Abstract
Background:
Philadelphia chromosome-positive (Ph+) advanced leukemias including acute myeloid leukemia (AML) and chronic myeloid leukemia myeloid blast phase (CML-MBP) have poor outcomes. Venetoclax (VEN) has shown synergism with BCR-ABL1 tyrosine kinase inhibitors (TKI) in pre-clinical studies. However, clinical activity of VEN+TKI-based regimens is unknown.
Methods:
We conducted a retrospective study on patients with Ph+ AML (n=7) and CML-MBP (n=9) who received a VEN+TKI-based regimens at our institution.
Results:
Median age was 42 years and the median number of prior lines of therapy was 5 (range 2–8). Nine patients received decitabine-based, and 7 received intensive chemotherapy-based regimens. Ten patients (63%) received ponatinib. The overall response rate (ORR) in 15 evaluable patients was 60% (6 CRi, 1 CR, 1 MLFS, 1 PR). The ORR was 43% in Ph+ AML and 75% in CML-MBP. The median overall survival (OS) for all patients was 3.6 months, for AML was 2.0 months, and for CML-MBP was 10.9 months. The median relapse-free survival (RFS) for AML and CML-MBP was 3.6 and 3.9 months, respectively. Patients achieving CR/CRi compared to non-responders had higher baseline Ph+ metaphases and BCR-ABL1 PCR.
Conclusions:
VEN+TKI-based combinations show encouraging activity in very heavily pre-treated, advanced Ph+ leukemias, particularly CML MBP.
Keywords: venetoclax, BCR/ABL TKI, decitabine, ponatinib, Philadelphia-chromosome positive acute myeloid leukemia, AML, chronic myeloid leukemia, CML, myeloid blast phase
INTRODUCTION
Acute myeloid leukemia (AML) and myeloid blast phase (MBP) of chronic myeloid leukemia (CML) are aggressive myeloid leukemias with overall poor outcomes.1,2 Both AML and CML-MBP are more frequently diagnosed in older patients with a median age of over 60 years at diagnosis.3,4 While AML is the most common acute leukemia in adults, CML is much less common, and only a minority of patients progress to the aggressive blast phase. The t(9;22) leads to the formation of the Philadelphia chromosome which is almost universally present in CML and leads to the development of the constitutively active BCR-ABL tyrosine kinase. However, in rare instances, Philadelphia chromosomes have been noted in AML.5–7 Patients with newly diagnosed Philadelphia chromosome-positive (Ph+) AML have poor outcomes with a reported median overall survival (OS) of 9 months, although there is a dearth of data on this entity given its rarity.5,6 Transformation to advanced phase CML occurs in 1–5% patients with chronic phase (CP) CML per year and CML-MBP has a median OS of 6–12 months, despite the use of second- or third-generation tyrosine kinase inhibitors (TKI).2,8,9 These poor outcomes highlight the need for novel therapies for these patients.
The anti-apoptotic BCL2 family of proteins regulate the mitochondrial apoptotic response. BCL2 is highly and almost universally expressed in leukemia cells and leukemic stem cells (LSC) in AML and CML, is upregulated by BCR-ABL1 signaling, and is overexpressed in CML blast phase.10–14 Venetoclax, a selective BCL2 inhibitor, is approved for use with low-dose cytarabine or hypomethylating agents for older or ‘unfit’ patients with newly diagnosed AML. Venetoclax has shown pre-clinical activity against TKI resistant CML cells and has shown synergism with BCR-ABL TKIs in eradicating LSCs in advanced CML.13,15,16 However, clinical activity of venetoclax and BCR-ABL TKI-based combinations is unknown. Hence, we conducted a retrospective study to summarize the activity of such combination therapies in Philadelphia chromosome-positive (Ph+) advanced myeloid leukemias.
MATERIALS AND METHODS
In this retrospective chart review study, we included patients with Philadelphia chromosome-positive AML and CML-MBP treated at our institution with venetoclax and BCR-ABL1 TKI-based combinations. Requirement for informed consent was waived due to the chart review nature of this study and it was conducted in accordance with the Declaration of Helsinki.
Treatment Regimens
Dosing of agents was performed per institutional guidelines and practice. Venetoclax was dosed at 400 mg daily with dose reduction in conjunction with CYP3A4 inhibitors e.g., azole antifungals for infection prophylaxis or treatment. Reduction of venetoclax duration from continuous to less than 2 weeks was allowed in cases of myelosuppression. TKI was administered concomitantly with venetoclax. Dasatinib was dosed at 100 mg daily (n=2) or 50 mg daily (n=2), bosutinib was dosed at 400 mg daily, nilotinib was dosed at 200 mg daily, and ponatinib was dosed at 30 mg daily (n=7), or 15 mg daily (n=2) or 45 mg daily (n=1). Two patients with concomitant FLT3-ITD received gilteritinib 80 mg daily and 120 mg daily, respectively. Decitabine dose was 20 mg/m2 for 5 to 10 days. CLIA2 regimen consisted of cladribine 5 mg/m2 on days 1–5, cytarabine 2000 mg/m2 IV on days 1–5, and idarubicin 10 mg/m2 IV days 1–3. CIA regimen consisted of clofarabine 22.5 mg/m2 IV on days 1–5, idarubicin 6 mg/m2 IV on days 1–3, and cytarabine 0.75 g/m2 IV on days 1–5. FIA regimen consisted of fludarabine 30 mg/m2 IV on days 1–5, idarubicin 6 mg/m2 IV on days 1–3, and cytarabine 0.75 g/m2 IV on days 1–5. Cladribine with HiDAC regimen consisted of cladribine 5 mg/m2 and cytarabine 1000 mg/m2 IV daily for days 1–3. CPX-351 was administered at 44 units/m2 on days 1, 3, 5. Patients could proceed to allogeneic stem-cell transplantation (allo-SCT) after achievement of a response if they were deemed fit for allo-SCT and a donor was available.
Response Assessment
The AML European LeukemiaNet 2017 criteria were used for determination of response.17 BCR-ABL1 kinase domain mutation analysis done on cDNA with a nested polymerase chain reaction (PCR) method covered codons 221 to 500.18 Flow cytometry for minimal residual disease (MRD) testing had a sensitivity of 0.1% or higher.19 Karyotype or fluorescence in situ hybridization were used for determination of proportion of Ph+ metaphases in bone marrow aspiration samples prior to starting therapy. Conventional karyotype was used for assessment of cytogenetic response after therapy.
Statistical Analysis
OS was determined from the date of start of therapy to date of death or censored at last follow-up. Relapse-free survival (RFS) was determined from date of achievement of response to relapse, or death, or censored at last follow-up. OS and RFS were calculated using the Kaplan-Meier method. Student’s t-test was used to determine the association between continuous variables and response. GraphPad Prism v7.0 (GraphPad Software, San Diego, CA) was used for statistical analyses.
RESULTS
Patients and Treatment
Between July 2017 and May 2019, 16 patients with Ph+ AML and CML-MBP were treated with venetoclax and BCR-ABL1 TKI-based regimens. Summary of baseline patient characteristics and prior therapies are shown in Table 1. Seven patients had Ph+ AML and nine patients had CML-MBP. The median age of the patients was 42 years (range 21 to 73) and 13 patients (81%) had ECOG performance status of ≤2. Eight out of 13 patients (62%) with evaluable karyotype had complex cytogenetics and seven out of 11 patients (64%) with next generation sequencing results had one or more adverse risk mutation as defined by AML ELN 2017 criteria. Three out of 11 tested patients (27%) had a TP53 mutation. Nine patients were refractory to prior therapy, three patients had relapsed disease, and four patients had transformed from CML CP while on TKI therapy. Three out of 12 patients tested prior to starting venetoclax plus TKI combination harbored an ABL1 kinase domain mutation including one mutation each of T315I, E255K, and E355G. Patients had received a median of 5 prior lines of therapies (range 2–8) and CML-MBP patients had received a median of 2 prior TKIs (range 1–3). Four of the seven AML patients had received prior BCR-ABL1 TKIs including dasatinib (n=3) and ponatinib (n=1). One patient with Ph+ ALL had received prior venetoclax. Summary of treatment regimens are shown in Table 2. Nine patients (57%) received decitabine-based regimens and the others received intensive chemotherapy-based regimens. Ten patients (63%) received ponatinib while the other patients received second-generation TKIs.
Table 1.
Baseline characteristics of patients with Philadelphia chromosome-positive (Ph+) AML and CML myeloid blast phase (MBP)
| Patient characteristic | Ph+ AML (n=7) | CML MBP (n=9) |
|---|---|---|
| Age, years | 47 [21–73] | 38 [26–75] |
| ECOG PS 0–2 | 6 (86) | 7 (78) |
| WBC count, ×109/L | 9.7 [1.1–25.7] | 6.9 [0.1–131.5] |
| Peripheral blasts, % | 52 [1–73] | 14 [2–88] |
| Bone marrow blasts, % | 52 [10–78] | 64 [2–79] |
| Ph+ metaphases, % | 5 [0–100] | 100 [86–100] |
| BCR-ABL1 PCR, % | 7.05 [0.03–17.01] | 71.74 [0.58–100] |
| Complex cytogenetics | 5 (71) | 3/6 (50) |
| Prior therapies | 5 [2–6] | 4 [2–8] |
| Prior TKIs | 3 (43) | 9 (100) |
| Prior venetoclax | 1 (14) | 0 (0) |
| BCR-ABL1 kinase domain mutation | 0/4 (0) | 3/8 (38) |
| Response to prior therapy | ||
| Refractory | 6 (86) | 4 (44) |
| Relapse | 1 (14) | 2 (22) |
| Transformation from CML-CP | 0 (0) | 3 (33) |
Results expressed as n (%) or median [range], ECOG PS = Eastern Co-operative Oncology Group Performance Status, Ph+ = Philadelphia chromosome-positive; TKI = tyrosine kinase inhibitor, CP = chronic phase, BCR-ABL1 RT-PCR reported as the percentage of BCR-ABL1 to ABL1 transcripts.
Table 2.
Venetoclax-based regimens in patients with Philadelphia chromosome-positive (Ph+) AML and CML myeloid blast phase (MBP)
| Regimen | Ph+ AML (n=7) | CML MBP (n=9) |
|---|---|---|
| Decitabine-based | 4 (57) | 5 (56) |
| Intensive chemotherapy-based | 3 (43) | 4 (44) |
| BCR-ABL TKI | ||
| Ponatinib | 6 (86) | 4 (44) |
| Dasatinib | 1 (14) | 3 (33) |
| Bosutinib | 0 (0) | 1 (11) |
| Nilotinib | 0 (0) | 1 (11) |
| Results expressed as n (%) |
Efficacy
Characteristics, treatment regimens, and outcomes of individual patients are shown in Table 3. Fifteen of the 16 patients were evaluable for response as one patient had early death due to pneumonia. The overall response rate (ORR) in 15 evaluable patients was 60%. One patient (7%) achieved CR, 6 (40%) achieved CRi, 1 patient (7%) achieved morphologic leukemia-free state (MLFS), and 1 patient (7%) had a partial response of extramedullary disease on positron emission tomography / computed tomography (PET/CT). Another patient had hypoplastic bone marrow but did not meet formal response criterion. The ORR in seven Ph+ AML patients was 43% (3/7) and in eight evaluable CML-MBP patients was 75% (6/8). Median time to response in these 9 responding patients was 2.1 months (range 0.8–4.2) with a median of 2 cycles of therapy to best response (range 1–4). Four patients proceeded to subsequent allo-SCT.
Table 3.
Characteristics and outcomes of individual patients with Philadelphia chromosome-positive advanced myeloid leukemia treated with venetoclax and tyrosine kinase inhibitor-based regimens.
| Pt No. | Age (yr) | Diagnosis | No. prior chemo | No. prior TKI | ABL Mut. | Ph+ meta (%)† | Bcr-Abl PCR (%)‡ | Regimen | TKI | Response MRD by FCM | CG Respo nse | MRD (PCR, %)‡ | FU mo | Relapse | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Philadelphia-chromosome positive acute myeloid leukemia | |||||||||||||||
| 1 | 21 | Refractory AML | 5 | 0 | Neg | 100 | 11.20 | DEC 10d | PON | CRi MRD+ | CCyR | 5.34 | 8.2 | Y | SCT > Dead |
| 2 | 47 | Refractory AML | 6 | 0 | ND | 5 | 7.05 | CPX-351+ Gilteritinib | PON | Hypocellular marrow | IS | 2.5 | 2.7 | N | SCT > Dead |
| 3 | 73 | Relapsed AML | 3 | 2 | Neg | 0 | 0.03 | DEC 5d | PON | MLFS | 0.02 | 1.4 | NA | Dead | |
| 4 | 29 | Refractory AML | 5 | 0 | ND | 0 | 0.06 | DEC 10d | DAS | NR | NA | NA | 1.8 | NA | Dead |
| 5 | 50 | Refractory AML | 6 | 1 | Neg | 20 | 8.20 | CIA | PON | NR | NA | NA | 2.4 | NA | Dead |
| 6 | 24 | Refractory AML | 4 | 0 | ND | 0 | 0.49 | FIA | PON | NR | NA | NA | 2.0 | NA | Dead |
| 7 | 70 | Refractory AML | 2 | 1 | Neg | 30 | 17.01 | DEC 10d + Gilteritinib | PON | CR MRD+ | IS | 7.12 | 9.2 | N | SCT > Alive |
| Chronic myeloid leukemia – myeloid blast phase | |||||||||||||||
| 1 | 42 | Refractory MBP | 4 | 1 | Neg | 100 | 100 | DEC 10d | DAS | CRi MRD+ | IS | 100 | 5.9 | N | Dead |
| 2 | 60 | Transformed CP | 2 | 1 | E355G | 100 | 100 | DEC 5d | BOS | CRi MRD− | PCyR | 23.7 | 2.7 | Unk | Dead |
| 3 | 34 | Transformed CP | 4 | 2 | T315I | 100 | 100 | CLIA2 | PON | CRi | CCyR | 0.23 | 2.8 | Y | Alive |
| 4 | 26 | Relapsed MBP | 5 | 2 | Neg | 100 | 8.00 | DEC 10d | PON | CRi MRD− | CCyR | 0 | 12.3 | Y | SCT > Dead |
| 5 | 65 | Relapsed MBP | 6 | 3 | Neg | 86 | 100 | DEC 5d | NIL | ED | NA | NA | 0.2 | NA | Dead |
| 6 | 30 | Refractory MBP | 8 | 3 | ND | 100 | 0.58 | Cladribine + HiDAC | DAS | NR | NA | NA | 13.7 | NA | Dead |
| 7 | 26 | Refractory MBP | 5 | 3 | E255K | 100 | 71.74 | CIA | PON | NR | NA | NA | 1.5 | NA | Dead |
| 8 | 61 | Transformed CP | 3 | 3 | Neg | IS | 9.42 | CLIA2 | PON | PR MRD− | NA | 0 | 10.2 | N | SCT > Alive |
| 9 | 75 | Transformed CP | 2 | 2 | Neg | 93 | 48.16 | DEC 10d | DAS | CRi MRD− | CCyR | 0.01 | 6.6 | N | Alive |
MBP: chronic myeloid leukemia-myeloid blast phase, CP: chronic phase, DEC: decitabine, CLIA2: cladribine, idarubicin, cytarabine, HiDAC: high dose cytarabine, CIA: clofarabine, idarubicin, cytarabine, PON: ponatinib, DAS: dasatinib, BOS: bosutinib, NIL: nilotinib, CR: complete remission, CRi: CR with incomplete hematologic recovery, PR: partial response of extramedullary disease on positron-emission tomography/computed tomography (PET/CT) MRD: minimal residual disease, ED: early death, NR: no response, NA: not applicable N: no, Neg=negative, Unk: unknown, lost to follow up, Y: yes, NA: not applicable, SCT: stem cell transplantation, FU: follow up, IS: insufficient sample, ND=not done.
Metaphases harboring t(9;21), reported as a percentage of karyotyped metaphases
BCR-ABL1 RT-PCR reported as the percentage of BCR-ABL1 to ABL1 transcripts. CCyR = complete cytogenetic response = 0% Ph+ metaphases, PCyR = partial cytogenetic response = 1–35% Ph+ metaphases
MRD negativity tested by flow cytometry was achieved in three out of seven (43%) responding and evaluable patients. Median BCR-ABL1 PCR at response was 1.4% (range, 0% to 23.7%) in eight responding patients tested. Four patients achieved a complete cytogenetic response (CCyR) including three patients with CML-MBP and one patient with Ph+ AML. Among three patients with TP53 mutation, two patients achieved a CRi and one patient achieved MLFS. Two patients with concomitant FLT3-ITD mutation treated with gilteritinib achieved CR with detectable MRD and hypocellular marrow, respectively.
Out of ten patients who received ponatinib, five patients achieved CRi/MLFS, one patient achieved PR on PET/CT, one patient had hypoplastic marrow with positive MRD, and three patients did not respond. Out of six patients who receive second generation TKIs, three patients achieved CRi, two patients did not respond, and one patient had early death. Patients treated with ponatinib had a trend towards longer survival, with a median OS of 5.3 months compared to patients treated with second-generation TKIs who had a median OS 3.6 months (hazard ratio [HR] 0.82, 95% confidence interval [CI, 0.25, 2.68], p=0.11). Patients achieving CR/CRi compared to non-responders had a higher number of baseline Ph+ metaphases (100% versus 20%, p=0.06) and baseline BCR-ABL1 PCR (48.16% versus 0.58%, p=0.05). The two patients in the AML cohort who achieved CR/CRi were those who had the highest proportion of Ph+ metaphases and highest BCR-ABL1 level by PCR. Both of these responders had 30% of higher Ph+ metaphases on karyotype and had BCR-ABL1 PCR higher than 10%.
Out of nine patients who received decitabine-based regimens, seven patients achieved CRi/MLFS, one patient did not respond, and one patient had early death. Out of seven patients who received intensive chemotherapy-based regimens, four patients did not respond, one patient achieved CRi, one patient achieved PR on PET/CT, and one patient had hypocellular marrow. Decitabine-based regimens yielded higher odds of achieving CRi/MLFS compared to intensive chemotherapy-based regimens (odds ratio 21.00, 95% CI [1.50, 293.25], p=0.02).
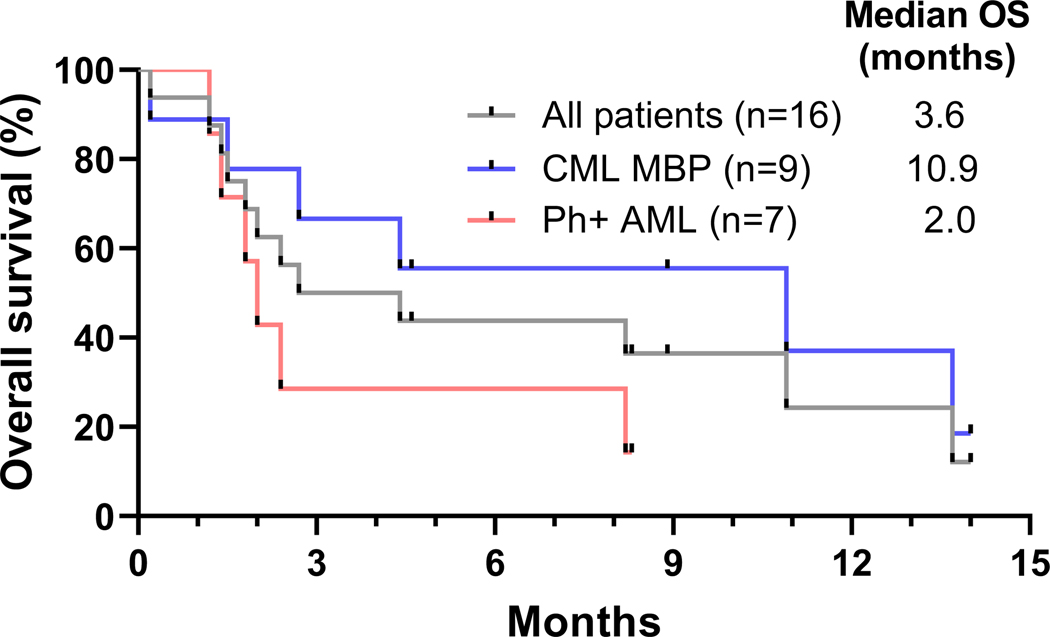
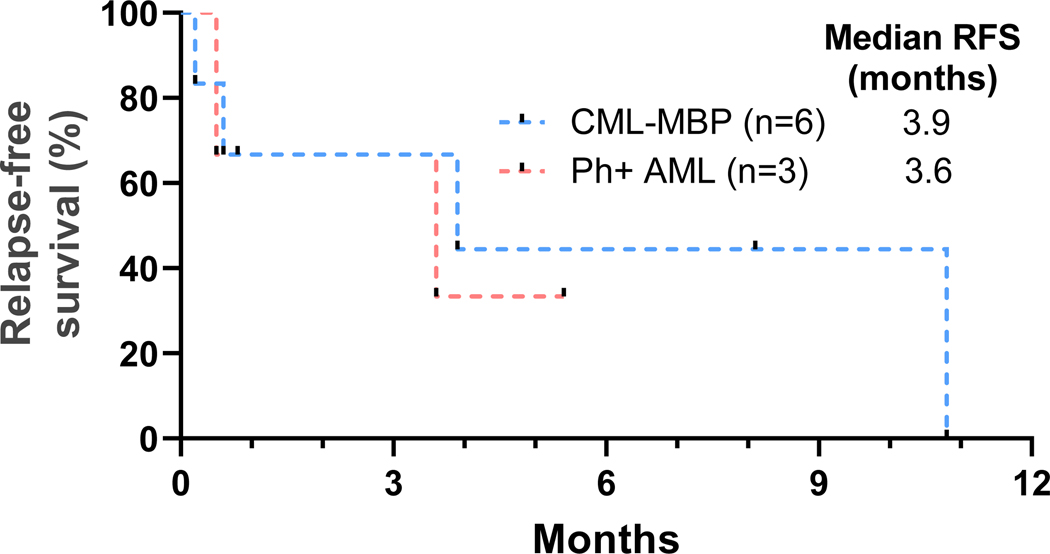
With a median follow-up of 14 months, four patients are alive, and two patients continue on therapy. Out of these four surviving patients, three patients received ponatinib, and two patients had undergone allo-SCT. There was one death within 30-days in a patient treated with decitabine-based regimen due to pneumonia. There were no early deaths in patients treated with intensive chemotherapy-based regimens. Median OS for all patients was 3.6 months, for patients with CML-MBP was 10.9 months, and for patients with Ph+ AML was 2.0 months (Fig. 1). Median RFS for patients with CML-MBP was 3.9 months and for patients with Ph+ AML was 3.6 months (Fig. 2). Patients treated with decitabine-based regimens had median OS of 4.4 months and those receiving intensive chemotherapy-based regimens was 2.4 months (HR 0.73, 95% CI [0.24, 2.26], p=0.56). Reasons for treatment discontinuation in 14 patients included no response in five patients, stem-cell transplantation in four patients, relapse in two patients, loss to follow-up in one patient, sepsis in one patient, and early death in one patient.
Fig 1.
Overall survival (OS) of patients with Philadelphia chromosome-positive (Ph+) advanced myeloid leukemias treated with venetoclax and BCR-ABL tyrosine kinase inhibitor-based regimens.
Fig 2.
Relapse-free survival (RFS) of patients with Philadelphia chromosome-positive (Ph+) advanced myeloid leukemias treated with venetoclax and BCR-ABL tyrosine kinase inhibitor-based regimens
DISCUSSION
In this retrospective study, we showed feasibility and activity of venetoclax and TKI-based regimens in relapsed/refractory (R/R) Ph+ AML and CML-MBP. This is the first report of clinical activity of this rational combination in a retrospective cohort of patients. In this heavily pretreated adverse risk group of patients with Ph+ advanced myeloid leukemias, venetoclax and TKI-based regimens showed an ORR of 60% and median OS of 2 months in Ph+ AML group and 10.9 months in CML-MBP group. The median OS of 10.9 months in this pre-treated CML-MBP group was comparable to previously reported median OS of 9–12 months in a cohort of patients with CML-MBP which included newly diagnosed patients, TKI-naive patients, both of which confer better outcomes compared to treated CML-MBP.2 In contrast, within our cohort of patients with CML-MBP, the median number of prior TKIs was 2 (range, 1–3).
Dasatinib has shown single agent activity in CML-MBP, however, VEN-TKI combination may be better than single agent dasatinib. With necessary precautions for cross-study comparisons, this combination of venetoclax and TKI-based regimens offered median OS of 10.9 months in patients with CML-MBP compared to 7.9 months with single agent dasatinib in a more favorable risk population.25 Patients with CML-MBP in our cohort were more heavily pre-treated and had received a median of two prior TKIs and included R/R patients while in comparison, the dasatinib START-B trial only included patients who were imatinib resistant or intolerant.
Ponatinib has shown favorable responses as single agent or in combination with non-chemotherapeutic agents in advanced Ph+ leukemias and R/R pre-B acute lymphoblastic leukemia (ALL).20,21 In our current report, three of the four surviving patients received ponatinib. We had previously reported that ponatinib showed the highest major hematologic and major molecular response rates of 67% and 27% respectively, compared to other TKIs in patients with CML-BP.2 Furthermore, pre-clinical studies have shown that ponatinib synergizes with venetoclax in Ph+ ALL samples through induction of LYN-mediated pro-apoptotic BIM and decreasing anti-apoptotic MCL1, and potentially abrogating venetoclax resistance.22 Hence, taken together, these data provide a strong rationale for combining venetoclax with ponatinib in this population. Decitabine-based regimens showed significantly higher response rate compared to intensive chemotherapy-based regimens, however, OS was comparable. Given how venetoclax synergizes with both decitabine and intensive chemotherapy, either of these backbones may be a reasonable option for such patients, depending on older versus younger age, and ‘fitness’ for intensive therapy.
Despite limited numbers, there was a trend towards higher clonal burden of Ph+ metaphases and higher baseline BCR-ABL1 PCR among patients who achieved CR/CRi compared to non-responders. The only two patients in the AML cohort who achieved CR/CRi had 30% or higher Ph+ metaphases and BCR-ABL1 PCR of 10% or higher. Response rates also appeared higher in CML-MBP, which is more strongly driven by BCR-ABL1 signaling than is Ph+ AML, which often only contains a subclonal BCR-ABL1-driven population. This suggests that venetoclax and TKI-based combinations may be particularly useful in patients where the Ph+ clone is a dominant clone without other major co-existing drivers. We speculate that minor subclones with Philadelphia chromosomes may develop in advanced leukemia due to chromosomal instability and addition of TKIs may not offer additional benefit in such cases when BCR-ABL is not the main driver.
This was a retrospective study with all inherent limitations thereof. We could not assess the impact of addition of venetoclax compared to TKI alone or with chemotherapy. The patient population and treatment regimens were heterogenous limiting broad-reaching conclusions from these data. Given the retrospective nature of this study, we could not provide assessment of treatment-emergent adverse events. Hence, we have opened a clinical trial to prospectively evaluate this combination of decitabine, venetoclax, and ponatinib in Ph+ AML and CML-MBP (NCT04188405).
In conclusion, venetoclax and TKI-based combination regimens are a feasible approach for treating advanced Ph+ advanced myeloid leukemias. Patients with CML-MBP may stand to benefit particularly from this combination due to BCR-ABL1 being the predominant driver. Previously published pre-clinical data of synergy, and clinical activity reported here warrant further evaluation of such combinations in these poor-risk populations.
Acknowledgments
Funding Sources
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 from the National Cancer Institute. NJS is supported by the K12 Paul Calabresi Clinical Oncology Scholar Award and the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Footnotes
Disclosure Statement
AM: Research funding from Celgene Corporation
MF: None
FR: Research funding from Amgen, Bristol-Myers Squibb, Merck, Seattle Genetics, Sunesis Pharmaceuticals, Honoraria from Amgen, Pfizer, Seattle Genetics, Sunesis Pharmaceuticals; Consulting or advisory role for Amgen, Seattle Genetics, Sunesis Pharmaceuticals.
JEC: Research funding from Ambit BioSciences, ARIAD, Arog, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, Celator, Celgene, Novartis, Pfizer, Sanofi, Sun Pharma, Teva; consultant for Ambit BioSciences, ARIAD, Astellas Pharma, BiolineRx, Bristol-Myers Squibb, Novartis; Pfizer.
EJJ: Consultancy Research funding from Takeda, BMS, Adaptive, Amgen, AbbVie, Pfizer, Cyclacel LTD
KS: Otsuka: Honoraria; Pfizer: Consultancy
KM: none
NGD: Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Immunogen: Research Funding; Pfizer Inc.: Consultancy, Research Funding; Incyte Corporation: Honoraria, Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Otsuka America Pharmaceutical, Inc.: Consultancy; Kiromic: Research Funding; Jazz: Consultancy.
GGM: None
MYK: Calithera Research Funding; Stemline Therapeutics Consultancy, Honoraria and Research Funding; Forty-Seven Consultancy and Honoraria; Eli Lilly Research Funding; AbbVie Consultancy, Honoraria and Research Funding; Cellectis Research Funding; Amgen Consultancy and Honoraria; F. Hoffman La-Roche Consultancy, Honoraria and Research Funding; Genentech Honoraria and Research Funding; Ascentage Research Funding; Kisoji Consultancy and Honoraria; Reata Pharmaceuticals Equity Ownership and Patents & Royalties; Ablynx Research Funding; Astra Zeneca Research Funding; Agios Research Funding.
CBB: none
LM: none
GB: AbbVie: Research Funding; Incyte: Research Funding; Janssen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy and Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Oncoceutics, Inc.: Research Funding.
CDD: AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding.
PB: Incyte Corporation Consultancy, Research Funding and Speakers Bureau; Celgene Corporation Consultancy and Research Funding; Blueprint Medicines Corporation Consultancy and Research Funding; Kartos Therapeutics Research Funding; Constellation Pharmaceuticals Research Funding; Pfizer Research Funding; Astellas Pharmaceuticals Research Funding; NS Pharma Research Funding; Promedior Research Funding; CTI BioPharma Research Funding;
KN: none
SP: none
HMK: Ariad Research Funding; Astex Research Funding; BMS Research Funding; Cyclacel Research Funding; Daiichi-Sankyo Research Funding; Pfizer Honoraria and Research Funding; Immunogen Honoraria and Research Funding; Jazz Research Funding; Actinium Honoraria; Novartis Research Funding; Takeda Honoraria.
NJS: Takeda Oncology: Consultancy and Research Funding; AstraZeneca: Consultancy: Amgen: Honoraria
Statement of Ethics
His work complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Patients signed written informed consent before receiving the treatments described here. This study was approved by the Institutional Review Board and given the retrospective nature of this study, requirement of informed consent for this study was waived.
Prior Presentation:
This data was published online, in part, as an abstract in the proceedings of the American Society of Clinical Oncology Annual Meeting 2019.
Data sharing:
At this time, we will not be able to share individual patient level data outside of our institution.
REFERENCES
- 1.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. The Lancet 2018;392(10147):593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017;123(22):4391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deschler B, Lübbert M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006;107(9):2099–107. [DOI] [PubMed] [Google Scholar]
- 4.Söderlund S, Dahlén T, Sandin F, et al. Advanced phase Chronic Myeloid Leukaemia in the TKI era – a report from the Swedish CML register. Eur J Haematol 2016;n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 5.Soupir CP, Vergilio J-A, Cin PD, et al. Philadelphia Chromosome–Positive Acute Myeloid LeukemiaA Rare Aggressive Leukemia With Clinicopathologic Features Distinct From Chronic Myeloid Leukemia in Myeloid Blast Crisis. Am J Clin Pathol 2007;127(4):642–50. [DOI] [PubMed] [Google Scholar]
- 6.Keung Y-K, Beaty M, Powell BL, Molnar I, Buss D, Pettenati M. Philadelphia chromosome positive myelodysplastic syndrome and acute myeloid leukemia—retrospective study and review of literature. Leuk Res 2004;28(6):579–86. [DOI] [PubMed] [Google Scholar]
- 7.Aoki J, Kakihana K, Kobayashi T, et al. Tyrosine kinase inhibitor therapy for acute myeloid leukemia with late-appearing Philadelphia chromosome. Leuk Res 2012;36(1):e41–2. [DOI] [PubMed] [Google Scholar]
- 8.DeFilipp Z, Khoury HJ. Management of Advanced-Phase Chronic Myeloid Leukemia. Curr Hematol Malig Rep 2015;10(2):173–81. [DOI] [PubMed] [Google Scholar]
- 9.Apperley JF. Chronic myeloid leukaemia. The Lancet 2015;385(9976):1447–59. [DOI] [PubMed] [Google Scholar]
- 10.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 2014;4(3):362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson CJ, Davids MS. BCL-2 Antagonism to Target the Intrinsic Mitochondrial Pathway of Apoptosis. Clin Cancer Res Off J Am Assoc Cancer Res 2015;21(22):5021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horita M, Andreu EJ, Benito A, et al. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J Exp Med 2000;191(6):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff DJ, Court Recart A, Sadarangani A, et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell 2013;12(3):316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintás-Cardama A, Qiu YH, Post SM, et al. Reverse phase protein array profiling reveals distinct proteomic signatures associated with chronic myeloid leukemia progression and with chronic phase in the CD34-positive compartment. Cancer 2012;118(21):5283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter BZ, Mak PY, Mu H, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med 2016;8(355):355ra117–355ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okabe S, Tauchi T, Tanaka Y, Ohyashiki K. Anti-Leukemic Effects of Venetoclax on Philadelphia Chromosome Positive Leukemia Cells. Blood 2016;128(22):5428–5428. [Google Scholar]
- 17.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luthra R, Sanchez-Vega B, Medeiros LJ. TaqMan RT-PCR assay coupled with capillary electrophoresis for quantification and identification of bcr-abl transcript type. Mod Pathol Off J U S Can Acad Pathol Inc 2004;17(1):96–103. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Jorgensen JL, Wang SA. How Do We Use Multicolor Flow Cytometry to Detect Minimal Residual Disease in Acute Myeloid Leukemia? Clin Lab Med 2017;37(4):787–802. [DOI] [PubMed] [Google Scholar]
- 20.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome–Positive Leukemias. N Engl J Med 2013;369(19):1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assi R, Kantarjian H, Short NJ, et al. Safety and Efficacy of Blinatumomab in Combination With a Tyrosine Kinase Inhibitor for the Treatment of Relapsed Philadelphia Chromosome-positive Leukemia. Clin Lymphoma Myeloma Leuk 2017;17(12):897–901. [DOI] [PubMed] [Google Scholar]
- 22.Leonard JT, Rowley JSJ, Eide CA, et al. Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med 2016;8(354):354ra114. [DOI] [PubMed] [Google Scholar]
- 23.Maiti A, DiNardo CD, Rausch CR, et al. Ten-Day Decitabine with Venetoclax (DEC10-VEN) in Acute Myeloid Leukemia: Updated Results of a Phase II Trial. Blood 2019;134(Supplement_1):2637–2637. [Google Scholar]
- 24.Aboudalle I, Konopleva MY, Kadia TM, et al. A Phase Ib/II Study of the BCL-2 Inhibitor Venetoclax in Combination with Standard Intensive AML Induction/Consolidation Therapy with FLAG-IDA in Patients with Newly Diagnosed or Relapsed/Refractory AML. Blood 2019;134(Supplement_1):176–176. [Google Scholar]
- 25.Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study. Cancer 2010;116(16):3852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]