Abstract
The Gram-positive bacterium Clavibacter nebraskensis (Cn) causes Goss’s wilt and leaf blight on corn in the North American Central Plains with yield losses as high as 30%. Cn strains vary in aggressiveness on corn, with highly aggressive strains causing much more serious symptoms and damage to crops. Since Cn inhabits the host xylem, we investigated differences in the secreted proteomes of Cn strains to determine whether these could account for phenotypic differences in aggressiveness. Highly and a weakly aggressive Cn strains (Cn14-15-1 and DOAB232, respectively) were cultured, in vitro, in the xylem sap of corn (CXS; host) and tomato (TXS; non-host). The secretome of the Cn strains were extracted and processed, and a comparative bottom-up proteomics approach with liquid chromatography–tandem mass spectrometry (LC–MS/MS) was used to determine their identities and concentration. Relative quantitation of peptides was based on precursor ion intensities to measure protein abundances. In total, 745 proteins were identified in xylem sap media. In CXS, a total of 658 and 396 proteins were identified in strains Cn14-5-1 and DOAB232, respectively. The unique and the differentially abundant proteins in the secretome of strain Cn14-5-1 were higher in either sap medium compared to DOAB232. These proteins were sorted using BLAST2GO and assigned to 12 cellular functional processes. Virulence factors, e.g., cellulase, β-glucosidase, β-galactosidase, chitinase, β-1,4-xylanase, and proteases were generally higher in abundance in the aggressive Cn isolate. This was corroborated by enzymatic activity assays of cellulase and protease in CXS. These proteins were either not detected or detected at significantly lower abundance levels in Cn strains grown in non-host xylem sap (tomato), suggesting potential factors involved in Cn–host (corn) interactions.
Keywords: secretome, Clavibacter, xylem sap, corn, mass spectrometry
1. Introduction
The phytopathogenic bacterial genus Clavibacter belongs to the Microbacteriaceae family (Class Actinobacteria). Members of this genus are pathogens of economically important agricultural crops [1,2], causing significant yield losses [1,3]. One of the species, Clavibacter nebraskensis, is the causal agent of the Goss’s wilt and leaf blight disease of corn [4,5,6]. This bacterial pathogen causes foliar infections by entering wounds in leaf tissues produced by mechanical damage, e.g., hail, blowing sandy soil, farm machinery, and/or herbivore grazing [7,8,9]. After a heavy rain or hail, bacteria in infected debris splash onto wounded leaves allowing Cn to enter plant tissues. Disease symptoms first appear in the form of gray lesions with streaks of small water-soaked spots (freckles), before turning necrotic. Bacterial exudates appear shiny after drying on the infected tissues [10]. In systemic infection phase of the disease, the pathogen colonizes the xylem vessels and spreads throughout the plant [11], which might lead to colonization of the seeds, providing another source of inoculum [5]. Infection in the absence of wounding can also occur via natural openings under high humidity conditions [12]. Cn overwinters in corn stubble and in alternate host plants such as barnyardgrass (Echinochloa crus-galli), foxtail (Setaria spp.), shattercane (Sorghum bicolor), annual ryegrass, Johnson grass (Sorghum halepense), and large crabgrass [5,7,13,14].
A few studies have investigated Clavibacter–host interactions at the proteome level [15,16]. The secretome of Clavibacter michiganensis subsp. michiganensis (Cmm), the causal agent of tomato stem canker, was investigated in minimal and xylem-mimicking media, revealing that Cmm responds to xylem sap similarly as to sugar-depleted media by secreting virulence factors such as Pat-1 and CelA [16]. In another study, Savidor and colleagues [15] investigated the secretome and showed that Cmm secretes multiple hydrolytic proteins, such as serine proteases and glycosyl hydrolases, which promote infection and disable plant defense components. Infected tomato responds by activating defense genes such as pathogenesis-related (PR) and other defense-related proteins [15]. These studies demonstrated the reliability of using xylem sap to better understand host–pathogen interactions, especially for Cmm. This xylem-sap approach could be a suitable medium for studying Cn–corn interactions in vitro. However, to date, no published reports exit on the use of xylem sap to study Cn.
Here, we report differences in the secreted proteomes between a highly aggressive (Cn14-5-1) and a weakly aggressive (DOAB232 = NCPPB2581 = LMG 5627T) strains of Cn grown in corn xylem sap (CXS). These differences were less marked or, even, not observed when the strains were grown in a non-host tomato xylem sap (TXS), indicating that the host sap might contain relevant factors involved in triggering or inducing Cn pathogenicity traits. Our results indicated that the Cn-secreted proteins might be involved in scavenging and mobilizing host plant nutrients for bacterial growth and proliferation. In addition, Cn deployed known virulence factors that affect the integrity of the host plant’s cell walls and membranes, e.g., cell wall-degrading enzymes (CWDEs) and proteases, especially in the aggressive isolate. The proteomics-based observations were supported by in vitro enzymatic assays of total cellulases and proteases, which showed significantly higher activities in the aggressive isolate, Cn14-5-1. Finally, secretion of chaperone proteins, part of a repair mechanism for stress-damaged proteins is potential evidence of the ability of Cn to counteract and evade the host’s defense strategies.
2. Materials and Methods
2.1. Bacterial Growth Conditions
Two Cn strains, the highly aggressive Cn14-5-1, and the weakly aggressive DOAB232T (=NCPPB2581T), were streaked on nutrient broth yeast extract (NBY), containing 0.8% (w/v) nutrient broth, 0.2% (w/v) yeast extract, 0.2% (w/v) K2HPO4, 0.05% (w/v) KH2PO4, 0.5% (w/v) glucose, and 1.5% (w/v) agar. After autoclaving, 1 mL of 1 M MgSO4·7H2O was added [17]. The bacterial cultures were incubated at 23–24 °C for 4–5 days. The term aggressiveness of Cn strains refers to the level of leaf damage induced by tested strains.
2.2. Aggressiveness Assay
Corn plants were inoculated with the two Cn strains (Cn14-5-1 and DOAB232). Corn hybrid A4631G2 (RIB Pride Seeds), rated as Goss’s wilt-tolerant (2015 Crop Production Services Seed guide, Loveland, CO, USA), was used to rate the ability of the two strains to induce leaf damage. In brief, Cn strains were grown on NBY plates for 4–5 days at 23–24 °C, and then loops of bacterial colonies were suspended and diluted in phosphate buffer (10 mM potassium phosphate dibasic, and 10 mM potassium phosphate monobasic, pH 6.7) and the concentration was adjusted to 7 × 108 cfu·mL−1. Three leaves (third, fourth, and fifth) of corn plants at the V4-V5 stage were wounded on both sides of the middle vein using a 1 mL disposable syringe plunger mounted with a 5 mm sand paper disk. Twenty microliters of inoculum were applied onto each wound. The wounded control plants were inoculated with phosphate buffer. Plants were placed in a humidity tent with 100% relative humidity overnight. The area under the disease progress curve (AUDPC) was calculated for both lesion length and disease severity as previously described [18]. The experiment was performed in 3 biological replicates and the entire experiment was repeated once.
2.3. Hydrogen Peroxide and Superoxide Localization
Hydrogen peroxide (H2O2) was visualized in the infected corn leaves using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St. Louis, MO, USA). Infected leaves were submerged in DAB solution (1 mg·mL−1 DAB in 50 mM Tris–acetate buffer (pH 5.0) and vacuumed for 5 min, and thereafter kept in the dark for 24 h. Infiltrated leaves were subsequently bleached using ethanol/glycerol/glacial acidic acid (3:1:1) for 15 min in a water bath at 95 °C. The latter step was repeated 3–4 times to clear out the remaining chlorophyll. Superoxide (O2−) was visualized using 0.5 mg·mL−1 nitroblue tetrazolium (NBT) (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 10 mM potassium phosphate buffer (pH 7.6) for 6 h in the dark and then chlorophyll was bleached out as described above and photographed [19].
2.4. Xylem Sap Collection
Corn xylem sap was collected from 6–8-week-old plants grown in a greenhouse (22 °C, 18 h light and 16 °C, 8 h dark). Stems were cut 10–15 cm above the soil and cleaned twice using autoclaved wipes to remove leaking sap. The sap was collected using a micropipette for 1 h, filter-sterilized using 0.22 µm filters (Millipore, Burlington, MA, USA), and stored at −20 °C. Tomato xylem sap was collected from 4-week-old plants grown under the same conditions and using the same procedure.
2.5. Bacterial Induction with Xylem Sap
The two Cn strains were grown in NBY liquid media for 12 h at 23–24 °C. The concentration of the bacterial cultures was adjusted to OD600 = 1.0, and then equal volumes of the cultures were centrifuged for 20 min at 6000× g. The bacterial pellets were washed twice with sterilized distilled water. Bacterial pellets were suspended and incubated on an orbital shaker at 200 rpm for 12 h at room temperature in 20 mL in either corn xylem sap (CXS) or tomato xylem sap (TXS). The experiment was carried out with 3 biological replicates and the entire experiment was repeated once.
2.6. Protein Extraction, Reduction, Alkylation and Digestion
Bacterial cells were removed from the xylem sap by centrifugation for 20 min at 12,000× g, and the supernatant was filtered through 0.22 μm filters to remove any remaining cells. The filtrates were concentrated to approximately 300 µL using centrifugal devices (10 kDa cut-off, Millipore Sigma, Burlington, MA, USA). Proteins were harvested by precipitation in 10% (w/v) trichloroacetic acid (TCA)-acetone and 0.07% (w/v) dithiothreitol (DTT) overnight at −20 °C. Samples were then centrifuged at 12,000× g for 20 min and protein pellets washed 3 times with acetone–DTT. The final pellet was air-dried and suspended in 100 mM ammonium bicarbonate.
Proteins were reduced in 5 mM DTT and incubated for 25 min at 56 °C. After cooling to room temperature, alkylation was performed in 14 mM iodoacetamide in the dark for 30 min. Protein samples were then dialyzed using Slide-A-Lyzer MINI Dialysis Device (ThermoFisher Scientific, Waltham, MA, USA) in 100 mM ammonium bicarbonate buffer for 2 h at room temperature, and then overnight in fresh dialysis buffer at 4 °C. Proteins were quantified using a Bradford assay [20] with bovine serum albumin (BioRad Laboratories, Hercules, CA, USA) as the standard. Approximately 2 µg of soluble protein was digested using Gold MS-grade trypsin (Promega, San Luis Obispo, CA, USA) at 37 °C for 18 h. The digest was terminated by adding 0.1% (v/v) formic acid (FA), and the samples were dried under vacuum. Tryptic peptides were dissolved in 0.1% (v/v) trifluoroacetic acid (TFA) in 2% (v/v) acetonitrile (ACN) desalted using C18 spin cartridges (ThermoFisher Scientific) and eluted using 0.1% (v/v) FA in ACN, vacuum dried, and stored at −20 °C.
2.7. LC–MS Analysis
Mass spectrometry analysis of 2 µg tryptic peptides was performed using a Q Exactive hybrid quadrupole-Orbitrap mass spectrometer (ThermoFisher Scientific) coupled with a nanoflow NLeasy1000 HPLC unit (Thermofisher Scientfic). All 3 biological replicates from the TXS- and CXS-treated bacterial samples were analyzed, creating raw MS files for further analysis. Peptides were separated on an in-house packed C18 column (5 μm particles, 300 Å pores, 10 cm) eluting at 300 nL/min. A 2% (v/v) ACN to 80% ACN (v/v) gradient in 0.1% (v/v) FA was used for HPLC, with a total run time of 2 h. After acquisition, spectra were converted to Mascot Generic Format (MGF) files using Mascot Distiller v2.0: (Matrix Science, London, UK) and queried against the C. nebraskensis genomic sequence using the Mascot search engine v2.4 (Matrix Science). The following parameters were applied: trypsin with 1 missed cleavage permitted, fixed modification of carbamidomethyl on Cys, variable modifications of oxidation on Met, and deamidation on Asn or Gln. The precursor ion tolerance was ±5 ppm, and MS/MS tolerance was ±0.1 Da. Decoy searches of reversed sequences was enabled. The spectra acquired during all of the LC–MS runs were deposited to the ProteomeXchange Consortium via the PRIDE partner repository [21], with the dataset identifier PXD014510.
2.8. LC–MS Data Analysis
Data from LC–MS were loaded into Scaffold (v4.8.6: Proteome Software Inc., Portland, OR, USA). Scaffold normalizes the runs by multiplying each spectrum count in each sample by the average count over total spectrum count of the replicate. Scaffold v4.8.6 (Proteome Software Inc.) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 5% probability to achieve a false discovery rate (FDR) less than 0.5% by the Peptide Prophet algorithm [22], with Scaffold delta-mass correction. Protein identifications were accepted if they could be established at greater than 95% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm [23]. Proteins that contained similar peptides and could not be differentiated on the basis of MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters. The differential abundant proteins were statistically analyzed using Fisher’s exact test with probability ˂0.05 based on total spectra counts.
2.9. Cellulase Activity Assay
Total cellulase activity assay was carried out as previously described [24] on the concentrated Cn filtrates with minor modifications. Where necessary, different Cn filtrate volumes (250, 500, and 1000 µL) were adjusted to 1 mL with filtered xylem sap and added to 0.5 mL of 1% carboxymethyl cellulose (CMC) as a substrate. The mixture was incubated at 40 °C in a water bath for 30 min. Thereafter, reactions were stopped by adding 3 mL of 3,5-dinitrosalicylic acid reagent (DNS) and then the color was developed by placing the samples in boiling water for 5 min. Reaction mixtures were placed in ice-cooled water to quench the reaction, and then 20 mL distilled water was added to each mixture. Absorbance was measured with the Harvard Biochrom Ultrospec 2100 pro UV/Visible Spectrophotometers (ThermoFisher Scientific) at 540 nm against the blank. The experiment was repeated once with 3 replications each. A standard curve was prepared using 0.2% (w/v) glucose.
2.10. Protease Activity Assay
Total protease activity was measured in the concentrated filtrates using casein as substrate [25]. In brief, different Cn filtrate volumes (250, 500, and 1000 µL) were adjusted to 1 mL with the enzyme solution (10 mM sodium acetate buffer with 5mM calcium, pH 7.5) to make a final volume of 1 mL. The assay was carried out by using 5 mL casein solution equilibrated at 37 °C in a water bath, and then 1 mL filtrate concentrates was added to casein solution and incubated at 37 °C for 10 min. After incubation, 5 mL TCA solution was added to stop the reaction. The final volume in the blank tubes was adjusted by adding 1 mL of the enzyme solution. Samples were incubated at 37 °C for 30 min in a water bath, then filtered using a 0.45 µm filter. Color was developed by adding 2 mL sample mixture with 5 mL sodium carbonate solution and 1 mL Folin’s reagent. Samples were incubated at 37 °C for 30 min in a water bath, then filtered using 0.45 µm filters. The absorbance of the developed blue color was measured with the Harvard Biochrom Ultrospec 2100 pro UV/Visible Spectrophotometers (ThermoFisher Scientific) at a wavelength of 660 nm. The activity was calculated on the basis of a standard curve generated using l-tyrosine (Sigma-Aldrich, St. Louis, MO, USA). The experiment was carried out with 3 biological replications and the entire experiment was repeated once.
2.11. Statistical Analysis
The analysis was performed on data collected from 3 biological replicates (n = 3) for each strain. Standard curves were used to convert spectra values into concentrations for total cellulases and proteases activities, while the disease severity scale values were converted into a percentage prior to statistical analysis. Statistical analysis was performed by the Statistical Analysis Software (SAS) (SAS Institute, Cary, NC, USA; release 9.1) using PROC MIXED module. Data were tested for normality using PROC UNIVARIATE and outliers were removed using Lund’s test [26] (Lund, 1975). The mean values were separated by least squared means and letters assigned by the macro PDMIX800.sas [27] (Saxton, Tadcaster, UK, 1998) with α = 0.05.
3. Results
3.1. Aggressiveness Assay
The total area under the disease progress curve (total AUDPC) of both disease severity and lesion length (Figure S1) indicated that the Cn strain Cn14-5-1 was significantly more aggressive than strain DOAB232. Strain Cn14-5-1 induced significant damage to corn leaves beginning with developing water-soaked spots, which later turned necrotic. Strain DOAB232 induced significantly low damage, limited to a few millimeters in size, from the inoculation site, leaving the rest of the inoculated leaves undamaged (Figure S1).
3.2. Characterisation of Cn Secretome by LC–MS Analysis
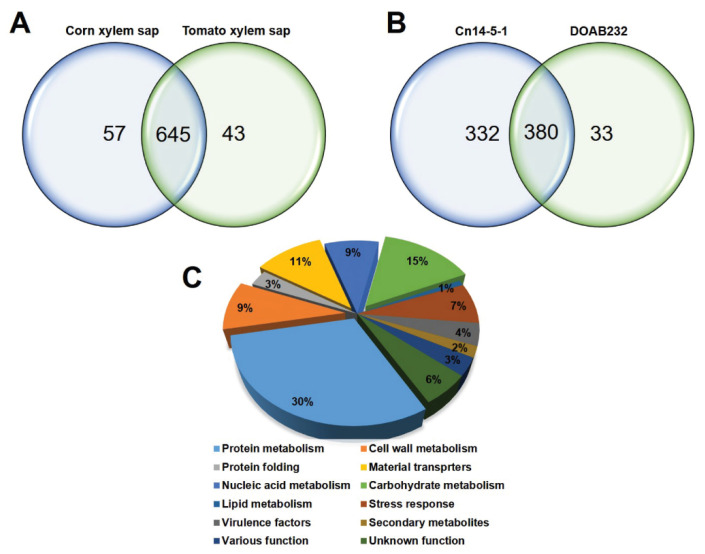
The total secretome profile of Cn strains Cn14-5-1 and DOAB232, grown in CXS and TXS, is shown in Table S1. A total of 745 proteins were identified in the bacterial secretomes for both strains in both xylem sap media (Table S1). A total of 645 proteins were observed in both sap media with only 57 and 43 proteins identified in CXS and TXS, respectively, for the two Cn strains (Figure 1A). On the other hand, in sap media, a total of 380 proteins were detected between the two Cn strains, with 332 and 33 proteins uniquely recorded by Cn14-5-1 and DOAB232, respectively (Figure 1B). Using the BLAST2GO algorithm, we categorized these secreted proteins into 12 cellular functional processes involved in protein metabolism (30.6%), cell wall metabolism, protein folding, material transporters, nucleic acid metabolism, carbohydrate metabolism, lipid metabolism, stress response, secondary metabolites, various functions, and a group of proteins with unknown function (Figure 1C).
Figure 1.
Categories of the identified secretome proteins of Cn strains and their abundance. (A) Venn diagram shows unique and differentially abundant proteins under corn xylem sap (CXS) and tomato xylem sap (TXS) media. (B) Venn diagram shows unique and differentially abundant proteins of Cn14-5-1 and DOAB232 strains. (C) Different classes and the abundance of the identified protein shown on the basis of the predicted biological function.
Table 1 shows the number of proteins on the basis of their status in the different secretome pools—presence/absence and uniquely or differentially abundance. The total identified proteins of Cn14-5-1 were approximately double those of DOAB232 in both sap media. Strains Cn14-5-1 and DOAB232 responded differently to CXS and TXS. The secretome patterns of strain Cn14-5-1 in both CXS and TXS were similar while strain DOAB232 exhibited more secreted proteins in CXS. Proteins that were identified only in CXS could more likely contribute to the bacterial colonization and/or pathogenicity on corn rather than those identified in both sap media.
Table 1.
Total secreted proteins in Cn isolates under different sap media.
| Identified Proteins | Corn Xylem Sap (CXS) | Tomato Xylem Sap (TXS) | ||
|---|---|---|---|---|
| Cn14-5-1 | DOAB232 | Cn14-5-1 | DOAB232 | |
| Total proteins | 658 | 396 | 678 | 229 |
| Unique proteins | 269 | 40 | 459 | 10 |
| Differentially abundant | 270 | 40 | 200 | 18 |
(CSX) corn xylem sap, (TXS) tomato xylem sap.
3.3. Abundance of Secreted Proteins in Xylem Sap Media
Proteins with various abundance levels were identified in the secretome of the Cn strains in the xylem sap media. Differentially or uniquely abundant proteins were identified in each strain under each sap medium. A total of 269 and 459 proteins were identified only in Cn14-5-1, the highly aggressive strain, under CXS (Table S2) and TXS (Table S3), respectively. These include well-characterized protein families involved in carbon metabolism (e.g., glucose-6-phosphate dehydrogenase), protein degradation (e.g., peptidases), and antibiotic resistance (e.g., putative multidrug export ABC transporters as well as a CelA-like protein, a known pathogenicity trait) (Tables S2 and S3). Further, a total of 270 and 200 proteins were differentially abundant in CXS (Table S4) and TXS (Table S5), respectively. Examples of uniquely and differentially abundant proteins secreted in both media are presented in Table 2 and Table 3, respectively, and were shown to exhibit the presence of a wide range of functional groups such as stress response, pathogenicity, protein degradation/folding, virulence, and carbohydrate metabolism. The majority of the unique proteins in Cn14-5-1 strain were identified under CXS conditions (Table 2). Some proteins with cellulase activity exhibited increased abundance in Cn14-5-1 under CXS, e.g., secreted cellulase, and cellulase-binding and expansin domain-containing proteins (Table 3). Unlike Cn14-5-1, only 40 and 10 secreted proteins were uniquely observed for DOAB232, the low aggressive strain, in CXS (Table S6) and TXS (Table S7), respectively. The number of differentially abundant proteins secreted in both sap media by DOAB232 also showed low counts of 40 and 18 for CXS (Table S8) and TXS (Table S9), respectively. The majority of uniquely abundant proteins of DOAB232 strain was identified in CXS, which mainly belonged to stress response proteins. A few proteins, such as endo-1,4-beta-xylanase and secreted serine protease, which are suggested to be potential pathogenicity factors, were identified in both sap media (Table 4). Several proteins that were differentially abundant in either sap media belonged to stress response and pathogenicity groups, e.g., 10 Kb chaperonin, serine protease, and endo-1,4-beta-xylanase (Table 5).
Table 2.
Proteins identified only in Cn14-5-1 in the different sap media.
| Accession No. | Identified Proteins | MW (KDa) | Gene/Locus | Type of Sap | Functional Group | |
|---|---|---|---|---|---|---|
| CXS | TXS | |||||
| CCE76040.1 | Serine peptidase, family S9X | 76 | CMN_02099 | + | − | Protein degradation |
| CCE75111.1 | pepP2 protein | 57 | pepP2 | + | − | Protein degradation |
| CCE76391.1 | Polyphosphate kinase | 82 | ppK | + | − | Protein phosphorylation |
| CCE75799.1 | Glucokinase | 33 | glkA | + | − | Catabolite repressor |
| CCE74648.1 | Radical SAM domain-containing protein | 46 | CMN_00689 | + | − | Stress response |
| CCE76022.1 | Protease II (oligopeptidase B) | 80 | ptrB | + | − | Protein degradation |
| CCE75620.1 | Metallopeptidase, peptidase family M20A | 47 | CMN_01675 | + | − | Protein degradation |
| CCE74827.1 | Putative tyrosine-protein kinase | 49 | CMN_00871 | + | − | Stress response |
| CCE74652.1 | Oxidoreductase | 35 | CMN_00693 | + | + | Stress response |
| CCE75671.1 | Transcriptional regulator, WhiA family | 35 | whiA | + | − | Signal transduction |
| CCE74496.1 | Thioredoxin | 21 | ccbD | + | − | Stress response |
| CCE75993.1 | Oxidoreductase | 36 | CMN_02051 | + | + | Stress response |
| CCE75476.1 | NUDIX hydrolase | 19 | CMN_01528 | + | + | Virulence |
| CCE76025.1 | Cupin_2 domain-containing protein | 32 | CMN_02083 | + | + | Virulence |
| AHN16207.1 | Cellulase | 78 | CelA | + | + | Pathogenicity |
| CCE75945.1 | Metallopeptidase, family M16B | 48 | pepR | + | − | Protein degradation |
| CCE76486.1 | Methionine aminopeptidase | 29 | mapA2 | + | − | Protein degradation |
| CAN00621.1 | Porphobilinogen deaminase | 34 | hemC | + | + | Pathogenicity |
| AJW80248.1 | Manganese catalase | 31 | DZF93_04220 | + | + | Stress response |
| CCE74906.1 | ManB protein | 61 | manB | + | + | Pathogenicity |
| CCE74104.1 | Putative glycosyl hydrolase, family 2 | 69 | CMN_00123 | + | − | Pathogenicity |
| AJW78817.1 | ATP-dependent Clp protease proteolytic subunit | 21 | clpP | + | + | Stress response |
| CCE76317.1 | Serine peptidase, family S1C | 51 | CMN_02381 | + | − | Protein degradation |
| CCE75886.1 | NTP pyrophosphatase | 22 | CMN_01943 | + | − | Signal transduction |
| CCE74756.1 | Putative secreted metalloprotease | 46 | CMN_00799 | + | − | Protein degradation |
| CCE76463.1 | Alanine racemase/kinase fusion protein | 59 | alr2 | + | − | Signal transduction |
| AJW79024.1 | HAD family phosphatase | 25 | VO01_07690 | + | − | Signal transduction |
| AJW80208.1 | MarR family transcriptional regulator | 17 | DZF93_09435 | + | − | Signal transduction |
| CCE74692.1 | Chloride anion channel | 25 | CMN_00734 | − | + | Pathogenicity |
| CCE74774.1 | ATP-dependent protease, ATPase subunit | 92 | clpC | − | + | Protein degradation |
| CCE74648.1 | Radical SAM domain-containing protein | 46 | CMN_00689 | − | + | Stress response |
| CCE76686.1 | Alpha-glucosidase, glycosyl hydrolase family 13 | 64 | aglC | − | + | Pathogenicity |
| CCE75391.1 | ATP-dependent protease, proteolytic subunit | 25 | clpP2 | − | + | Stress response |
| CCE76068.1 | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase | 38 | dxr | − | + | Pathogenicity |
| CCE75358.1 | Glucan debranching enzyme | 82 | treX | − | + | Pathogenicity |
| CCE74827.1 | Putative tyrosine-protein kinase | 49 | CMN_00871 | − | + | Stress response |
| CCE76839.1 | 2-Keto acid dehydrogenase | 41 | CMN_02915 | − | + | Energy production |
| CCE74969.1 | Lipoprotein | 59 | lpqB | − | + | Pathogenicity |
| CCE74801.1 | Beta-glycosidase | 54 | bglJ | − | + | Pathogenicity |
| CCE76286.1 | Alpha glycosidase | 81 | CMN_02350 | − | + | Pathogenicity |
| AJW79410.1 | Sugar kinase | 32 | VO01_09940 | − | + | Signal transduction |
| CCE74250.1 | Alpha-L-arabinofuranosidase | 55 | abfA1 | − | + | Pathogenicity |
| AJW78269.1 | Organic hydroperoxide resistance protein | 14 | Ohr | − | + | Stress response |
(CSX) corn xylem sap, (TXS) tomato xylem sap, (+) presence, (−) absence.
Table 3.
Differentially abundant proteins in Cn14-5-1 in different sap media.
| Accession No. | Identified Proteins | MW (KDa) | Gene/Locus | Fisher’s Exact Test | Type of Sap | Fold Change | Functional Group | |
|---|---|---|---|---|---|---|---|---|
| p-Value (p < 0.05) | CXS | TXS | ||||||
| CCE76069.1 | Secreted peptidyl-prolyl cis-trans isomerase | 34 | CMN_02128 | 0.00013 | − | + | 3.2 | Protein folding |
| ALD12817.1 | Cold-shock protein | 7 | AES38_07750 | 0.00018 | − | + | 38 | Stress response |
| CCE74756.1 | Putative secreted metalloprotease | 46 | CMN_00799 | 0.00029 | − | + | 80 | Protein degradation |
| CCE75860.1 | Secreted lipase | 30 | CMN_01917 | 0.00047 | + | − | 10 | Pathogenicity |
| CCE76016.1 | Beta-galactosidase, lactase | 113 | lacZ | 0.001 | − | + | 42 | Pathogenicity |
| CCE75767.1 | Metallopeptidase | 49 | CMN_01822 | 0.0016 | + | − | 14 | Protein degradation |
| CCE74047.1 | Chaperone protein dnaK | 67 | dnaK | 0.0017 | + | − | 6.1 | Protein folding |
| CCE74654.1 | Catalase | 57 | katA | 0.0018 | + | − | 5.8 | Stress response |
| CCE75796.1 | Non-specific serine/threonine protein kinase | 69 | pknE | 0.0051 | − | + | 3.3 | Virulence |
| CCE75569.1 | Leucine aminopeptidase, family M17 | 52 | pepA | 0.006 | + | − | 6.9 | Protein degradation |
| CCE74438.1 | Transcriptional regulator, LytR family | 43 | CMN_00472 | 0.009 | + | − | 13 | Virulence |
| CCE74438.1 | Transcriptional regulator, LytR family | 43 | CMN_00472 | 0.013 | − | + | 48 | Virulence |
| CCE75659.1 | Transketolase | 75 | tktA | 0.015 | + | − | 5.8 | Stress response |
| CCE74692.1 | Chloride anion channel | 25 | CMN_00734 | 0.038 | + | − | 11 | Virulence |
| CCE75389.1 | Peptidyl-prolyl cis-trans isomerase | 52 | tig | 0.043 | − | + | 14 | Pathogenicity |
| CCE74969.1 | Lipoprotein | 59 | lpqB | 0.044 | + | − | 22 | Virulence |
| CCE74654.1 | Catalase | 57 | katA | 0.071 | − | + | 9.2 | Stress response |
| CCE75600.1 | Endo-1,4-beta-xylanase | 71 | xysB | 0.084 | − | + | 2.5 | Pathogenicity |
| AJW79410.1 | Sugar kinase | 32 | VO01_09940 | 0.089 | + | − | 18 | Signal transduction |
| CCE76365.1 | 60 KDa chaperonin | 57 | groEL | <0.00010 | + | − | 19 | Protein folding |
| CCE74122.1 | Cellulose-bindingand an expansin domain | 37 | CMN_00144 | <0.00010 | + | − | 16 | Pathogenicity |
| CCE76326.1 | Secreted cellulase | 58 | cel | <0.00010 | + | − | 11 | Pathogenicity |
| CCE75788.1 | FtsI protein | 62 | ftsI | <0.00010 | + | − | 30 | Pathogenicity |
| CCE75200.1 | Serine protease, family S1C | 50 | CMN_01248 | <0.00010 | + | − | 18 | Protein degradation |
| CCE76016.1 | Beta-galactosidase, lactase | 113 | lacZ | <0.00010 | + | − | 13 | Pathogenicity |
| CCE75860.1 | Secreted lipase | 30 | CMN_01917 | <0.00010 | − | + | 41 | Pathogenicity |
| CCE76365.1 | 60 KDa chaperonin | 57 | groEL | <0.00010 | − | + | 37 | Protein folding |
| CCE75200.1 | Serine protease, family S1C | 50 | CMN_01248 | <0.00010 | − | + | 29 | Protein degradation |
| CCE74047.1 | Chaperone protein dnaK | 67 | dnaK | <0.00010 | − | + | 22 | Protein folding |
| CCE74122.1 | Cellulose-binding and an expansin domain | 37 | CMN_00144 | <0.00010 | − | + | 12 | Pathogenicity |
| CCE76326.1 | Endoglucanase | 58 | cel | <0.00010 | − | + | 3 | Pathogenicity |
| CCE76173.1 | Secreted serine peptidase family S8 | 43 | CMN_02235 | <0.00010 | − | + | 2.1 | Protein degradation |
| CAQ02078.1 | Glucose-6-phosphate 1-dehydrogenase | 58 | Zwf | 0.025 | + | − | 4.9 | Carb. Metabolism |
| CCE75669.1 | Glyceraldehyde 3-phosphate dehydrogenase | 36 | gapA | <0.00010 | + | − | 7.9 | Carb. Metabolism |
| CCE74538.1 | Putative levansucrase | 65 | sacB | 0.00051 | − | + | 4.3 | Carb. Metabolism |
| AJW79067.1 | OpcA protein | 35 | DZF93_01230 | 0.013 | + | − | 14 | Virulence |
| CCE75796.1 | PASTA domain containing Ser/Thr kinase | 69 | pknE | 0.025 | + | − | 2.3 | Virulence |
| CCE74059.1 | Glycosyl hydrolase, (chitinase) family 18 | 39 | CMN_00077 | 0.0026 | + | − | 1.6 | Pathogenicity |
(CSX) corn xylem sap, (TXS) tomato xylem sap, (+) presence, (−) absence.
Table 4.
Proteins identified only in DOAB232 in different sap media.
| Accession No. | Identified Proteins | MW (KDa) | Gene/Locus | Type of Sap | Functional Group | |
|---|---|---|---|---|---|---|
| CXS | TXS | |||||
| CCE75047.1 | Putative secreted 5’-nucleotidase | 73 | CMN_01095 | + | + | Nucleotide catabolism |
| CAQ00484.1 | Putative solute-binding lipoprotein | 46 | CMS0363 | + | + | Virulence |
| AJW80270.1 | Integration host factor | 10 | VO01_15120 | + | − | Stress response |
| CCE74134.1 | Rhodanese domain-containing protein | 10 | CMN_00157 | + | − | Stress response |
| CCE74199.1 | Sugar ABC transporter | 43 | CMN_00224 | + | − | Cell surface |
| CCE75732.1 | Rhodanese domain-containing protein | 12 | CMN_01787 | + | − | Stress response |
| AJW79484.1 | Exodeoxyribonuclease VII small subunit | 9 | xseB | + | − | DNA catabolic |
| AJW78093.1 | General stress protein CsbD | 6 | DZF93_05670 | + | − | Stress response |
| CCE74980.1 | Alkyl hydroperoxide reductase | 17 | bcp | + | − | Stress response |
| AJW79401.1 | Antibiotic biosynthesis monooxygenase | 11 | DZF93_04600 | + | − | Stress response |
| CCE74048.1 | Heat shock chaperone GrpE | 24 | grpE | + | − | Protein folding |
| CCE76425.1 | Secreted serine protease, peptidase family S8A | 121 | sbtB | + | + | Protein degradation |
| CCE75599.1 | Endo-1,4-beta-xylanase | 45 | xysA | + | + | Pathogenicity |
| CCE76583.1 | Endoglucanase, glycosyl hydrolase family 26 | 47 | CMN_02651 | − | + | Pathogenicity |
| CCE74634.1 | Esterase | 25 | CMN_00675 | − | + | Virulence |
(CSX) corn xylem sap, (TXS) tomato xylem sap, (+) presence, (−) absence.
Table 5.
Differentially abundant proteins in strain DOAB232 grown in the different sap media.
| Accession No. | Identified Proteins | MW (KDa) | Gene/Locus | Fisher’s Exact Test | Type of Sap | Fold Change | Functional Group | |
|---|---|---|---|---|---|---|---|---|
| p-Value (p < 0.05) | CXS | TXS | ||||||
| CCE74709.1 | Putative extracellular nuclease | 74 | CMN_00751 | <0.00010 | + | − | 6.0 | Nucleotide degradation |
| A5CU64.1 | 10 kDa chaperonin | 11 | groES | <0.00010 | + | − | 1.8 | Protein folding |
| ALD12817.1 | Cold-shock protein | 7 | AES38_07750 | <0.00010 | + | − | 2.1 | Stress response |
| CCE76393.1 | Phosphate-binding protein PstS | 37 | pstS | <0.00010 | + | − | 1.2 | Transport protein |
| CCE76173.1 | Secreted serine peptidase family S8 | 43 | CMN_02235 | <0.00010 | + | − | 1.4 | Protein degradation |
| CCE74340.1 | Levan fructotransferase | 57 | CMN_00371 | <0.00010 | + | − | 4.1 | Carb. metabolism |
| CCE76401.1 | Putative hydrolase | 22 | CMN_02466 | <0.00010 | + | − | 3.4 | Protein degradation |
| CCE76077.1 | Putative RTX toxin | 204 | CMN_02136 | <0.00010 | + | − | 10.0 | Virulence |
| CCE76590.1 | Sugar ABC transporter | 48 | CMN_02658 | <0.00010 | + | − | 7.5 | Transport protein |
| CCE75600.1 | Endo-1,4-beta-xylanase | 71 | xysB | <0.00010 | + | − | 6.0 | Pathogenicity |
| CCE75697.1 | FKBP-type peptidyl-prolyl cis-trans isomerase | 13 | CMN_01752 | <0.00010 | + | − | 8.0 | Protein folding |
| CCE76397.1 | Anti-sigma factor | 29 | CMN_02462 | 0.0017 | + | − | 1.8 | Stress response |
| CCE75288.1 | Membrane-associated serine peptidase | 40 | CMN_01337 | 0.0057 | + | − | 1.5 | Protein degradation |
| CCE74709.1 | Putative extracellular nuclease | 74 | CMN_00751 | <0.00010 | − | + | 8.4 | Pathogenicity |
| CCE75697.1 | FKBP-type peptidyl-prolyl cis-trans isomerase | 13 | CMN_01752 | 0.001 | − | + | 4.0 | Protein folding |
| CCE76165.1 | Ferritin-like domain-containing protein | 32 | CMN_02227 | <0.00010 | − | + | 3.5 | Element acquisition |
| CCE74340.1 | Levan fructotransferase | 57 | CMN_00371 | <0.00010 | − | + | 2.7 | Carb. metabolism |
| CCE75152.1 | Agglutinin receptor precursor | 48 | CMN_01200 | <0.00010 | − | + | 2.5 | Signal transduction |
| CCE76077.1 | Putative RTX toxin | 204 | CMN_02136 | <0.00010 | − | + | 2.0 | Pathogenicity |
| CCE76397.1 | Anti-sigma factor | 29 | CMN_02462 | <0.00010 | − | + | 1.3 | Stress response |
| CCE74757.1 | NPL/P60 family endopeptidase | 45 | CMN_00800 | <0.00010 | − | + | 1.3 | Protein degradation |
| AJW79074.1 | Superoxide dismutase | 23 | SOD | 0.027 | − | + | 5.2 | Stress response |
| CCE76077.1 | Putative RTX toxin | 204 | CMN_02136 | <0.00010 | + | − | 10 | Virulence |
| CCE75795.1 | Secreted LysM peptidoglycan-binding protein | 43 | CMN_01850 | <0.00010 | + | − | 2.4 | Virulence |
(CSX) corn xylem sap, (TXS) tomato xylem sap, (+) presence, (−) absence.
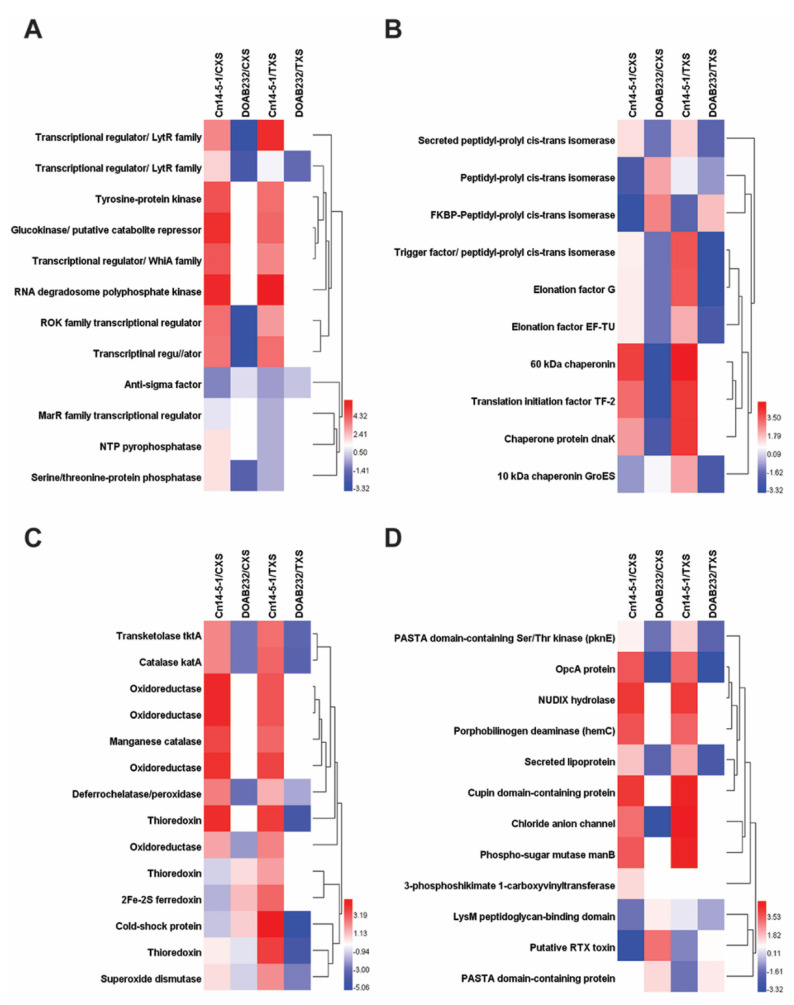
Figure 2 shows the fold change patterns of key stress-related protein families of the two Cn strains treated with CXS and TXS media. Figure 2A shows the changes in 12 secreted proteins that are related to signal perception and transduction. All the secreted proteins exhibited an increment with the exception of the anti-sigma factor, which showed a differentially reduced abundance in strain Cn14-5-1. RNA degradosome polyphosphate kinase and glucokinase (putative catabolite repressor) exhibited the highest fold changes in both xylem sap media for the highly aggressive strain (Figure 2B). The weakly pathogenic strain (DOAB232) either showed no fold change or a reduced abundance for all the 12 secreted proteins. With respect to the 10 protein folding enzymes analyzed, significant increases were observed only in 3 (60 kDa chaperonin, translation initiation factor TF-2, and chaperone protein dnaK) for strain Cn-14-5-1, while DOAB232 had significant increases only for FKBP-peptidyl-polyl-cis-transisomerase in both xylem sap media (Figure 2B).
Figure 2.
Stress-related proteins patterns of the two strains of Clavibacter nebraskensis treated with different xylem sap media. Secretome profile of Cn14-5-1 was normalized by that of the DOAB232 strain, and vice versa in each sap medium to identify the differential stress-related protein profile under corn xylem sap (CXS) and tomato xylem sap (TXS) induction. (A) Signal perception and transduction; (B) protein folding; (C) reactive oxygen species (ROS) scavenging proteins; and (D) virulence-related proteins. Hierarchical clustering was performed using the group average (unweighted pair-group method (UWPGM)) method.
In addition, proteins with predicted antioxidant activities were identified in both strains when treated with both xylem sap media (Figure 2C). Of the 14 secreted proteins, 9 differentially increased in both xylem sap media for strain Cn14-5-1, while none exhibited significant increase for DOAB232 (Figure 2C). These include catalase (KatA), transketolase tktA, oxidorectuxtase, manganese catalase, organic hydroperoxide resistance protein (Ohr), and thioredoxins differentially increased abundance levels in both xylem sap media in Cn14-5-1 (Figure 2C).
Figure 2D shows the changes in patterns of 12 secreted proteins reported to have potential virulence activities. Strain Cn14-5-1 exhibited significant fold increases in 7 (OpcA protein, NUDIX hydrolase, porphobilinogen deaminase, secreted lipoprotein, Cupin domain-containing protein, chloride anion channel, and phospo-sugar mutase manB) of the 12 virulence-related proteins in CXS and TXS (Figure 2D). On the other hand, fibronectin type III domain-containing protein (putative RTX toxin) was the only virulence-related protein out of 12 that exhibited a differentially increased abundance in DOAB232 in CXS (Figure 2D). PASTA domain-containing protein was the only secreted protein that showed moderate (about 1.5-fold change) in both xylem sap media by strain DOAB232 (Figure 2D).
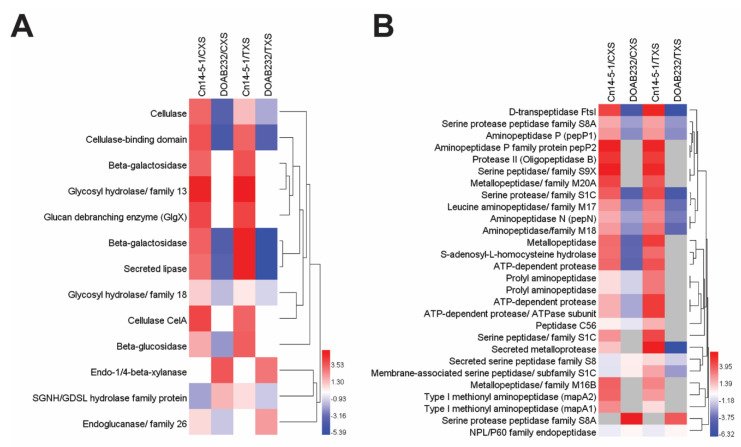
Different cell wall-degrading enzymes (CWDEs), belonging to four glycosyl hydrolase families (GH3, GH5, GH18, and GH26), varied in abundance in the Cn secetome (Figure 3). However, the abundance levels of the proteins with hydrolytic activity were higher in the secretome of the Cn14-5-1 than DOAB232 (Figure 3A). Significant fold changes were recorded in 10 of the 13 secreted proteins detected in the secretome of strain Cn14-5-1 in both sap media (Figure 3A). The 10 proteins included cellulase, beta-galactosidase, glycosyl hydrolase, glucan debrabching enzyme (GigX), CelA, and beta-glucosidase (Figure 3A). On the other hand, the weakly aggressive strain (DOAB232) exhibited significant positive changes for only endo-1,4-β-xylanases anddetected in both sap media (Figure 3A).
Figure 3.
Hydrolytic proteins patterns of the two strains of Clavibacter nebraskensis treated with different xylem sap media. Secretome profile of Cn14-5-1 was normalized by that of the DOAB232 strain, and vice versa in each sap medium to identify the differential stress-related protein profile under corn xylem sap (CXS) and tomato xylem sap (TXS) induction. (A) Cell wall-degrading enzymes, (B) proteases. Hierarchical clustering was performed using the group average (unweighted pair-group method (UWPGM)) method.
Two classes of peptidases, serine peptidase and metallopeptidase families, with seven different peptidase families from each class, were identified in the Cn secretomes (Figure 3B). In general, the majority of the proteins with proteolytic activity were detected in the secretome of the Cn14-5-1 strain (Figure 3B). Different protein peptidases were observed in the secretome of strain Cn14-5-1 only. However, some aminopeptidase classes belonging to various subfamilies, and metallopeptidase were differentially abundant in Cn14-5-1 (Figure 3B). Some serine protease peptidase family S8A such as secreted subtilase B (sbtB) were differentially abundant only in DOAB232 in both xylem sap media (Figure 3B).
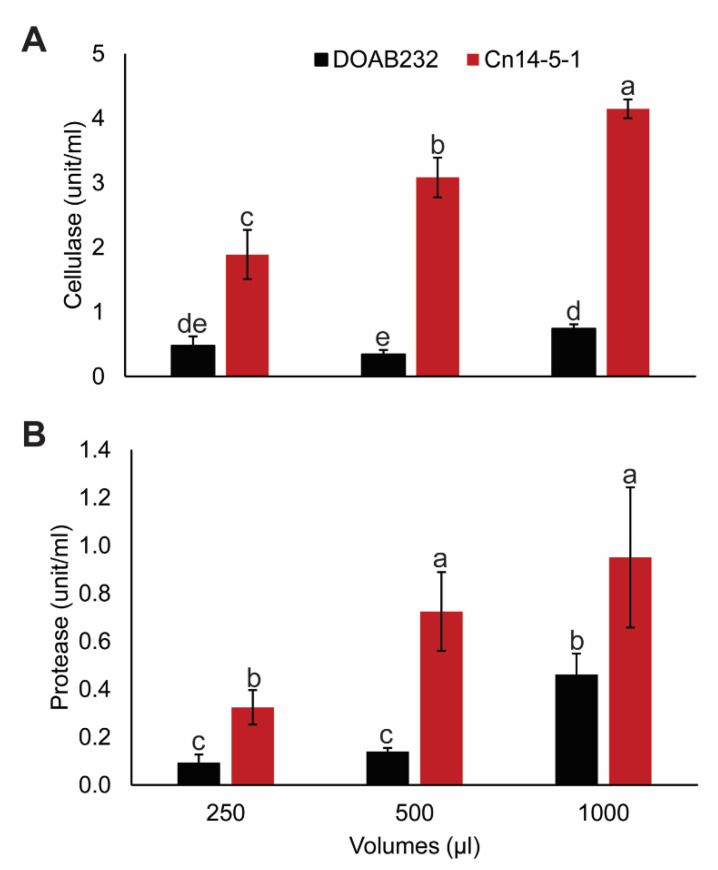
3.4. Hydrolytic Enzyme Activity Assays
Cellulase activity in the Cn14-5-1 strain increased significantly with increasing volume of CXS filtrates, which ranged from 2–4 units·mL−1, while the cellulose activity in the filtrate of the DOAB232 strain showed significantly lower increases in activity (Figure 4A). A similar trend was observed for protease activity with strain Cn14-5-1, showing significantly higher activity of 0.3 to 0.9 units·mL−1 compared to 0.09 to 0.4 units·mL−1 for DOAB232 (Figure 4B). Both strains showed increases in protease activity with increasing filtrate volumes (Figure 4B).
Figure 4.
Enzymatic activity of secreted cellulases and proteases in filtrates of Clavibacter nebraskensis strains. The weakly aggressive DOAB232 strain in black and the highly aggressive Cn14-5-1 strain in red. (A) Enzymatic activity of total cellulase, and (B) protease were assayed in Cn filtrates. The mean values (n = 3) were separated by least squared means, and values with the same letter are not significantly different at (p < 0.05).
3.5. Hydrogen Peroxide and Superoxide Assays
Reactive oxygen species, hydrogen peroxide (H2O2), and superoxide (O2−) were detected using DAB and NBT, respectively, and localized in corn leaves infected with Cn strains Cn14-5-1 and DOAB232. They were compared to non-infected controls, which exhibited very low staining. Leaves infected with either Cn strains showed deep brown and blue staining for H2O2 and O2−, respectively, around the inoculation sites (Figure S2), suggesting the host cell response by the production of reactive oxygen species.
4. Discussion
This study profiled the secretomes of two Cn strains that differ in the level of aggressiveness or pathogenicity to corn. This is the first report of the use of LC–MS analysis to investigate the secreted proteins of the corn pathogen, C. nebraskensis, and the data presented here will enable a better understanding of the pathogenicity traits in Cn. The generated proteomic data would be a step forward to refine the annotation of the sequenced genome of C. nebraskensis. A recent study conducted by Peritore-Galve and colleagues, investigated the implement of proteome peptide sequences to refine the genome annotations of the Clavibacter michiganensis susp. michiganensis, the causal agent of bacterial canker of tomato, to create a system called proteogenomics [28].
The discrepancies in aggressiveness between Cn14-5-1 and DOAB232, which presented different symptoms clearly on corn, and resulted in different levels of plant damage, provided a platform to study, in vitro, Cn aggressiveness at the protein level using natural xylem sap media. Strain Cn14-5-1 was isolated in 2014, while DOAB 232 was isolated over 40 years ago. Agarkova and colleagues [9] indicated that strain DOAB 232 was pathogenic by direct injection of the bacterial suspension into corn stem. In our study, we used minimal foliar abrasion method, but only Cn14-5-1 inflicted typical symptoms of the Goss’s disease on corn. The differences in aggressiveness could be due to a combination of factors including the effect of long-term storage on DOAB 232 as well as changes at the genome level. Comparative genomics analysis of DOAB 232 and two strains, DOAB395 and DOAB397, isolated at the same year with Cn14-5-1 in 2014, revealed a proteome homology of only 92%, an indication of potential differences in genome arrangements [29]. The LC–MS data presented here contribute to the understanding of this genome-level differences and may explain the variation in aggressiveness of the two Cn strains.
This study identified secreted proteins reported to be involved in potential virulence of Cn strains in response to the corn xylem sap (CXS, host plant) and the tomato xylem sap (TXS, non-host plant) as an alternative medium. Tomato xylem sap (TXS) was used as an alternative induction medium because tomato is a host for C. michiganensis subsp. michiganensis that does not infect corn plants. The use of synthetic media such as minimal media or rich media, e.g., NBY, would either stress Cn or produce high background noise that might impair reliable mass spectrometric analyses, respectively. We hypothesized that Cn proteins secreted in equal abundance in both CXS and TXS were less likely to be directly involved in the pathogenicity of corn. The identified Cn secreted proteins were assigned into five different categories as follows: adaptation to new ecological niches, signal perception and transduction, stress-responsive proteins, virulence-related proteins, and hydrolytic enzymes for cell wall or protein degradation.
Degradation of plant cell walls is usually a major part of plant pathogen infections [30,31], since it can be a critical step in establishing a successful infection. Plant pathogens, including bacteria, secrete cell wall-degrading enzymes (CWDEs) to break down the cell walls of host plants, which releases nutrients and promotes colonization [30,32,33].
We identified different CWDEs belonging to four glycosyl hydrolase families (GH3, GH5, GH18, and GH26) showing variation in abundance. These enzymes are involved in pectin, hemicellulose, and cellulose hydrolysis [15], and could be required for Cn to colonize corn tissues resulting in the establishment of C. nebraskensis secreted cellulase (CelA, GH5), β-glucosidase (GH3), β-galactosidase (GH5), and chitinase (GH18), which hydrolyze cellulose, pectin, and chitin, and were found in Cn14-5-1 grown in CXS. In contrast, only β-1,4-xylanase was identified in DOAB232, which could contribute to its lower aggressiveness and milder symptoms of this strain. In the potato–Clavibacter sepedonicus system, cellulases (celA and celB) and xylanases were reported to be upregulated [34]. The putative plasmid-encoded celA gene was also implicated in the pathogenicity of C. sepedonicus strains to eggplant [35,36], and concluded that cellulase is a major plasmid-borne virulence determinant [36]. It is unclear whether the lack of plasmid in strain DOAB232 may contribute to its low aggressiveness. The identification of secreted CelA in extracts of Cn14-5-1 in CXS suggests the presence of a plasmid or an unknown production pathway. Some Cn strains contain single large plasmids with a proximate size of 70 kb, which have shown no correlation with Cn strain pathogenicity [37]. Further research is required to determine the involvement of any such plasmid in the virulence of Cn14-5-1. However, our results clearly showed that Cn14-5-1 is a very aggressive strain. The watery-soaked spots seen in symptomatic infected corn leaves infected by Cn14-5-1 suggested the efficiency of the CWDEs and can be considered as direct evidence of the role of CWDEs as virulence factors in this system. Furthermore, the high activity of total cellulase in Cn14-5-1 compared to DOAB232, measured in the filtrates of Cn grown in CXS, provided additional support to the assumption that CWDEs are involved in Cn aggressiveness.
Establishing a successful infection relies not only on degrading host plant cell walls but also on neutralizing host protein-based defense factors. We identified proteins belonging to two peptidase families in Cn14-5-1 and DOAB232. Secreted peptidases are frequently implicated in overcoming host protein defense components, and as such a large arsenal of such enzymes could lead to higher aggressiveness [38]. Secreted proteases are used by bacterial pathogens to evade plant immune systems and maintain homeostasis [39]. CXS medium boosted the secretion of proteases, e.g., subtilase C (sbtC, S8A) and subtilase B (subtB, S8A), especially in Cn14-5-1, which secreted more proteases and peptidases than DOAB232. Similar proteolytic enzymes, specifically from the serine proteases families Ppa, Sbt. and Chp, were identified in tomato infected by C. michiganensis subsp. michiganensis [15]. The authors concluded that these enzymes were likely targeting host immune components as well as structural components to facilitate disease progression. The protease assay indicated significantly higher levels of proteases in Cn14-5-1, which may contribute to degradation of plant defense proteins and promote colonization. A similar conclusion was reported on the involvement of proteases in colonization of Clavibacter michiganensis and Clavibacter sepedonicus [3]. An important next step will be to find targets for these enzymes, as these would be host factors potentially involved in resistance to Goss’s wilt disease. Besides secreted hydrolytic proteins, other secreted proteins with various cellular functions might also be important in Cn–host interaction.
The ability of bacteria to utilize carbohydrates from the surrounding environment is a positive sign of adaptation. Unlike DOAB232, secretion of carbohydrate metabolism-related proteins was obvious in strain Cn14-5-1 treated with CXS. Furthermore, Cn14-5-1 secreted some carbohydrate metabolism-related proteins in TXS medium, an indication of the ability of Cn14-5-1 to utilize carbohydrates from non-host plants. Some of these identified proteins, e.g., glucokinase (GLK) and glycerol kinase, function as transcriptional regulators or catalytic enzymes for carbohydrate metabolism, respectively [40,41,42]. Identifying these proteins in the highly aggressive Cn14-5-1 strain but not in DOAB232 provided further evidence that this isolate utilizes host carbon resources.
Activity of transcriptional and translational machineries in bacterial cells are essential for cellular perception and signal transduction. The ability to perceive and transmit signals is crucial to prepare and respond to plant defense factors. The majority of transcriptional regulators and DNA-binding proteins were identified in Cn14-5-1 treated with CXS, e.g., antibiotic resistance regulator (MarR) protein family, a one-component signal transduction [43], transcriptional regulator (TetR) [44,45], and ROK family transcriptional regulator [46,47], may provide evidence of the efficiency of strain Cn14-5-1 to recognize and respond to plant stimuli.
Furthermore, phosphorylation is a crucial process that modulates protein activities. Secreted proteins with high abundance in Cn14-5-1 that are involved in protein phosphorylation, e.g., serine/threonine protein phosphatase, HAD family phosphatase, and NTP phosphatase, when treated with CXS may indicate the preference to the host stimuli.
The Cn–corn interaction occurs in the apoplast and extracellular spaces, where bacterial cells secrete virulence factors to evade plant defense factors and promote infection. These secreted virulence factors are translocated via transporter proteins [15,48]. We identified a larger number of transporters in Cn14-5-1 than DOAB232, which could be another potential piece of evidence to explain variations in Cn strain aggressiveness. Plant pathogens negatively affect the integrity of host plant cell walls and cell membranes to cause cell death and release nutrients [49]. Thereafter, pathogens employ different strategies to take up these nutrients, including passive diffusion and active uptake. Various types of membrane transporters facilitate the latter, e.g., ABC transporters [50,51] and ATP-binding cassette ABC transporters [52].
Under stress conditions, e.g., environmental changes, bacterial cellular proteins are subjected to alterations. Chaperones are responsible for folding either newly synthesized polypeptides or refolding stress-denatured proteins, or both [53,54]. The secretome of Cn14-5-1 had various types of chaperones with different levels of abundance, e.g., peptidyl-poly cis/trans isomerase (PPIase), which reacts with the chaperone DnaK system to fold newly synthesized polypeptides [55]. In addition, 60 kDa chaperonin and 10 kDa chaperonin (GroES) are essential in newly synthesized polypeptides, as well as stress-denatured proteins [56,57]. Abundance of these types of chaperones in Cn14-5-1 treated with CXS may demonstrate efficient re-use of resources, which may enhance its ecological fitness leading to high aggressiveness.
Phytopathogens cope with host plant reactive oxygen species (ROS)-based defenses [58] by secreting antioxidant enzymes. Corn plants accumulate ROS in the infection site to restrict bacterial growth and to hinder disease progress. High abundance of secreted ROS scavenging proteins in Cn14-5-1 secretome, e.g., catalase (KatA), and manganese catalase were deployed to mitigate the deleterious effect of ROS as the first host plant chemical defense barrier [59]. Finally, lipoproteins [60,61], cupin domain-containing proteins [62], and Stk1 family PASTA domain-containing Ser/Thr kinase [63] were also observed in Cn14-5-1, and these may also contribute to the higher aggressiveness of this strain.
5. Conclusions and Future Perspectives
Host and non-host xylem sap represent a convenient and biologically relevant model for culturing Cn and performing comparative proteomics of the Cn–corn interaction in vitro. We found that the pathogen secretome was altered in response to host xylem sap compared to non-host xylem sap. We identified significant changes in the abundance of key proteins implicated in pathogenicity. Since these changes are in response to host xylem, it is likely that these proteins could play important roles in the corn-pathogen interaction through responding to chemical stimuli present in the host xylem sap. The xylem sap system has the advantage that the host proteome is very minute from the protein extracts. This minimizes background noise from LC–MS data and greatly simplifies downstream proteomic analyses. A comparison of the secretomes of weakly and highly aggressive strains of Cn indicated that cell wall-degrading enzymes, proteases, and other types of proteins might have important roles in Goss’s wilt disease establishment. Further experiments on Cn gene knockout for CWDEs and/or proteases encoding genes will be useful in deciphering the potential role of these two major secreted proteins in Cn aggressiveness.
Acknowledgments
We would like to thank Lorne Adam for his assistance in reviewing the manuscript, and Bryan Waller, Pride Seeds, for providing hybrid A4631G2 RIB.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-7382/9/1/1/s1: Figure S1: Aggressiveness assay of the two strains of Clavibacter nebraskensis. Two disease assessment methods were used to evaluate the aggressiveness of Cn14-5-1 and DOAB232 on the basis of the total area under the disease progress curve (AUDPC) using (A) lesion length and (B) disease severity. Figure S2: Reactive oxygen species (ROS) localization in corn leaves inoculated with the two strains of Clavibacter nebraskensis. Localization of hydrogen peroxide (A) and superoxide (B) in corn leaves. 3,3′-Diaminobenzidine tetrahydrochloride (DAB) (brown) staining of the infected corn leaves with Cn strains Cn14-5-1 and DOAB232 vs. non-infected leaf (control). Nitroblue tetrazolium (NBT) (blue) staining of the infected corn leaves with Cn strains Cn14-5-1 and DOAB232 vs. non-infected leaf (control). Table S1: Seven hundred and forty-five total secreted proteins identified in the corn xylem sap (host) and tomato xylem sap (non-host). Table S2: Secreted proteins identified only for strain Cn14-5-1 in corn xylem sap medium. Table S3: Secreted proteins identified only for strain Cn14-5-1 in tomato xylem sap medium. Table S4: Increased differentially abundant secreted proteins of Cn14-5-1 in corn xylem sap medium. Table S5: Differentially abundant proteins of Cn14-5-1 with an increased fold change in tomato xylem sap medium. Table S6: Secreted proteins identified only for strain DOAB232 in corn xylem sap medium. Table S7: Secreted proteins identified only for strain DOAB232 in tomato xylem sap medium. Table S8: Differentially abundant proteins of DOAB232 with an increased fold change in corn xylem sap medium. Table S9: Differentially abundant proteins of DOAB232 with an increased fold change in tomato xylem sap medium.
Author Contributions
A.S. contributed to the idea, carried out experiments, and contributed to data analysis and writing the manuscript; C.R. processed samples for mass spectrometry, helped in data analysis, and revised the manuscript; J.T.T. contributed to experimental design and revised the manuscript; F.D. contributed the idea, supervised the work, and contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Manitoba Corn Growers Association and the Manitoba Growing Innovation Capacity and Knowledge Development program, Grain Innovation Hub—Corn Development Project # 1000135719.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and the supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tambong J.T. Comparative genomics of Clavibacter michiganensis subspecies, pathogens of important agricultural crops. PLoS ONE. 2017;12:e0172295. doi: 10.1371/journal.pone.0172295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacques M.A., Durand K., Orgeur G., Balidas S., Fricot C., Bonneau S., Quillévéré A., Audusseau C., Olivier V., Grimault V., et al. Phylogenetic analysis and polyphasic characterization of Clavibacter michiganensis strains isolated from tomato seeds reveal that nonpathogenic strains are distinct from C. michiganensis subsp. michiganensis. Appl. Environ. Microbiol. 2012;78:8388–8402. doi: 10.1128/AEM.02158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichenlaub R., Gartemann K.H. The Clavibacter michiganensis subspecies: Molecular investigation of Gram-positive bacterial plant pathogen. Annu. Rev. Phytopathol. 2011;49:445–464. doi: 10.1146/annurev-phyto-072910-095258. [DOI] [PubMed] [Google Scholar]
- 4.Vidaver A.K., Mandel M. Corynebacterium nebraskense, a new orange-pigmented phytopathogenic species. Int. J. Syst. Bacteriol. 1974;24:482–485. doi: 10.1099/00207713-24-4-482. [DOI] [Google Scholar]
- 5.Schuster M.L. Leaf Freckles and Wilt of Corn Incited by Corynebacterium nebraskense Schuster, Hoff, Mandel, Lazar, 1972. Agricultural Experiment Station, University of Nebraska; Lincoln, NE, USA: 1975. [Google Scholar]
- 6.Li X., Tambong J., Yuan K.X., Chen W., Xu H., Lévesque C.A., De Boer S.H. Reclassification of Clavibacter michiganensis subspecies on the basis of whole-genome and multi-locus sequence analyses. Int. J. Syst. Evol. Microbiol. 2018;68:234–240. doi: 10.1099/ijsem.0.002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysong D.S., Doupnik J.B., Lane L. Proceedings of the Annual Corn Sorghum Research Conference. 36th American Seed Trade Association; Washington, DC, USA: 1981. Goss’s wilt and corn lethal necrosis—Can they become a major problem? pp. 104–130. [Google Scholar]
- 8.Rocheford T.R., Vidaver A.K., Gardner C.O., Arbust D.L. Effect of wind-generated sand abrasion on infection of corn (Zea mays L.) by Corynebacterium michiganense ssp. nebraskense. Phytopathology. 1985;75:1378. [Google Scholar]
- 9.Agarkova I.V., Lambrecht P.A., Vidaver A.K. Genetic diversity and population structure of Clavibacter michiganensis subsp. nebraskensis. Can. J. Microbiol. 2011;57:366–374. doi: 10.1139/w11-016. [DOI] [PubMed] [Google Scholar]
- 10.Langemeier C.B., Robertson A.E., Wang D., Jackson-Ziems T.A. Factors affecting the development and severity of Goss’s bacterial wilt and leaf blight of corn, caused by Clavibacter michiganensis subsp. nebraskensis. Plant Dis. 2017;101:54–61. doi: 10.1094/PDIS-01-15-0038-RE. [DOI] [PubMed] [Google Scholar]
- 11.Claflin L.E. Goss’s bacterial wilt and blight. In: White D.H., editor. Compendium of Corn Diseases. American Phytopathological Society; St. Paul, MN, USA: 1999. [Google Scholar]
- 12.Mallowa S., Mbofung G., Eggenberger S., Den Adel R., Sheiding S.R., Robertson A.E. Infection of maize by Clavibacter michiganensis subsp. nebraskensis does not require severe wounding. Plant Dis. 2016;100:724–731. doi: 10.1094/PDIS-08-15-0923-RE. [DOI] [PubMed] [Google Scholar]
- 13.Langemeier C.B., Jackson-Ziems T.A., Kruger G.R. Four common Setaria Species are alternative hosts for Clavibacter michiganensis subsp. nebraskensis, causal agent of Goss’s bacterial wilt and blight of corn. Plant Health Prog. 2014;15:57–60. doi: 10.1094/PHP-RS-12-0160. [DOI] [Google Scholar]
- 14.Ikley J.T., Wise K.A., Johnson W.G. Annual ryegrass (Lolium multiflorum), Johnsongrass (Sorghum halepense), and large crabgrass (Digitaria sanguinalis) are alternative hosts for Clavibacter michiganensis subsp. nebraskensis, causal agent of Goss’s wilt of corn. Weed Sci. 2015;63:901–909. [Google Scholar]
- 15.Savidor A., Teper D., Gartemann K.-H., Eichenlaub R., Chalupowicz L., Manulis-Sasson S., Barash I., Tews H., Mayer K., Giannone R., et al. The Clavibacter michiganensis subsp. michiganensis–tomato interactome reveals the perception of pathogen by the host and suggests mechanisms of infection. J. Proteome Res. 2012;11:736–750. doi: 10.1021/pr200646a. [DOI] [PubMed] [Google Scholar]
- 16.Hiery E., Poetsch A., Moosbauer T., Amin B., Hofmann J., Burkovski A. A proteomic study of Clavibacter michiganensis subsp. michiganensis culture supernatants. Proteomes. 2015;3:411–423. doi: 10.3390/proteomes3040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross D.C., Vidaver A.K. A selective medium for isolation of Corynebacterium nebraskense from soil and plant parts. Phytopathology. 1979;69:82–87. doi: 10.1094/Phyto-69-82. [DOI] [Google Scholar]
- 18.Soliman A., Gulden R.H., Tambong J.T., Bajracharya P., Adam L.R., Xu R., Cott M., Daayf F. Developed and validated inoculation and disease assessment methods for Goss’s bacterial wilt and leaf blight disease of corn. Crop Prot. 2018;112:59–167. doi: 10.1016/j.cropro.2018.05.022. [DOI] [Google Scholar]
- 19.Campbell M.T., Proctor C.A., Dou Y., Schmitz A.J., Phansak P., Kruger G.R., Walia H. Genetic and molecular characterization of submergence response identifies Subtol6 as a major submergence tolerance locus in maize. PLoS ONE. 2015;10:e0120385. doi: 10.1371/journal.pone.0120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1979;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller A., Nesvizhskii A.I., Kolker E., Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 23.Nesvizhskii A.I., Keller A., Kolker E., Aebersold R.A. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y.H.P., Hong J., Ye X. Cellulase Assays. In: Mielenz J., editor. Biofuels: Methods in Molecular Biology. Volume 581. Humana Press; Totowa, NJ, USA: 2009. [DOI] [PubMed] [Google Scholar]
- 25.Cupp-Enyard C. Sigma’s non-specific protease activity assay—Casein as a substrate. JoVE. 2008;19:899. doi: 10.3791/899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund R.E. Tables for an approximate test for outliers in linear models. Technometrics. 1975;17:473–476. doi: 10.1080/00401706.1975.10489374. [DOI] [Google Scholar]
- 27.Saxton A.M. Proceedings of the 23rd SAS Users Group International. SAS Institute; Cary, NC, USA: 1998. A macro for converting mean separation output to letter groupings in PROC MIXED; pp. 1243–1246. [Google Scholar]
- 28.Peritore-Galve F.C., Schneider D.J., Yang Y., Thannhauser T.W., Smart C.D., Stodghill P. Proteome profile and genome refinement of the tomato-pathogenic bacterium Clavibacter michiganensis subsp. michiganensis. Proteomics. 2019;19:e1800224. doi: 10.1002/pmic.201800224. [DOI] [PubMed] [Google Scholar]
- 29.Tambong J.T., Xu R., Daayf F., Brière S., Bilodeau G.J., Tropiano R., Hartke A., Reid L.M., Cott M., Cote T., et al. Genome analysis and development of a multiplex TaqMan real-time PCR for specific identification and detection of Clavibacter michiganensis subsp. nebraskensis. APS Phytopathol. 2016;106:1473–1485. doi: 10.1094/PHYTO-05-16-0188-R. [DOI] [PubMed] [Google Scholar]
- 30.Bellincampi D., Cervone F., Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant–pathogen interactions. Front. Plant Sci. 2014;28:228. doi: 10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalaoui F., Halama P., Dumortier V., Paul B. Cell wall-degrading enzymes produced in vitro by isolates of Phaeosphaeria nodorum differing in aggressiveness. Plant Pathol. 2000;49:727–733. doi: 10.1046/j.1365-3059.2000.00491.x. [DOI] [Google Scholar]
- 32.Barras F., Gijsegem F.V., Chatterjee A.K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 1994;32:201–234. doi: 10.1146/annurev.py.32.090194.001221. [DOI] [Google Scholar]
- 33.Melotto M., Kunkel B.N. Virulence strategies of plant pathogenic bacteria. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes. Springer; Berlin/Heidelberg, Germany: 2013. pp. 61–82. [Google Scholar]
- 34.Holtsmark I., Takle G., Brurberg M. Expression of putative virulence factors in the potato pathogen Clavibacter michiganensis subsp. sepedonicus during infection. Arch. Microbiol. 2008;189:131–139. doi: 10.1007/s00203-007-0301-2. [DOI] [PubMed] [Google Scholar]
- 35.Laine M.J., Haapalainen M., Wahlroos T., Kankare K., Nissinen R., Kassuwi S., Metzler M.C. The cellulase encoded by the native plasmid of Clavibacter michiganensis ssp. sepedonicus plays a role in virulence and contains an expansin-like domain. Phys. Mol. Plant Pathol. 2000;57:221–233. [Google Scholar]
- 36.Nissinen R., Kassuwi S., Peltola R., Metzler M.C. In planta—Complementation of Clavibacter michiganensis subsp. sepedonicus strains deficient in cellulase production or HR induction restores virulence. Eur. J. Plant Pathol. 2001;107:175–182. doi: 10.1023/A:1011270926064. [DOI] [Google Scholar]
- 37.Ahmad A., Mbofung G.Y., Acharya J., Schmidt C.L., Robertson A.E. Characterization and comparison of Clavibacter michiganensis subsp. nebraskensis strains recovered from epiphytic and symptomatic infections of maize in Iowa. PLoS ONE. 2015;10:e0143553. doi: 10.1371/journal.pone.0143553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoston A., Mudgett M.B. Cysteine proteases in phytopathogenic bacteria: Identification of plant targets and activation of innate immunity. Curr. Opin. Plant Biol. 2004;7:384–390. doi: 10.1016/j.pbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Figaj D., Ambroziak P., Przepiora T., Skorko-Glonek J. The role of proteases in the virulence of plant pathogenic bacteria. Int. J. Mol. Sci. 2019;20:672. doi: 10.3390/ijms20030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boos W., Shuman H. Maltose/maltodextrin system of Escherichia coli: Transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 1998;62:204–229. doi: 10.1128/MMBR.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lengsfeld C., Schönert S., Dippel R., Boos W. Glucose-and Glucokinase-controlled mal gene expression in Escherichia coli. J. Bacteriol. 2009;191:701–712. doi: 10.1128/JB.00767-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh J.I., Kettering R., Saxl R., Bourand A., Darbon E., Joly N., Briozzo P., Deutscher J. Structural characterizations of glycerol kinase: Unraveling phosphorylation-induced long-range activation. Biochemistry. 2009;48:346–356. doi: 10.1021/bi8009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grove A. Regulation of metabolic pathways by MarR family transcription factors. Comput. Struct. Biotechnol. J. 2017;15:366–371. doi: 10.1016/j.csbj.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos J.L., Martínez-Bueno M., Molina-Henares A.J., Terán W., Watanabe K., Zhang X., Gallegos M.T., Brennan R., Tobes R. The TetR family of transcriptional repressors. Mol. Biol. Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuthbertson L., Nodwella J.R. The TetR Family of regulators. Microbiol. Mol. Biol. Rev. 2013;77:440–475. doi: 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titgemeyer F., Reizer J., Reizer A., Saier M.H.J. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology. 1994;140:2349–2354. doi: 10.1099/13500872-140-9-2349. [DOI] [PubMed] [Google Scholar]
- 47.Kazanov M.D., Li X., Gelfand M.S., Osterman A.L., Rodionov D.A. Functional diversification of ROK-family transcriptional regulators of sugar catabolism in the Thermotogae phylum. Nucleic Acids Res. 2012;41:790–803. doi: 10.1093/nar/gks1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thapa S.P., Pattathil S., Hahn M.G., Jacques M.-A., Gilbertson R.L., Coaker G. Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a Gram-positive bacterial pathogen. Mol. Plant Microbe Interact. 2017;30:786–802. doi: 10.1094/MPMI-06-17-0146-R. [DOI] [PubMed] [Google Scholar]
- 49.Laluk K., Mengiste T. Necrotroph attacks on plants: Wanton Destruction or Covert Extortion? Arabidopsis Book. 2010;8 doi: 10.1199/tab.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatima U., Senthil-Kumar M. Plant and pathogen nutrient acquisition strategies. Front. Plant Sci. 2015;6:750. doi: 10.3389/fpls.2015.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delmotte N., Knief C., Chaffron S., Innerebner G., Roschitzki B., Schlapbach R., von Mering C., Vorholt J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA. 2009;106:16428–16433. doi: 10.1073/pnas.0905240106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J., Ge J., Heuveling J., Schneider E., Yang M. Structural basis for substrate specificity of an amino acid ABC transporter. Proc. Natl. Acad. Sci. USA. 2015;112:5243–5248. doi: 10.1073/pnas.1415037112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braakman I., Hebert D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013;5:a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park C.-J., Seo Y.-S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015;31:323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoller G., Rucknagel K.P., Nierhaus K.H., Schmid F.X., Fischer G., Rahfeld J.-U. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 1995;20:4939–4948. doi: 10.1002/j.1460-2075.1995.tb00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rospert S., Glick B.S., Jenö P., Schatz G., Todd M.J., Lorimer G.H., Viitanen P.V. Identification and functional analysis of chaperonin 10, the groES homolog from yeast mitochondria. Proc. Natl. Acad. Sci. USA. 1993;90:10967–10971. doi: 10.1073/pnas.90.23.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy-Rimler G., Viitanen P., Weiss C., Sharkia R., Greenberg A., Niv A., Lustig A., Delarea Y., Azem A. The effect of nucleotides and mitochondrial chaperonin 10 on the structure and chaperone activity of mitochondrial chaperonin 60. Eur. J. Biochem. 2001;268:3465–3472. doi: 10.1046/j.1432-1327.2001.02243.x. [DOI] [PubMed] [Google Scholar]
- 58.Fones H., Preston G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 2012;327:1–8. doi: 10.1111/j.1574-6968.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- 59.Hasset D.J., Cohen M.S. Bacterial adaptation to oxidative stress: Implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989;3:2574–2582. doi: 10.1096/fasebj.3.14.2556311. [DOI] [PubMed] [Google Scholar]
- 60.Kovacs-Simon A., Titball R.W., Michell S.L. Lipoproteins of bacterial pathogens. Infect. Immun. 2011;79:548–561. doi: 10.1128/IAI.00682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zückert W.R. Secretion of bacterial lipoproteins: Through the cytoplasmic membrane, the periplasm and beyond. Biochim. Biophys. Acta. 2014;1843:1509–1516. doi: 10.1016/j.bbamcr.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Hadrami A., Islam M.R., Adam L.R., Fouad D. A cupin domain-containing protein with a quercetinase activity (VdQase) regulates Verticillium dahliae’s pathogenicity and contributes to counteracting host defenses. Front. Plant Sci. 2015;6:440. doi: 10.3389/fpls.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Débarbouillé M., Dramsi S., Dussurget O., Nahori M.-A., Vaganay E., Jouvion G., Cozzone A., Msadek T., Duclos B. Characterization of a serine/threonine kinase involved in virulence of Staphylococcus aureus. J. Bacteriol. 2009;191:4070–4081. doi: 10.1128/JB.01813-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and the supplementary materials.