Abstract
Prenatal exposure to maternal immune activation (MIA) has been implicated as a risk factor for the development of autism spectrum disorder (ASD), though the conditions under which this elevated risk occurs are unclear. Animal literature demonstrates that antibiotic use, which affects the composition of the maternal gut microbiota, modifies the effect of MIA on neurodevelopmental outcomes in the offspring. The aim of this study was to assess whether antibiotic use during pregnancy modifies the association between MIA and subsequent risk of ASD, in a prospective birth cohort with 116 ASD cases and 860 typically developing child controls. There was no evidence of interaction between fever or genitourinary infection and antibiotic use on the odds of ASD in unadjusted or adjusted analyses. However, we found evidence of an interaction between flu, specifically in second trimester, and antibiotic use at any point during pregnancy on the odds of ASD in the child. Among women who received an antibiotic during pregnancy, flu in trimester two was not associated with ASD (aOR=0.99 [0.43–2.28]). Among women who were not exposed to an antibiotic at any point during pregnancy, flu in second trimester was significantly associated with increased odds of ASD (aOR=4.05 [1.14–14.38], p=0.03), after adjustment for child sex, child birth year, maternal age, gestational age, C-section delivery, and low birth weight. These findings should be treated as hypothesis-generating and suggest that antibiotic use may modify the influence that maternal immune activation has on autism risk in the child.
Keywords: Autism spectrum disorder (ASD), environmental exposure, anti-bacterial agents, maternal exposure, epidemiology, risk factors, minority health
Lay summary
We looked at whether the association between activation of the immune system during pregnancy and risk of the child developing autism ASD differed among women who did or did not take an antibiotic at any point during pregnancy. We examined 116 children with ASD and 860 without ASD and found that flu in second trimester was associated with increased ASD, but only among women who did not take an antibiotic during pregnancy. No other immune activation exposures seemed to interact with antibiotic use.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by two core domains of symptoms: impairment of social communication, and restricted, repetitive behaviors, interests, or activities (American Psychiatric Association, 2013). The prenatal period and early-life period are considered to be the critical windows for the development of ASD, given the brain’s susceptibility to environmental stressors (Landrigan, 2010; Lyall et al., 2014; Rice & Barone Jr, 2000; Rodieret al., 1996; Schlotz & Phillips, 2009).
Prenatal exposure to maternal immune activation (MIA) has been implicated as a risk factor for the development of neuropsychiatric disorders, and, in particular, schizophrenia and ASD (Careaga et al., 2017; Croen et al., 2019; Köhler-Forsberg et al., 2018; Lydholm et al., 2019). Animal studies have demonstrated that maternal immune activation leads to behavioral abnormalities such as decreased social approach and ultrasonic vocalizations, and increased repetitive grooming and marble burying behavior in the offspring (Bauman et al., 2014; Harvey & Boksa, 2012; Malkovaet al., 2012; Schwartzer et al., 2013; Smithet al., 2007). These behavioral changes are paralleled by alterations in the immune profiles of offspring, in particular levels of IL-6 and IL-17α (Choi et al., 2016; Hsiao et al., 2012; Mandal et al., 2011; Smith et al., 2007).
Human studies have also found a strong link between prenatal exposure to MIA and risk of ASD in the offspring. Bacterial (Atladóttir et al., 2010; Fang et al., 2015; Lee et al., 2015), viral (Atladóttir et al., 2010; Lee et al., 2015), and genitourinary infections (Fang et al., 2015; Zerbo et al., 2013), as well as fever (Brucato et al., 2017; Croen et al., 2019; Hornig et al., 2018; Zerbo et al., 2013) have been implicated as risk factors for ASD. However, there is substantial heterogeneity in the findings across these studies. A meta-analysis and systematic review by Jiang et al. found that overall, maternal infections, particularly those requiring hospitalization, increase the risk for ASD (Jiang et al., 2016). Specifically they concluded that any maternal infection in the second trimester, bacterial infections overall and in particular during the second and third trimesters, and viral infections over the entire pregnancy (but not within a particular trimester) were associated with higher risk of ASD (Jiang et al., 2016). Despite the evidence that an activated immune system in the pregnant mother is associated with higher risk for neurodevelopmental abnormalities in the child, the conditions under which this elevated risk occurs, such as the responsiveness of a mom’s immune system or gut microbiome, are unclear.
Human gut microbiota—the assemblage of microbes living in our gastrointestinal tract—and their genetic material (the gut microbiome) have emerged as key players in health and disease (Shreiner et al., 2015). Exposure to antibiotics, often taken for infection, can lead to long-term changes to the gut microbiome (Jakobsson et al., 2010). Animal studies suggest that maternal exposure to antibiotics, by modulating the maternal gut microbiome, may lead to the neurodevelopmental and behavioral abnormalities observed in the context of maternal immune activation (Kim et al., 2017; Lammert et al., 2018; Wang et al., 2019). For example, Kim et al. found that MIA-associated phenotypes (i.e. increased repetitive behaviors, anxiety, social interaction deficits, cortical patches) manifested only in the presence of specific maternal gut microbiota that promote differentiation of Th17 cells and subsequent section of Il-17a. Treatment of pregnant dams with a broad-spectrum antibiotic altered the composition of the gut microbiota, leading to a decrease in the amount of Th17 cells in the intestine, decreasing IL-17a levels in the circulating maternal plasma. These microbial and immune changes prevented the neurodevelopmental and behavioral abnormalities typically seen in the context of MIA. In other words, among pregnant dams exposed to immune activation, antibiotic use protects against the MIA-associated neurodevelopmental abnormalities seen in the offspring (Kim et al., 2017). However, this has not been assessed in humans.
The aim of this study was to assess whether antibiotic use during pregnancy modifies the association between prenatal exposure to maternal immune activation and subsequent risk of autism spectrum disorder, in a prospective cohort with high rates of autism. We hypothesized that antibiotic use would be protective against ASD in the presence of maternal immune activation.
Methods
Sample
Our sample consisted of mother-child pairs from the Boston Birth Cohort (BBC), a prospective birth cohort recruited from the Boston Medical Center (Wang et al., 2014; Wang et al., 2002). Initiated in 1998, the BBC was designed to investigate environmental and genetic determinants for preterm delivery. Subsequently, the BBC was extended to postnatal follow-up to study child health outcomes including ASD. Because it is enriched for preterm deliveries, it is a useful cohort for studying autism epidemiology, given that preterm delivery is a risk factor for the development of ASD (Kuzniewicz et al., 2014). The BBC has been described in detail elsewhere (Brucato et al., 2017; Wang et al., 2002), but briefly: Women with a live, singleton birth at Boston Medical Center are eligible for recruitment. Pregnancies involving IVF, chromosomal abnormalities, major birth defects, and preterm deliveries due to maternal trauma are excluded. Eligible participants are contacted 24–72 hours following birth for consent and study enrollment. Since 2003, children who seek postnatal care at Boston Medical Center are able to be followed up for developmental outcomes, such as ASD status. Most pregnant women in the Boston Medical Center are urban, low-income minority individuals who receive healthcare through public assistance based insurance programs (Brucato et al., 2017; Wang et al., 2014; Wang et al., 2002). The institutional review board of Johns Hopkins Bloomberg School of Public Health and Boston University Medical Center approved the study.
Outcome Classification
Electronic medical record ICD-9-CM diagnostic codes from inpatient, outpatient, and emergency department visits at the Boston Medical Center (October 1, 2003 through September 31, 2015) were used to classify children as ASD cases or neurotypical controls. ICD-9 codes were used since the majority of the diagnostic window occurred when ICD-9 was in use. Children were classified as having ASD if their medical recordings contained any of the ICD-9-CM codes at least once: 299.00, 299.01, 299.80, 299.81, 299.90, or 299.91 (Brucato et al., 2017). Children were classified as neurotypical controls if they were never diagnosed with any of the following conditions: ASD, attention deficit hyperactivity disorder (ADHD), intellectual disability (ID), developmental delay (DD), oppositional defiant disorder (ODD), conduct disorder (CD), or congenital anomalies.
Definition of maternal immune activation
Maternal immune activation is defined as an elevated inflammatory state that follows one of multiple possible exposures, such as infection (e.g. bacterial, viral), fever, psychosocial stress or psychopathology, and other inflammatory conditions such as obesity or gestational diabetes (Boulanger-Bertolus et al., 2018; Knuesel et al., 2014; Pantham et al., 2015). The details of defining maternal immune activation in these BBC data have previously been described elsewhere (Brucato et al., 2017). Briefly, maternal immune activation was defined as one of any of the following exposures: prenatal flu, prenatal fever (excluding intrapartum), prenatal genitourinary (GU) tract infections, and intrapartum (labor and delivery) fever. A standardized self-report postpartum questionnaire was administered to the mother 24–72 hours following delivery for self-report of flu, fever, and GU infection. Intrapartum fever was defined as a >38° C maternal temperature during labor and delivery and was abstracted from electronic medical records (EMR). For flu and fever, overall as well as trimester-specific variables were used, and an overall variable only was used for genitourinary infection. All individuals were dichotomized as “exposed” or “unexposed” for these MIA variables. Women who did not have self-report information on prenatal flu, fever, or GU infections or who were missing information on intrapartum fever were treated as missing that exposure.
Definition of antibiotic use during pregnancy
Mothers were classified as exposed versus unexposed to antibiotic use during any time of pregnancy including labor and delivery. This dichotomous exposure was determined from three sources of information. First, in the standardized postpartum questionnaire, mothers were asked ‘What medicines did you take during your pregnancy excluding vitamins?’. Women could list up to 5 medications and indicate which trimester the medication was taken. Entries listing any of the antibiotics found in Table S12 or including the word “antibiotic” were classified as being an antibiotic, and the mother designated “exposed”. Second, inpatient and outpatient antibiotic treatments during the entire pregnancy were extracted from the EMR. Similarly, medications were classified as antibiotics if they contained the word “antibiotic” or any of the medications in Table S12. Intrapartum (labor and delivery) antibiotics were extracted from medical charts. Women with inpatient or outpatient antibiotic prescriptions that overlapped any pregnancy period were also considered to be exposed. We combined these three sources of information to obtain one aggregate, binary variable representing whether or not a woman was exposed to an antibiotic during pregnancy or the labor and delivery period, if any of these three sources indicated exposure (Table S1). Women who did not have EMR antibiotic information and who did not report antibiotic use on the standardized questionnaire and who did not receive an intrapartum antibiotic were classified as not exposed to antibiotics during pregnancy.
Correspondence between medical charts/EMR and maternal self-report was high among women with no exposure recorded in a medical chart/EMR: only 4 of 282 (1.5%) self-reported antibiotic use discordant with their EMR and medical charts. However, among the 684 women with medical chart/EMR record of exposure, 471 (68.9%) did not self-report antibiotic use, indicating either true discordance (such as not taking prescribed antibiotics) or lack of familiarity with antibiotics among women self-reporting.
While EMR data included information on the specific type of antibiotic prescribed and at what point during pregnancy, data were too sparse after stratifying by ASD, MIA, and antibiotic use to incorporate timing or type of antibiotic as part of the analysis. Importantly, we looked at any antibiotic exposure throughout pregnancy, not exposure to an antibiotic for treatment of the MIA. Therefore, antibiotic use could have been prescribed for a number of other reasons (e.g. receiving intravenous ampicillin for group B strep prophylaxis), For a list of which antibiotics were extracted from the postpartum maternal questionnaire and the EHR records, see Supplemental Table 12.
Covariate definitions
Given the relatively small number of observations and complicated relationships between perinatal factors, we sought to minimize the number of covariates in analytic models. We considered all potential confounders and effect modifiers, but chose only covariates with observed marginal associations with ASD as adjustment factors with parsimony in mind. These included: maternal age at delivery, child birth year, child sex, gestational age (weeks), mode of delivery (C-section or vaginal birth), and low birth weight (<2500 grams). These variables were all obtained from medical charts.
Race was self-reported by choosing one of the nine categories: Black/African American (Black, African American, Haitian, Cape Verdean, Caribbean), Asian (Asian and Pacific Islander), White, Hispanic, mixed race, and all others. However, race was not included as a covariate in our regression models because of sparseness across racial categories, even when collapsing across the two most predominant racial groups in our sample (Black and White, non-Hispanic).
Analytic dataset
A complete-case analysis was carried out. Out of 1,196 mother-child pairs (ASD or neurotypical control), 24 were excluded for missing information on any covariates, 58 for missing antibiotic use, and 150 for missing any of the overall-pregnancy MIA exposures (i.e. trimester level MIA exposures could still be missing). This left 976 pairs in our analytic sample (n=108 ASD, n=841 neurotypical). Individuals retained in the analytic sample (n=976) did not differ significantly from those excluded (n=220) on any of our predictors, outcomes, or covariates (Table S2).
Statistical analyses
All data cleaning and analyses were performed in R Studio version 1.1.383 (R version 3.4.3). Descriptive characteristics of the analytic sample were stratified by ASD and antibiotic use. We first carried out unadjusted and adjusted logistic regression models examining the main effect of each of 10 MIA exposures (flu overall and in each trimester, fever overall and in each trimester, GU infection overall, intrapartum fever) with ASD as the outcome. Then, we conducted a series of 10 logistic regression models with each MIA exposure as a predictor, antibiotic use as an interaction term, and ASD diagnosis as the outcome. We ran both unadjusted models and models adjusted for child sex, child birth year, maternal age, gestational age, C-section delivery, and low birth weight. Models with an interaction term p-value < 0.10 were suggestive of possible interaction and were followed up with stratified analyses. These models estimated the association between MIA and odds of ASD, stratified by antibiotic use (unadjusted and adjusted for the covariates above). For significant associations, we also performed Fisher’s exact test of independence between the MIA exposure and ASD, stratified by antibiotic use, given our small sample size.
Lastly, we carried out sensitivity analyses. First, we repeated our regression modeling excluding children less than three years old to account for the possibility that ASD diagnosis would be much less likely before the age of three (n=113 ASD remaining). Second, we used a more stringent classification of ASD, requiring 2 or more ICD-9 CM codes for ASD diagnosis on different clinical visits (n=94 ASD remaining).
Results
Sample characteristics
Out of 976 children, 116 (11.9%) had a diagnosis of ASD, and 70.1% of mothers used an antibiotic during pregnancy or in labor and delivery. Sixty one percent (61%) of this study population was Black, non-Hispanic, 8.5% were white, non-Hispanic, and the remainder (30%) were other races. The age of ASD diagnosis ranged from 1 to 15 years, with a mean of 7.8 (SD 3.3). Three children were diagnosed prior to the age of 3.
Children with ASD were significantly more likely to be exposed to antibiotics during gestation, to be male, to have a more recent birth year, to have an older mother, to be born at an earlier gestational age, to be delivered by C-section, and to be born at a low birth weight (Table 1). Children with maternal exposure to antibiotics were more likely to be male, to be younger/have a later birth year, to have an earlier gestational age, and to be born by C-section.
Table 1.
Descriptive Characteristics of Total Sample, Stratified by ASD and Antibiotic Use in Pregnancy (% or Mean (SD))
| Total Sample (n=976) | TD (n=860) | ASD (n=116) | No Antibiotic (n=288) | Antibiotic (n=688) | |
|---|---|---|---|---|---|
| ASD ‡ | 11.9 | 0% | 100.0 | 8.7 | 13.2 |
| Antibiotic use during pregnancy † | 70.5 | 69.4 | 78.4 | 0% | 100.0 |
| Male child †,‡ | 44.3 | 40.5 | 72.4 | 39.9 | 46.1 |
| Maternal age (years) | 28.4 (6.4) | 28.2 (6.4) | 29.8 (6.1) | 27.9 (6.4) | 28.5 (6.4) |
| Gestational age (months) †,‡ | 38.1 (3.1) | 38.4 (2.8) | 36.5 (4.6) | 38.4 (3.2) | 38.0 (3.1) |
| Child birth year †,‡ | 2006.4 (3.5) | 2006.3 (3.5) | 2006.9 (3.3) | 2004.5 (3.8) | 2007.2 (3) |
| Maternal education: high school grad or below | 61.6 | 62.3 | 56.6 | 65.6 | 60.0 |
| Maternal marital status: not married | 65.1 | 65.2 | 64.6 | 64.9 | 65.2 |
| Maternal Race | |||||
| Black | 61.4 | 61.6 | 59.5 | 63.9 | 60.3 |
| White, non-Hispanic | 8.5 | 9.0 | 5.2 | 7.3 | 9.0 |
| Other races | 30.0 | 29.4 | 35.4 | 28.8 | 30.7 |
| Maternal smoking | |||||
| Smoked 6 months prior to pregnancy | 84.2 | 85.1 | 77.7 | 85.7 | 83.6 |
| Quit during pregnancy | 3.2 | 3.1 | 3.6 | 2.9 | 3.3 |
| Smoked prior and during pregnancy | 12.6 | 11.8 | 18.8 | 11.5 | 13.1 |
| C-section delivery †,‡ | 32.1 | 30.8 | 41.4 | 21.2 | 36.6 |
| Low birth weight (<2500 grams) † | 22.4 | 20.9 | 33.6 | 20.1 | 23.4 |
P-value <0.10 for Chi-square or T-test/ Wilcoxon rank sum test difference between ASD and TD group;
P-value <0.10 for Chi-square or T-test/Wilcoxon rank sum test difference between Antibiotic and No Antibiotic use group
The frequency of maternal immune activation exposures, stratified by ASD status and by antibiotic use during pregnancy, are shown in Table 2. The most common type of maternal immune activation was genitourinary infection (30.7%), followed by flu (19.5%) fever (9.3%), and intrapartum fever (6.7%).
Table 2.
Count (%) of maternal immune activation exposures in total sample and stratified by ASD, Antibiotic Use in Pregnancy
| TD (n=860) | ASD (n=116) | |||||
|---|---|---|---|---|---|---|
| Antibiotic during Pregnancy | Antibiotic during Pregnancy | |||||
| Total (n=) | No (n=263) | Yes (n=597) | No (n=25) | Yes (n=91) | ||
| GU Infection | No | 676 (69.3%) | 199 (75.7%) | 395 (66.2%) | 21 (84.0%) | 61 (67.0%) |
| Yes | 300 (30.7%) | 64 (24.3%) | 202 (33.8%) | 4 (16.0%) | 30 (33.0%) | |
| Flu Overall | No | 786 (80.5%) | 209 (79.5%) | 486 (81.4%) | 18 (72.0%) | 73 (80.2%) |
| Yes | 190 (19.5%) | 54 (20.5%) | 111 (18.6%) | 7 (28.0%) | 18 (19.8%) | |
| Flu Trim 1 | No | 921 (94.8%) | 243 (93.5%) | 567 (95.1%) | 24 (96.0%) | 87 (95.6%) |
| Yes | 51 (5.2%) | 17 (6.5%) | 29 (4.9%) | 1 (4.0%) | 4 (4.4%) | |
| Flu Trim 2 | No | 894 (92.0%) | 244 (93.8%) | 547 (91.8%) | 20 (80.0%) | 83 (91.2%) |
| Yes | 78 (8.0%) | 16 (6.2%) | 49 (8.2%) | 5 (20.0%) | 8 (8.8%) | |
| Flu Trim 3 | No | 884 (90.9%) | 232 (89.2%) | 546 (91.6%) | 24 (96.0%) | 82 (90.1%) |
| Yes | 88 (9.1%) | 28 (10.8%) | 50 (8.4%) | 1 (4.0%) | 9 (9.9%) | |
| Fever Overall | No | 885 (90.7%) | 242 (92.0%) | 542 (90.8%) | 22 (88.0%) | 79 (86.8%) |
| Yes | 91 (9.3%) | 21 (8.0%) | 55 (9.2%) | 3 (12.0%) | 12 (13.2%) | |
| Fever Trim 1 | No | 943 (96.9%) | 253 (96.9%) | 577 (96.8%) | 24 (96.0%) | 89 (97.8%) |
| Yes | 30 (3.1%) | 8 (3.1%) | 19 (3.2%) | 1 (4.0%) | 2 (2.2%) | |
| Fever Trim 2 | No | 942 (96.8%) | 255 (97.7%) | 577 (96.8%) | 24 (96.0%) | 86 (94.5%) |
| Yes | 31 (3.2%) | 6 (2.3%) | 19 (3.2%) | 1 (4.0%) | 5 (5.5%) | |
| Fever Trim 3 | No | 943 (96.9%) | 255 (97.7%) | 578 (97.0%) | 24 (96.0%) | 86 (94.5%) |
| Yes | 30 (3.1%) | 6 (2.3%) | 18 (3.0%) | 1 (4.0%) | 5 (5.5%) | |
| Intrapartum fever | No | 911 (93.3%) | 252 (95.8%) | 552 (92.5%) | 25 (100.0%) | 82 (90.1%) |
| Yes | 65 (6.7%) | 11 (4.2%) | 45 (7.5%) | 0 (0.0%) | 9 (9.9%) | |
Main effect of maternal immune activation on odds of ASD
In unadjusted models, flu in overall pregnancy (OR 1.16, 95% CI 0.72–1.86) and second trimester (OR 1.54, 95% CI 0.82–2.88) and fever overall (OR=1.53, 95% CI 0.85–2.77), in second trimester (OR=1.82, 95% CI 0.73–4.52), and third trimester (OR=1.89, 95% CI 0.76–4.73), as well as intrapartum fever (OR=1.21, 95% CI 0.58–2.51) were associated with higher odds of ASD, though none of these associations were significant. In adjusted models, flu overall (OR=1.43, 95% CI 0.86–2.36), in trimester 2 (OR=1.50, 95% CI 0.77–2.93), and trimester 3 (OR=1.34, 95% CI 0.65–2.76) as well as fever overall (OR=1.78, 95% CI 0.95–3.33), in trimester 2 (OR=1.55, 95% CI 0.58–4.15), in trimester 3 (OR=2.63, 95% CI 1.00–6.89), and intrapartum fever (OR=1.27, 95% CI 0.59–2.71) were all associated with increased odds of ASD, particularly fever in trimester 3 (p=0.05). Genitourinary infection was not associated with elevated odds of ASD in either unadjusted or adjusted models (Table 3, Figure 1).
Table 3.
Regression Models Estimating Association between Maternal Immune Activation Exposures and Odds of ASD
| Unadjusted | Adjusted § | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Genitourinary Infection | 0.93 (0.61 , 1.42) | 0.72 | 0.84 (0.54 , 1.33) | 0.46 |
| Flu | 1.16 (0.72 , 1.86) | 0.55 | 1.43 (0.86 , 2.36) | 0.17 |
| Flu Trimester 1 | 0.79 (0.31 , 2.04) | 0.63 | 1.02 (0.38 , 2.72) | 0.97 |
| Flu Trimester 2 | 1.54 (0.82 , 2.88) | 0.18 | 1.50 (0.77 , 2.93) | 0.23 |
| Flu Trimester 3 | 0.94 (0.47 , 1.87) | 0.86 | 1.34 (0.65 , 2.76) | 0.42 |
| Fever | 1.53 (0.85 , 2.77) | 0.16 | 1.78 (0.95 , 3.33) | 0.07 |
| Fever Trimester 1 | 0.82 (0.24 , 2.73) | 0.74 | 1.06 (0.3 , 3.67) | 0.93 |
| Fever Trimester 2 | 1.82 (0.73 , 4.52) | 0.20 | 1.55 (0.58 , 4.15) | 0.39 |
| Fever Trimester 3 | 1.89 (0.76 , 4.73) | 0.17 | 2.63 (1.00 , 6.89) | 0.05 |
| Intrapartum Fever | 1.21 (0.58 , 2.51) | 0.61 | 1.27 (0.59 , 2.73) | 0.53 |
Adjusted for child sex, child year of birth, maternal age, gestational age, delivery type, and low birthweight.
Figure 1. Regression Models Estimating Association between Maternal Immune Activation Exposures and Odds of ASD.
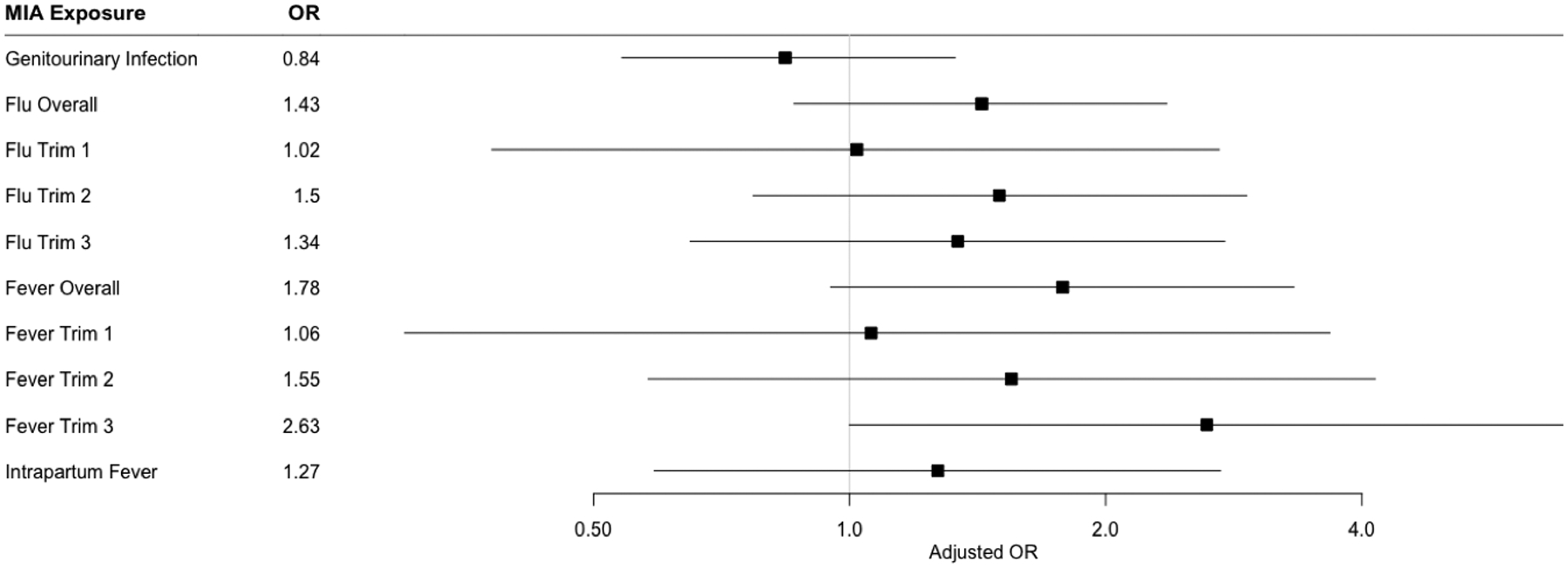
Forest plot showing adjusted odds ratio (OR) and 95% confidence intervals for the association between prenatal GU infection, flu (overall and trimester-specific), and fever (overall and trimester-specific), and intrapartum fever and Autism Spectrum Disorder. Models are adjusted for child sex, child year of birth, maternal age, gestational age, delivery type, and low birthweight.
Interaction between maternal immune activation and antibiotic use
In unadjusted and adjusted models, there was evidence of an interaction between flu in second trimester and antibiotic use on odds of ASD (adjusted interaction term OR=0.23, 95% CI 0.05–0.96, p=0.04) (Table S9). No other regression models showed evidence of any significant interaction at p<0.10. We could not carry out interaction models for intrapartum fever because of sparse data (Table S3–S11).
We further examined the association between flu in second trimester and ASD risk, stratified by antibiotic use in pregnancy or labor and delivery. Among women who received an antibiotic, there was no significant association between flu in trimester two and odds of ASD, in either unadjusted (OR=1.08, 95% CI 0.49–2.35, p=0.85) or adjusted (OR=0.99, 95% CI 0.43–2.28, p=0.99) models (Figure 2 and Table 4). In this subgroup, male sex was associated with higher odds of ASD (OR=3.37, 95% CI 2.05–5.53, p<0.0001) and greater gestational age with reduced odds of ASD (OR=0.81, 95% CI 0.73–0.89, p<0.0001).
Figure 2. Log Odds (95% CI) for Association between Flu in Trimester 2 and ASD, Stratified by Antibiotic Use during Pregnancy.
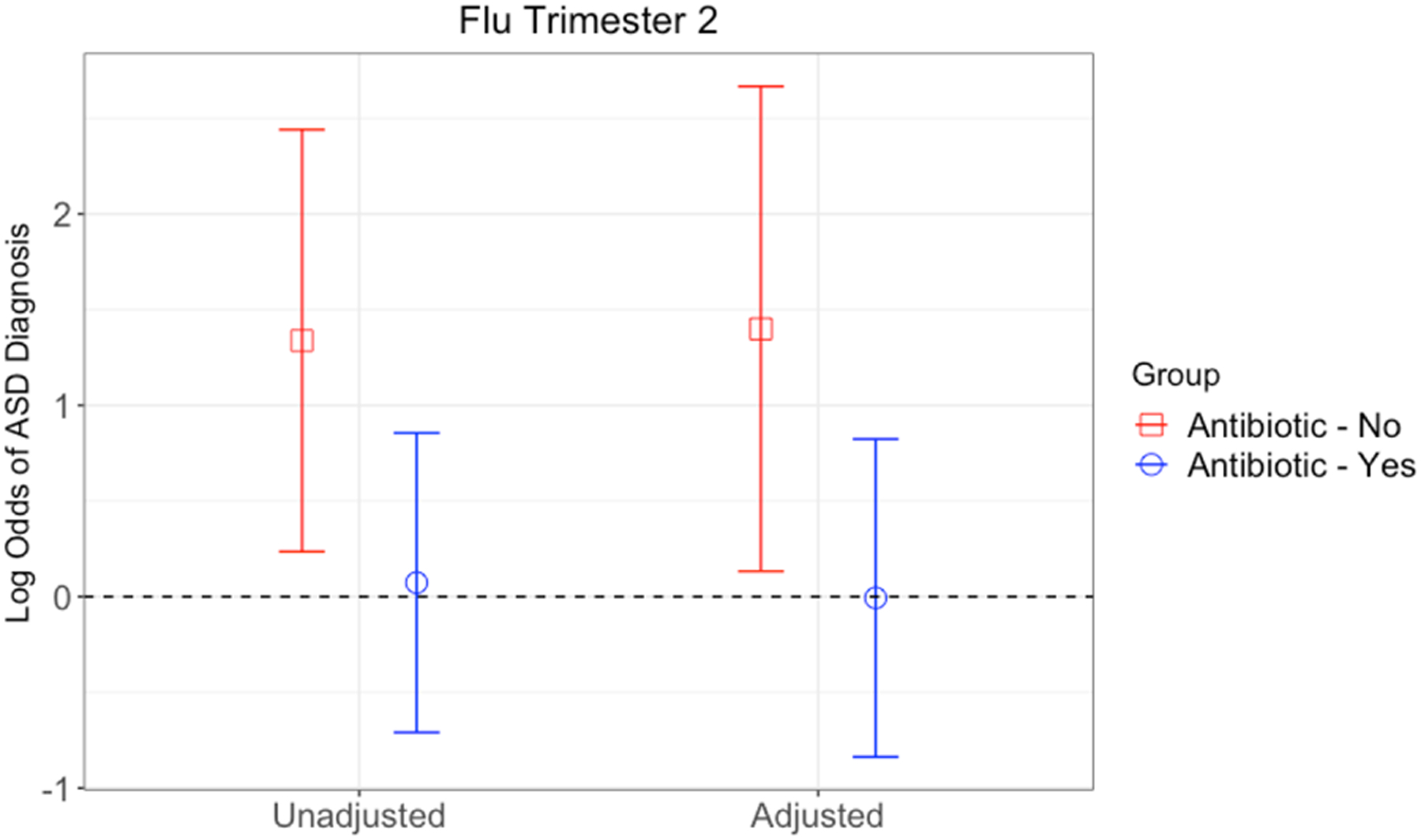
Model 1 was unadjusted. Model 2 was adjusted for child sex, child year of birth, maternal age, gestational age, delivery type, and low birthweight.
Table 4.
Logistic Regression Models Estimating Association between Flu in Second Trimester and Odds of ASD, Stratified by Antibiotic Use during Pregnancy
| Antibiotic use during Pregnancy | No Antibiotic use during Pregnancy | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Flu Trim 2 (unadjusted) | 1.08 (0.49, 2.35) | 0.85 | 3.81 (1.27 , 11.49) | 0.02 |
| Flu Trim 2 (adjusted §) | 0.99 (0.43 , 2.28) | 0.99 | 4.05 (1.14 , 14.38) | 0.03 |
| Male child | 3.37 (2.05 , 5.53) | <0.0001 | 5.83 (2.14 , 15.86) | <0.001 |
| Child’s year of birth | 1.03 (0.96 , 1.12) | 0.41 | 1.11 (0.99 , 1.26) | 0.09 |
| Maternal age (years) | 1.02 (0.99 , 1.06) | 0.19 | 1.05 (0.98 , 1.12) | 0.20 |
| Gestational age (weeks) | 0.81 (0.73 , 0.89) | <0.0001 | 0.93 (0.79 , 1.09) | 0.37 |
| C-section delivery | 1.08 (0.67 , 1.76) | 0.75 | 1.85 (0.65 , 5.28) | 0.25 |
| Low birth weight (<2500 grams) | 0.54 (0.25 , 1.15) | 0.11 | 2.46 (0.58 , 10.44) | 0.22 |
Adjusted for child sex, child year of birth, maternal age, gestational age, delivery type, and low birthweight.
However, among women who did not receive an antibiotic during pregnancy, flu in the second trimester significantly increased the odds of ASD in the child, in the unadjusted model (OR=3.81, 95% CI 1.27–11.49, p=0.02) and after adjusting for confounders (OR=4.05, 95% CI=1.14–14.38, p=0.03), though the confidence intervals are wide, reflecting the small sample size in this stratum (Table 2). In this subgroup, male sex was also associated with increased odds of ASD (OR=5.83, 95% CI 2.14–15.86, p<0.001). The fisher’s exact test for independence between flu in trimester 2 and ASD resulted in an OR of 1.08 (95% CI 0.58–1.92), p=0.77 among women who took an antibiotic and an OR of 1.5 (95% CI 0.50–4.01), p=0.44 among those that did not take an antibiotic (data not shown).
Lastly, we carried out two sensitivity analyses. First, we restricted our analysis to children 3 years of age or older. The magnitude, significance, and interpretation of the interaction finding did not change (data not shown). Second, we restricted ASD cases to those with 2 or more ICD-9-CM codes instead of one at two separate clinical visits (n=88 ASD). The association between flu in trimester 2 and odds of ASD remained positive and significant for the group with no antibiotic use during pregnancy but in unadjusted models only (OR=3.4, 95% CI 1.03–11.20, p<0.05). In the adjusted model, the association was no longer significant (OR=3.15, 95% CI 0.80–12.41, p=0.10).
Discussion
In this birth cohort, as previously reported by Brucato et al., we observed increased risk for ASD in children born to mothers who experienced maternal immune activation (MIA) during pregnancy, specifically fever. Flu exposure also showed increased risk for ASD, but this was not statistically significant. However, among women who experienced MIA due to flu, and were exposed to antibiotics during pregnancy, the risk for ASD was attenuated; in contrast, the association between flu exposure and increased risk of ASD remained among those without antibiotic use. This effect modification was statistically significant for the combination of second trimester flu exposure and antibiotic use, implying exposure to an antibiotic during pregnancy in the context of MIA may protect against the increased risk for ASD in the child. Our finding was specific to second trimester, and was not replicated in first or third trimester, or in overall pregnancy. We also did not find any evidence of significant interactions between other non-flu MIA exposures and antibiotic use.
The findings from our study support recent animal literature demonstrating that antibiotics mitigate the maternal immune activation insult on the risk for ASD in the developing offspring, and suggest that the maternal gut microbiome may play a critical role in modulating the immune system’s response on the developing brain (Kim et al., 2017; Lammert et al., 2018; Wang et al., 2019).
The present study was motivated specifically by the animal experiments performed by Kim et al. 2017. The MIA-associated phenotypes (increased repetitive behaviors, anxiety, social interaction deficits, as well as cortical patches) that are typically considered to be autism-like symptoms, require secretion of Il-17a, which occurs through differentiation of Th17 cells, which themselves are promoted by the presence of specific maternal gut bacteria. Kim et al. demonstrated that treatment of pregnant dams with vancomycin, a broad-spectrum antibiotic, prevented the neurodevelopmental abnormalities associated with MIA by decreasing the amount of Th17 cells in the small intestine, thus decreasing levels of IL-17a circulating in the maternal plasma. Critically, transplantation of the mouse intestinal microbiome with commensal bacteria from humans previously found to induce Th17 cells also led to the neurodevelopmental abnormalities (Kim et al., 2017). In sum, this demonstrates that the composition of the maternal gut microbiome, as well as factors that influence it such as antibiotics or probiotics, seem to determine whether exposure to maternal immune activation leads to increased risk of neurodevelopmental abnormalities in the offspring (Kim et al., 2017; Lammert et al., 2018; Wang et al., 2019). Notably, animal research has shown that the influence of MIA on the offspring is modified by the genetic strain of the mice (Morais et al., 2018; Schwartzer et al., 2013), and that strains differ in their immune and microbiota profiles, in a sex-dependent manner (Coretti et al., 2017).
In this study, we found that flu in the second trimester increased risk for ASD in the child, among women who did not receive any antibiotic during pregnancy or labor and delivery. Viral infections during pregnancy have previously been implicated with increased for ASD, though findings have not been specific to a particular trimester, and bacterial infections during second and third trimester in particular, have been associated with increased ASD risk (Atladóttir et al., 2010; Fang et al., 2015; Jiang et al., 2016; Lee et al., 2015). One possibility is that women who self-reported having the flu during pregnancy may not have truly had influenza, but might have had a different infection, perhaps of bacterial nature. Influenza infection can also increase risk for a secondary bacterial pneumonia, which would be appropriately treated by antibiotics. We also cannot rule out that the possibility that other trimester-specific flu/fever exposures would have been implicated had enough observations been present. There were more flu exposures across and within each trimester, compared to fever exposures, among each of the four groups (ASD/TD × antibiotic use; see Table 2). Further, because this study was enriched for preterm births, infections in third trimester might not be adequately captured due to early delivery. Further, women may not recall first-trimester infections or may not have known they were pregnant during that time. For these reasons, our second-trimester finding may not necessarily reflect a true critical window compared to other trimesters. Prior literature stresses the importance of considering ASD risks in the context of specific infectious agents as well as the timing in pregnancy.
In this study, antibiotic exposure could have happened at any point during pregnancy. It is most intuitive that antibiotics would have the strongest modifying effect for more proximal infection exposures. Therefore, the signal we observe may be ‘noisier’ due to collapsing antibiotic exposure across all trimesters. However, we were not powered enough to look at both MIA and antibiotic use within each trimester. It is plausible that an earlier infection/fever might have long lasting immune consequences that could still be modified by later exposure to antibiotics. This remains to be tested in future studies, however.
To our knowledge, this is the first prospective birth cohort study in humans to test for interaction between maternal immune activation and antibiotic use in pregnancy on child ASD risk. However, this study was motivated by a number of earlier publications examining antibiotic use or treatment of infection on ASD. Fever-associated risk for ASD or developmental disabilities has been found to be decreased among mothers who took antipyretic medications during pregnancy, implying that treatment for the maternal immune activation was protective (Zerbo et al., 2013). The literature regarding treatment with antibiotic use in particular on ASD risk has been mixed. A 2012 study of the Danish National Birth Cohort found a small but elevated risk of ASD and infantile autism among women who self-reported taking an antibiotic in pregnancy, though the study did not disentangle whether the association was causal or due to confounding by indication (Atladóttir et al, 2012).
A much larger number of studies have examined the associations between infections, antibiotic use and ASD risk in the postnatal period. While these studies may involve a different mechanism of action than that in the pregnancy period, they do add further evidence that inflammatory processes and medications influence the developing brain. A 2018 study using Danish registry data found that infections since birth treated with anti-infective agents were associated with increased risk of being diagnosed with a mental disorder or redeeming a prescription for psychotropic medication. Antibiotic use in particular was associated with increased risk for a mental disorder (Köhler-Forsberg et al., 2018). A study out of the Autism Treatment Network similarly found that antibiotic use and ear infections were associated with increased risk for ASD, and the antibiotic in particular seemed to account for the association between ear infection and ASD (Bittker & Bell, 2018). However, another recent study also using Danish registry data aimed to assess whether otitis media, previously associated with risk for ASD (Adams et al., 2016), was itself associated with ASD or whether antibiotic treatment for otitis media was the true risk factor (Wimberley et al., 2018). The authors found that exposure to exposure to otitis media and antibiotic use were both independently associated with increased risk for ASD. Otitis media remained significantly associated with ASD even after controlling for exposure to antibiotics. There was no evidence of any interaction between otitis media and antibiotic use on ASD, given that the risk for ASD among children who were exposed to both otitis media and antibiotics did not change meaningfully from the risk among children only exposed to antibiotics (Wimberley et al., 2018).
This study should be interpreted in the context of several limitations. First, our sample size was small when looking at the interaction of MIA with antibiotic use on ASD status, resulting in large confidence intervals. When we assessed for interaction between flu in trimester 2 and antibiotic use on odds of ASD using Fisher’s Exact Test, we saw qualitative evidence of an interaction, but neither strata were significant. The sensitivity analysis using a more stringent ASD case definition (2 or more codes) also rendered our findings not statistically significant. It is also possible that children were diagnosed with ASD outside of the Boston Medical Center, in which case this information may not be included in the EMR data. This is a limitation of the study, though we note there is considerable contact between children and BMC medical providers, with each child on averaging having an average of 39 visits, with a range of 1 to 463, as indicated in prior publication by this group (Brucato et al., 2017). Therefore, findings should be treated as preliminary, hypothesis-generating, and in need of replication in a larger cohort. Our small sample also meant that we could not separate antibiotic use by type of antibiotic or by trimester. Future studies in larger cohorts are needed to replicate our findings and more fully explore these associations. Another limitation of this study is that we used a complete-case analysis, which has the potential to introduce biases. However, complete cases did not differ significantly from those excluded, and ASD outcome was not associated with complete case status either unadjusted or after adjusting for covariates (Hughes et al, 2019).
Next, while medical charts/EMR demonstrated high sensitivity in detecting antibiotic use during pregnancy, sensitivity by self-report was low. This could reflect low awareness of medications taken during labor and delivery, lack of familiarity with types of medications during pregnancy, or low adherence to prescriptions. Prior literature has also shown that while concordance between self-report and health record data is strong for medications used chronically, there tends to be much lower concordance for shorter-term medication use, including antibiotic use during pregnancy (Cheung et al., 2017; Laursen et al., 2019; Palmsten et al., 2018; Pisa et al., 2015; Sarangarm et al., 2012). Lastly, we relied on self-report information on infections. Therefore, our data may be susceptible to recall errors. However, we do not think recall errors, if any, differ by ASD status, since women recalled their infectious exposures just after the birth of their child, years prior to ASD diagnosis. For women who did not have antibiotic information in EMR (because only women with an antibiotic prescription are included in EMR), we assumed that these women were not exposed to antibiotics, provided that they also did not self-report antibiotic use on the standardized questionnaire and did not receive intrapartum antibiotic. In light of these limitations, our findings should be interpreted with caution and serve as hypothesis-generating.
Our study had a number of strengths, however. First, we assessed for the first time the interaction between maternal immune activation and antibiotic use on ASD. Within a prospective, enriched-risk birth cohort, we demonstrated that the association between maternal immune activation is modified by the use of antibiotics during pregnancy. Antibiotic use and ASD were both assessed by EMR data, and we relied on self-report of infections shortly after birth. The reason we used maternal self-report of infection is because infections recorded in EMR data would likely only capture the most severe infections that required treatment. We felt that using self-report information would increase sensitivity and decrease misclassification.
The interaction effect we found was maintained after adjusting for demographic factors and clinical variables. The findings from this study are in line with recent animal literature showing that antibiotic use affects the maternal gut microbiome, modifying the influence of maternal immune activation on neurodevelopment and autism-like symptoms in the offspring.
Given the animal literature showing the interaction between maternal infection/fever and antibiotic use, we sought to examine this question specifically, and did not assess for the main effect of antibiotic use on ASD risk. However, antibiotics also carry risks to the mother and developing fetus, and prior literature has shown that antibiotics themselves might increase the risk of ASD (Adams et al., 2016; Atladóttir et al., 2012; Bittker & Bell, 2018; Champagne-Jorgensen et al., 2018; Eshraghi et al., 2018; Köhler-Forsberg et al., 2018; Moya-Pérez et al., 2017). Another important consideration is that this study did not look at the effect of treatment of an infection with an antibiotic on ASD risk. Rather, antibiotics could have been prescribed for any reason. Future studies are needed to assess whether treatment of infection with antibiotics modifies the risk of ASD in the child.
The gut microbiome is malleable across the life course, which makes it an attractive target for intervention (Clemente et al., 2012). Early immunological and metabolic programming by the microbiota and their metabolites may have long-term consequences for human health, including neurodevelopment (Ajslev et al., 2011; Borre et al., 2014; Johansson et al., 2011; Kalliomäki et al., 2008; C. H. Kim, 2018; Matamoros et al., 2013; Penders et al., 2006; Rautava et al., 2012; Sylvia & Demas, 2018).
Supplementary Material
Funding Acknowledgments
This study is supported in part by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number R40MC27443, Autism Field-initiated Innovative Research Studies Program; and grant number UJ2MC31074, Autism Single Investigator Innovation Program. The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (R21ES011666, R21HD066471, U01AI090727, R21AI079872, R01HD086013, 2R01HD041702, and R01HD098232). CBH was supported by the National Institute of Mental Health Psychiatric Epidemiology Training Program (5T32MH014592; PI Peter Zandi). NTM was supported by National Heart, Lung, and Blood Institute of the NIH grant K01HL141589. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. The funding agencies had no involvement in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest to declare.
References
- Adams DJ, Susi A, Erdie-Lalena CR, Gorman G, Hisle-Gorman E, Rajnik M, Elrod M, & Nylund CM (2016). Otitis media and related complications among children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 46(5), 1636–1642. [DOI] [PubMed] [Google Scholar]
- Ajslev TA, Andersen CS, Gamborg M, Sørensen TIA, & Jess T (2011). Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. International Journal of Obesity, 35(4), 522. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Atladóttir Hjördis Ó, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, & Parner ET (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(12), 1423–1430. [DOI] [PubMed] [Google Scholar]
- Atladóttir Hjördis Ósk, Henriksen TB, Schendel DE, & Parner ET (2012). Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics, 130(6), e1447–e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Iosif A-M, Smith SEP, Bregere C, Amaral DG, & Patterson PH (2014). Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biological Psychiatry, 75(4), 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittker SS, & Bell KR (2018). Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and autism: an epidemiological study. Neuropsychiatric Disease and Treatment, 14, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, & Cryan JF (2014). Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine, 20(9), 509–518. 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Boulanger-Bertolus J, Pancaro C, & Mashour GA (2018). Increasing role of maternal immune activation in neurodevelopmental disorders. Frontiers in Behavioral Neuroscience, 12, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, Stuart EA, Fallin MD, & Wang X (2017). Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Research, 10(11), 1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Murai T, & Bauman MD (2017). Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biological Psychiatry, 81(5), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne-Jorgensen K, Kunze WA, Forsythe P, Bienenstock J, & Neufeld K-AM (2018). Antibiotics and the nervous system: More than just the microbes? Brain, Behavior, and Immunity. [DOI] [PubMed] [Google Scholar]
- Cheung K, El Marroun H, Elfrink ME, Jaddoe VWV, Visser LE, & Stricker BHC (2017). The concordance between self-reported medication use and pharmacy records in pregnant women. Pharmacoepidemiology and Drug Safety, 26(9), 1119–1125. [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, & Huh JR (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science, 351(6276), 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente JC, Ursell LK, Parfrey LW, & Knight R (2012). The impact of the gut microbiota on human health: An integrative view. Cell, 148(6), 1258–1270. 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coretti L, Cristiano C, Florio E, Scala G, Lama A, Keller S, Cuomo M, Russo R, Pero R, & Paciello O (2017). Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Scientific Reports, 7, 45356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, Daniele Fallin M, Levy S, Schieve LA, & Yeargin-Allsopp M (2019). Infection and fever in pregnancy and autism spectrum disorders: findings from the study to explore early development. Autism Research, 12(10), 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi RS, Deth RC, Mittal R, Aranke M, Kay S-IS, Moshiree B, & Eshraghi AA (2018). Early disruption of the microbiome leading to decreased antioxidant capacity and epigenetic changes: Implications for the rise in autism. Frontiers in Cellular Neuroscience, 12, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Wang S, Huang N, Yeh H, & Chen C (2015). Prenatal Infection and Autism Spectrum Disorders in Childhood: A Population-Based Case–Control Study in T aiwan. Paediatric and Perinatal Epidemiology, 29(4), 307–316. [DOI] [PubMed] [Google Scholar]
- Harvey L, & Boksa P (2012). Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Developmental Neurobiology, 72(10), 1335–1348. [DOI] [PubMed] [Google Scholar]
- Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, Hirtz D, Gunnes N, Lie KK, & Magnus P (2018). Prenatal fever and autism risk. Molecular Psychiatry, 23(3), 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, & Patterson PH (2012). Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences, 109(31), 12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Heron J, Sterne JAC, & Tilling K (2019). Accounting for missing data in statistical analyses: multiple imputation is not always the answer. International Journal of Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, & Engstrand L (2010). Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PloS One, 5(3), e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xu L, Shao L, Xia R, Yu Z, Ling Z, Yang F, Deng M, & Ruan B (2016). Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain, Behavior, and Immunity, 58, 165–172. [DOI] [PubMed] [Google Scholar]
- Johansson MA, Sjögren YM, Persson J-O, Nilsson C, & Sverremark-Ekström E (2011). Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PloS One, 6(8), e23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki M, Carmen Collado M, Salminen S, & Isolauri E (2008). Early differences in fecal microbiota composition in children may predict overweight. The American Journal of Clinical Nutrition, 87(3), 534–538. [DOI] [PubMed] [Google Scholar]
- Kim CH (2018). Immune regulation by microbiome metabolites. Immunology, 154(2), 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, & Huh JR (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, & Prinssen EP (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology, 10(11), 643. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, Mors O, & Benros ME (2018). A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry. 10.1001/jamapsychiatry.2018.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, & Croen LA (2014). Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. The Journal of Pediatrics, 164(1), 20–25. [DOI] [PubMed] [Google Scholar]
- Lammert CR, Frost EL, Bolte AC, Paysour MJ, Shaw ME, Bellinger CE, Weigel TK, Zunder ER, & Lukens JR (2018). Cutting Edge: Critical Roles for Microbiota-Mediated Regulation of the Immune System in a Prenatal Immune Activation Model of Autism. Journal of Immunology (Baltimore, Md. : 1950), 201(3), 845–850. 10.4049/jimmunol.1701755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ (2010). What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics, 22(2), 219–225. [DOI] [PubMed] [Google Scholar]
- Laursen M, Hallgreen CE, Dreyer N, Bourke A, Mt-Isa S, & Blackburn S (2019). Comparison of electronic self-reported prescription medication use during pregnancy with the national prescription register in Denmark. Pharmacoepidemiology and Drug Safety. [DOI] [PubMed] [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, Karlsson H, & Dalman C (2015). Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, Behavior, and Immunity, 44, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, & Hertz-Picciotto I (2014). Maternal lifestyle and environmental risk factors for autism spectrum disorders. International Journal of Epidemiology, 43(2), 443–464. 10.1093/ije/dyt282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydholm CN, Köhler-Forsberg O, Nordentoft M, Yolken RH, Mortensen PB, Petersen L, & Benros ME (2019). Parental infections before, during, and after pregnancy as risk factors for mental disorders in childhood and adolescence: A nationwide Danish study. Biological Psychiatry, 85(4), 317–325. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, & Patterson PH (2012). Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, Behavior, and Immunity, 26(4), 607–616. 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Marzouk AC, Donnelly R, & Ponzio NM (2011). Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain, Behavior, and Immunity, 25(5), 863–871. [DOI] [PubMed] [Google Scholar]
- Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, & de La Cochetiere M-F (2013). Development of intestinal microbiota in infants and its impact on health. Trends in Microbiology, 21(4), 167–173. [DOI] [PubMed] [Google Scholar]
- Morais LH, Felice D, Golubeva AV, Moloney G, Dinan TG, & Cryan JF (2018). Strain differences in the susceptibility to the gut–brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behavioural Pharmacology, 29(2), 181–198. [DOI] [PubMed] [Google Scholar]
- Moya-Pérez A, Luczynski P, Renes IB, Wang S, Borre Y, Anthony Ryan C, Knol J, Stanton C, Dinan TG, & Cryan JF (2017). Intervention strategies for cesarean section–induced alterations in the microbiota-gut-brain axis. Nutrition Reviews, 75(4), 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmsten K, Hulugalle A, Bandoli G, Kuo GM, Ansari S, Xu R, & Chambers CD (2018). Agreement between maternal report and medical records during pregnancy: medications for rheumatoid arthritis and asthma. Paediatric and Perinatal Epidemiology, 32(1), 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantham P, Aye ILMH, & Powell TL (2015). Inflammation in maternal obesity and gestational diabetes mellitus. Placenta, 36(7), 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, & Stobberingh EE (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics, 118(2), 511–521. [DOI] [PubMed] [Google Scholar]
- Pisa FE, Casetta A, Clagnan E, Michelesio E, Brumatti LV, & Barbone F (2015). Medication use during pregnancy, gestational age and date of delivery: agreement between maternal self-reports and health database information in a cohort. BMC Pregnancy and Childbirth, 15(1), 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautava S, Luoto R, Salminen S, & Isolauri E (2012). Microbial contact during pregnancy, intestinal colonization and human disease. Nature Reviews Gastroenterology & Hepatology, 9(10), 565. [DOI] [PubMed] [Google Scholar]
- Rice D, & Barone S Jr (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives, 108(suppl 3), 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, & Romano J (1996). Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. Journal of Comparative Neurology, 370(2), 247–261. [DOI] [PubMed] [Google Scholar]
- Sarangarm P, Young B, Rayburn W, Jaiswal P, Dodd M, Phelan S, & Bakhireva L (2012). Agreement between self-report and prescription data in medical records for pregnant women. Birth Defects Research Part A: Clinical and Molecular Teratology, 94(3), 153–161. [DOI] [PubMed] [Google Scholar]
- Schlotz W, & Phillips DIW (2009). Fetal origins of mental health: evidence and mechanisms. Brain, Behavior, and Immunity, 23(7), 905–916. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, & Ashwood P (2013). Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational Psychiatry, 3(3), e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner AB, Kao JY, & Young VB (2015). The gut microbiome in health and in disease. Current Opinion in Gastroenterology, 31(1), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, & Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience, 27(40), 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia KE, & Demas GE (2018). A gut feeling: microbiome-brain-immune interactions modulate social and affective behaviors. Hormones and Behavior, 99, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, & Caruso D (2014). Preterm birth and random plasma insulin levels at birth and in early childhood. Jama, 311(6), 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Xiao, Yang J, Zhang H, Yu J, & Yao Z (2019). Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Research, 12(4), 576–588. [DOI] [PubMed] [Google Scholar]
- Wang Xiaobin, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, & Xu X (2002). Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Jama, 287(2), 195–202. [DOI] [PubMed] [Google Scholar]
- Wimberley T, Agerbo E, Pedersen CB, Dalsgaard S, Horsdal HT, Mortensen PB, Thompson WK, Köhler-Forsberg O, & Yolken RH (2018). Otitis media, antibiotics, and risk of autism spectrum disorder. Autism Research, 11(10), 1432–1440. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, & Hertz-Picciotto I (2013). Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. Journal of Autism and Developmental Disorders, 43(1), 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


