement in these patients was due to concurrent lupus nephritis or isolated TMA. Third, the incidence of TMA in SLE patients may be underestimated, as less severe cases may have been managed in the outpatient setting. It would thus follow that the associated factors and morbidity identified in this study is specific to SLE patients requiring hospitalization. Lastly, there was no data on drug treatment in the NIS database. Consequently, we could not assess the treatment effects of medications, such as eculizumab, on outcomes in SLE patients with TMA [22].
5. Conclusions
In conclusion, TMA infrequently occurred in less than 1% of patients admitted for SLE, but it was associated with a significantly higher morbidity, mortality, and resource use. History of chronic kidney and Asian/Pacific Islanders were significantly associated with increased risk of TMA while age ≥ 40 years and Hispanics were significantly associated with decreased risk of TMA.
Acknowledgments
All authors had access to the data and played essential roles in writing of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-9721/9/1/3/s1.
Author Contributions
Conceptualization, A.I.P., C.T., P.H., W.K., F.Q., B.B., T.B., M.A.M., S.V. and W.C.; data curation, C.T. and W.K.; formal analysis, C.T. and W.K.; investigation, A.I.P., C.T., P.H., W.K. and W.C.; methodology, A.I.P., C.T., W.K. and W.C.; project administration, B.B. and T.B.; resources, W.K., B.B. and T.B.; software, W.K.; supervision, F.Q., M.A.M., S.V. and W.C.; validation, C.T., P.H., W.K., B.B. and W.C.; visualization, M.A.M. and W.C.; writing—original draft, A.I.P.; writing—review and editing, A.I.P., C.T., P.H., W.K., F.Q., B.B., T.B., M.A.M., S.V. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brocklebank V., Wood K.M., Kavanagh D. Thrombotic Microangiopathy and the Kidney. Clin. J. Am. Soc. Nephrol. 2018;13:300. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 3.Descombes E., Droz D., Drouet L., Grünfeld J.P., Lesavre P. Renal vascular lesions in lupus nephritis. Medicine (Baltim.) 1997;76:355–368. doi: 10.1097/00005792-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Banfi G., Bertani T., Boeri V., Faraggiana T., Mazzucco G., Monga G., Sacchi G. Renal vascular lesions as a marker of poor prognosis in patients with lupus nephritis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL) Am. J. Kidney Dis. 1991;18:240–248. doi: 10.1016/S0272-6386(12)80885-7. [DOI] [PubMed] [Google Scholar]
- 5.Barber C., Herzenberg A., Aghdassi E., Su J., Lou W., Qian G., Yip J., Nasr S.H., Thomas D., Scholey J.W., et al. Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clin. J. Am. Soc. Nephrol. 2012;7:757–764. doi: 10.2215/CJN.02870311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song D., Wu L.H., Wang F.M., Yang X.W., Zhu D., Chen M., Yu F., Liu G., Zhao M.H. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res. Ther. 2013;15:R12. doi: 10.1186/ar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridoux F., Vrtovsnik F., Noël C., Saunier P., Mougenot B., Lemaitre V., Dracon M., Lelièvre G., Vanhille P. Renal thrombotic microangiopathy in systemic lupus erythematosus: Clinical correlations and long-term renal survival. Nephrol. Dial. Transpl. 1998;13:298–304. doi: 10.1093/oxfordjournals.ndt.a027822. [DOI] [PubMed] [Google Scholar]
- 8.Couture P., Hié M., Pineton de Chambrun M., Galicier L., Costedoat-Chalumeau N., Deroux A., Kanouni T., Roumier M., Melki I., Mathian A., et al. FRI0302 Systemic lupus erythematosus-associated thrombotic microangiopathies in 60 patients: Clinical features, prognosis and treatment in a french multicentric cohort. Ann. Rheum. Dis. 2018;77:688. doi: 10.1136/annrheumdis-2018-eular.6871. [DOI] [Google Scholar]
- 9.Chen M.-H., Chen M.-H., Chen W.-S., Chang P.M.-H., Lee H.-T., Lin H.-Y., Huang D.-F. Thrombotic microangiopathy in systemic lupus erythematosus: A cohort study in North Taiwan. Rheumatology. 2011;50:768–775. doi: 10.1093/rheumatology/keq311. [DOI] [PubMed] [Google Scholar]
- 10.Park M.H., Caselman N., Ulmer S., Weitz I.C. Complement-mediated thrombotic microangiopathy associated with lupus nephritis. Blood Adv. 2018;2:2090–2094. doi: 10.1182/bloodadvances.2018019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kello N., Khoury L.E., Marder G., Furie R., Zapantis E., Horowitz D.L. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: Case series and review of literature. Semin. Arthritis Rheum. 2019;49:74–83. doi: 10.1016/j.semarthrit.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Hamasaki K., Mimura T., Kanda H., Kubo K., Setoguchi K., Satoh T., Misaki Y., Yamamoto K. Systemic lupus erythematosus and thrombotic thrombocytopenic purpura: A case report and literature review. Clin. Rheumatol. 2003;22:355–358. doi: 10.1007/s10067-003-0742-1. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Puerta J.A., Barbhaiya M., Guan H., Feldman C.H., Alarcón G.S., Costenbader K.H. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: A Hispanic and asian paradox. Arthritis Rheumatol. 2015;67:752–760. doi: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almaani S., Meara A., Rovin B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017;12:825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letchumanan P., Ng H.J., Lee L.H., Thumboo J. A comparison of thrombotic thrombocytopenic purpura in an inception cohort of patients with and without systemic lupus erythematosus. Rheumatology (Oxf.) 2009;48:399–403. doi: 10.1093/rheumatology/ken510. [DOI] [PubMed] [Google Scholar]
- 16.Kaslow R.A. High rate of death caused by systemic lupus erythematosus among U.S. residents of Asian descent. Arthritis Rheum. 1982;25:414–418. doi: 10.1002/art.1780250409. [DOI] [PubMed] [Google Scholar]
- 17.Serdula M.K., Rhoads G.G. Frequency of systemic lupus erythematosus in different ethnic groups in Hawaii. Arthritis Rheum. 1979;22:328–333. doi: 10.1002/art.1780220403. [DOI] [PubMed] [Google Scholar]
- 18.Barrera-Vargas A., Rosado-Canto R., Merayo-Chalico J., Arreola-Guerra J.M., Mejía-Vilet J.M., Correa-Rotter R., Gómez-Martín D., Alcocer-Varela J. Renal Thrombotic Microangiopathy in Proliferative Lupus Nephritis: Risk Factors and Clinical Outcomes: A Case-Control Study. J. Clin. Rheumatol. 2016;22:235–240. doi: 10.1097/RHU.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 19.Peigne V., Perez P., Resche Rigon M., Mariotte E., Canet E., Mira J.-P., Coppo P., Veyradier A., Azoulay E. Causes and risk factors of death in patients with thrombotic microangiopathies. Intensive Care Med. 2012;38:1810–1817. doi: 10.1007/s00134-012-2638-5. [DOI] [PubMed] [Google Scholar]
- 20.Matsuyama T., Kuwana M., Matsumoto M., Isonishi A., Inokuma S., Fujimura Y. Heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thromb. Haemost. 2009;102:371–378. doi: 10.1160/TH08-12-0825. [DOI] [PubMed] [Google Scholar]
- 21.Yue C., Su J., Gao R., Wen Y., Li C., Chen G., Zhang X., Li X. Characteristics and Outcomes of Patients with Systemic Lupus Erythematosus-associated Thrombotic Microangiopathy, and Their Acquired ADAMTS13 Inhibitor Profiles. J. Rheumatol. 2018;45:1549–1556. doi: 10.3899/jrheum.170811. [DOI] [PubMed] [Google Scholar]
- 22.Wright R.D., Bannerman F., Beresford M.W., Oni L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 2020;21:245. doi: 10.1186/s12882-020-01888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.