Abstract
BACKGROUND
Melatonin is reported to be related to diabetes mellitus (DM) risk; however, the effect of melatonin on diabetic retinopathy (DR) risk remains unclear.
AIM
The aim of this study was to determine the effect of melatonin on DR risk.
METHODS
A hospital-based case-control study was conducted from January 2020 to June 2020. DR was assessed using the Diabetic Retinopathy preferred practice pattern (PPP)-updated 2019 criteria. The participants were divided into the DM cases without DR (NDR) group, non-proliferative DR (NPDR) group and proliferative DR (PDR) group. Plasma melatonin concentration was detected with the enzyme-linked immunosorbent assay kit. The relationship between plasma melatonin concentration and DR risk as well as severity was assessed.
RESULTS
It was found that plasma melatonin was 72.83 ± 16.25, 60.38 ± 13.43, 44.48 ± 10.30 and 44.69 ± 8.95 pg/mL in healthy controls, NDR group, NPDR and PDR group, respectively. In addition, it was found that plasma melatonin could be used as a potential diagnostic biomarker for DR (AUC = 0.893, P < 0.001). There was a significant positive relationship between total bilirubin and melatonin content (P < 0.001) based on the correlation assay. Significant associations between total bilirubin and melatonin content were also detected in the NPDR (R2 = 0.360, P < 0.001) and PDR (R2 = 0.183, P < 0.001) groups.
CONCLUSION
The data obtained in this study demonstrated that plasma melatonin concen-tration was decreased in DR cases and could be used as a sensitive and specific marker for the diagnosis of DR. A significant positive relationship between total bilirubin and melatonin was detected. More related studies are required to understand the role of melatonin in DR.
Keywords: Melatonin, Diabetic retinopathy, Case-control study, Risk factor, Type 2 diabetes
Core Tip: Melatonin is reported to be related to diabetes mellitus risk; however, the effect of melatonin on diabetic retinopathy (DR) risk remains unclear. The data obtained in this study demonstrated that plasma melatonin concentration was decreased in DR cases and could be used as a sensitive and specific marker for the diagnosis of DR. A significant positive relationship between total bilirubin and melatonin was detected. More related studies are required to understand the role of melatonin in DR.
INTRODUCTION
The global prevalence of diabetes mellitus (DM) has continued to increase in the past decade and it is now regarded as a huge public health problem[1]. Diabetic retinopathy (DR), is the most common microvascular complication of DM, and has affected more than 400 million patients and is the main cause of blindness in individuals of working age[2]. The primary prevention measures for DR include stricter control of blood glucose, blood pressure and blood lipid, adjustment of diet and exercise, and establishment of effective screening strategies[3]. However, a considerable number of patients still progress to the advanced stage of DR. Advanced DR patients often require intravitreal injection of anti-vascular endothelial growth factor drugs and corticosteroids, as well as vitrectomy. Although the treatments for DR have improved in recent years, the long-term prognosis of late stage DR is not optimistic, and highlights the importance of controlling the progression of DR from the early to late stage.
Biomarkers are biochemical indicators that can be used to mark changes in the structure or function of a system, organ, tissue and cell, and effective biomarkers have been used in the diagnosis and classification of various disorders, including DM and DR[4]. A recent study involved the detection of dual biomarkers for the sensitive screening of DR based on a delicate microbead enrichment technique[5]. Another study demonstrated that peripheral fractal dimension could be calculated automatically and therefore be used as a useful surrogate biomarker in DR patients[6]. Blood samples, such as serum and plasma, have huge diagnostic potential as they are noninvasive and easy to obtain. In a case-control study, researchers aimed to investigate the expression of plasma levels of miR-29b and miR-200b in DR secondary to type 2 diabetes[7]. Melatonin is an indoleamine synthesized by pineal cells and it is synthesized and secreted at night[8]. Once synthesized, melatonin is released into the blood. Previous studies have shown that melatonin can demonstrate significant immune regulation, anti-tumor, and anti-aging effects and provides protective effects on the brain, heart, liver and other important organs[9]. Plasma melatonin was reported to be associated with the risk of several diseases. In a cross-sectional study of 61 patients with idiopathic Parkinson’s disease (PD), it was reported that plasma melatonin levels in PD patients were significantly higher than those in healthy controls[10].
Melatonin has been reported to be associated with several metabolic diseases and might protect different organs during DM development. Melatonin, which was an anti-oxidant and anti-apoptosis factor, demonstrated a potential effect in the treatment of DR based on previous experimental DR models[11]. To date, there is limited knowledge on the association between plasma melatonin and the risk of DR. In the present cross-sectional study, plasma melatonin was determined in DR cases and the related clinical characteristics were investigated.
MATERIALS AND METHODS
The current study was approved by the Ethical Committee of the Changshu No. 2 People’s Hospital. The subjects had the opportunity to familiarize themselves with the blood test results. Additional information is provided and the test results may be used for research purposes only and not for diagnostic procedures. All procedures were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants before the study.
Study population
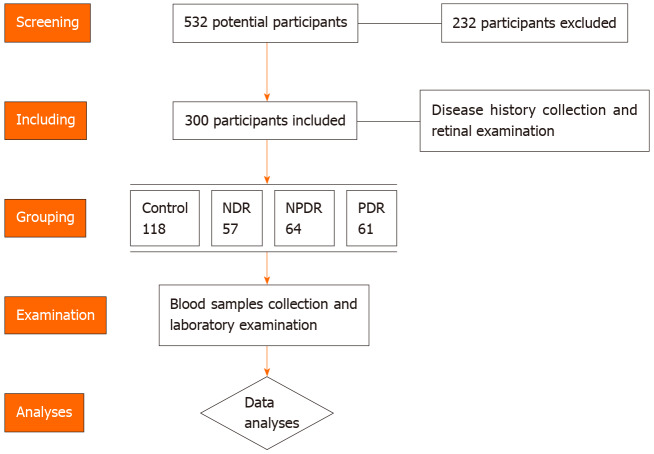
A total of 532 healthy volunteers, DM and DR patients were screened for potential inclusion in this study between January 2020 and June 2020. A total of 232 participants were excluded as they did not meet the inclusion criteria or were unwilling to participate. The inclusion criteria were as follows: (1) Healthy controls or type 2 DM cases with/without DR; (2) Able to cooperate with the research protocol; and (3) Signed the informed consent before the study. The exclusion criteria were as follows: (1) Diagnosed with type 1 DM; (2) Patients with psychiatric disorders or severe sleeping disorder; and (3) Oral melatonin supplementation use in the recent 2 wk. Participants without a history of DM were included in the control group. The DM patients were divided into the DM cases without DR (NDR) group, non-proliferative DR (NPDR) group and the proliferative DR (PDR) group. In this study, a total of 118 healthy controls, 57 NDR cases, 64 NPDR cases and 61 PDR cases were enrolled. The diagnosis of type 2 DM was made according to the American Diabetes Association (ADA 2019) criteria[12]. The diagnosis and classification of DR cases were conducted based on retinal photographs according to were taken and graded according to the Diabetic Retinopathy preferred practice pattern (PPP)-updated 2019 guideline. After collecting clinical data and blood samples, the clinical information and laboratory data were used in advanced analyses. The flow chart of patients and controls screening is shown in Figure 1.
Figure 1.
Flowchart demonstrates the number of patients enrolled, grouping, examination and analyses in the study. NDR: Without diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
Clinical data
Baseline information, such as age, diabetes duration, use of oral hypoglycemic agents and insulin, history of hypertension, smoking and drinking was obtained by consulting the participants. Body mass index (BMI) was calculated as weight (kg)/height (m2). The laboratory data, including physical indicators and biochemical examination results were extracted from the in-hospital examination system. The diagnosis of DR was conducted by fundus photography by two ophthalmologists specialized in the retina using digital Canon retinography. All the data were processed by two independent researchers and the consistency was checked before advanced analyses.
Collection of blood samples and melatonin detection
After collecting the clinical data and laboratory outcomes, a total of 5 mL venous blood was obtained and placed in a vacuum tube at 8:00 am. The venous blood samples were placed in tubes containing ethylenediaminetetraacetic acid (EDTA) on ice and were sent to obtain plasma as soon as possible. Whole blood was mixed thoroughly in the tubes and then centrifuged for 15 min at 20000 g at 4°C to obtain the plasma samples. These plasma samples were placed in Eppendorf tubes (1.5 mL) and stored at -80°C until use. Plasma concentrations of melatonin were detected using a human melatonin enzyme-linked immunosorbent assay (ELISA) Kit (Glory Science Co., Ltd., TX, United States) in accordance with the manufacturer's instructions. Each sample was examined three times and the mean value was used in the final data analyses.
Statistical analysis
Continuous measurements are presented as mean ± standard difference (SD). The independent t test was used to compare indicators between the two groups, and one-way ANOVA was used to determine the differences between three groups. The chi square test was used for qualitative data comparison. The receiver operating characteristic (ROC) curve was used to test the efficacy of related indicators. The Pearson correlation coefficient was used to analyze the correlation between different parameters. P < 0.05 was considered statistically significant. This study used GraphPad 8 statistical software for the statistical analysis.
RESULTS
Characteristics of the study participants by the presence and severity of DR
In this study, 62 females/56 males, 30 females/27 males, 38 females/26 males, and 32 females/29 males were included in the healthy controls group, NDR group, NPDR group and PDR group, respectively, and there was no significant gender distribution among the groups (P = 0.947). Age in the healthy controls group, NDR group, NPDR group and PDR group was 59.84 ± 9.60, 58.21 ± 7.75, 60.89 ± 9.93 and 65.72 ± 8.36 years, and those in the NPDR and PDR group were older (P < 0.001). When the BMI was considered, there was a slight but significant difference between the different groups (P = 0.047). An advanced study on blood pressure and hypertension statu, showed that higher blood pressure and a greater incidence of hypertension were detected in the DR group. No significant differences in smoking and drinking history were detected among the groups (P = 0.801 and 0.730, respectively) (Table 1).
Table 1.
Baseline characteristics, disease information and laboratory data of the healthy controls, without diabetic retinopathy, non-proliferative diabetic retinopathy and proliferative diabetic retinopathy groups
| Healthy controls (n = 118) |
DM
|
P value | |||
|
|
NDR (n = 57)
|
NPDR (n = 64)
|
PDR (n = 61)
|
||
| Baseline characteristics | |||||
| Gender (male, %) | 56 (48.70) | 27 (47.37) | 26 (40.63) | 29 (47.54) | 0.947 |
| Age (yr) | 59.84 ± 9.60 | 58.21 ± 7.75 | 60.89 ± 9.93 | 65.72 ± 8.36 | < 0.001 |
| BMI (kg/m2) | 26.11 ± 3.18 | 27.39 ± 3.71 | 26.86 ± 2.76 | 25.93 ± 3.89 | 0.047 |
| SBP (mmHg) | 123.53 ± 14.47 | 126.82 ± 17.62 | 131.66 ± 10.63 | 136.34 ± 11.87 | < 0.001 |
| DBP (mmHg) | 73.97 ± 12.08 | 73.54 ± 12.69 | 81.14 ± 15.42 | 77.52 ± 13.09 | 0.0019 |
| MAP (mmHg) | 90.49 ± 9.41 | 91.30 ± 10.86 | 97.98 ± 10.78 | 97.13 ± 10.04 | < 0.001 |
| Disease information | |||||
| Diabetes duration (yr) | - | 5.72 ± 2.64 | 10.92 ± 6.01 | 12.52 ± 4.96 | < 0.001 |
| Fasting blood glucose (mg/dL) | 84.73 ± 23.85 | 133.81 ± 24.98 | 143.28 ± 36.25 | 167.86 ± 40.42 | < 0.001 |
| HbA1C (%) | 5.87 ± 1.32 | 7.85 ± 1.69 | 8.61 ± 2.11 | 9.27 ± 1.80 | < 0.001 |
| Use of oral hypoglycemic agents | - | 38 (66.67) | 50 (78.13) | 44 (72.13) | 0.369 |
| Use of insulin | - | 15 (26.31) | 24 (37.50) | 21 (34.43) | 0.408 |
| Hypertension (n, %) | 52 (44.07) | 25 (43.86) | 38 (59.38) | 41 (67.21) | 0.009 |
| Smoking (n, %) | 37 (31.36) | 14 (24.56) | 18 (28.13) | 19 (31.15) | 0.801 |
| Drinking (n, %) | 41 (34.75) | 20 (30.09) | 19 (29.69) | 24 (39.34) | 0.730 |
| Laboratory data | |||||
| Triglycerides (mmol/L) | 4.70 ± 1.50 | 4.79 ± 1.28 | 4.55 ± 1.28 | 4.88 ± 1.20 | 0.567 |
| Total cholesterol (mmol/L) | 4.57 ± 1.54 | 5.01 ± 1.64 | 4.25 ± 1.77 | 4.77 ± 1.79 | 0.077 |
| LDL cholesterol (mmol/L) | 3.12 ± 1.12 | 2.96 ± 1.20 | 2.98 ± 1.09 | 2.87 ± 0.94 | 0.504 |
| HDL cholesterol (mmol/L) | 1.18 ± 0.27 | 1.14 ± 0.26 | 1.19 ± 0.29 | 1.16 ± 0.28 | 0.740 |
| Total bilirubin (mmol/L) | 12.79 ± 3.34 | 13.44 ± 3.89 | 11.55 ± 4.04 | 11.21 ± 3.89 | 0.002 |
| AST (U/L) | 21.76 ± 9.08 | 22.62 ± 9.15 | 22.38 ± 8.78 | 22.40 ± 9.60 | 0.930 |
| ALT (U/L) | 21.16 ± 8.66 | 22.78 ± 9.35 | 23.16 ± 8.92 | 22.61 ± 8.33 | 0.4251 |
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; MAP: Mean arterial pressure; FBG: Fasting blood glucose; HbA1C: Hemoglobin A1C; BMI: Body mass index; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; AST: Aspartate transaminase; ALT: Alanine aminotransferase; DM: Diabetes mellitus.
Diabetes-related characteristics, such as diabetes duration, fasting blood glucose, hemoglobin A1C (HbA1C) content, oral hypoglycemic agent use and insulin administration, were detected in the NDR, NPDR and PDR groups. It was found that longer diabetes duration, severe fasting blood glucose and higher HbA1C content were detected in the DR group (P < 0.001). Treatments for diabetes, including oral hypoglycemic agents and insulin use, were not related to the risk of NPDR or PDR incidence in all type 2 DM cases (P < 0.369 and P < 0.408, respectively) .
When the relationship between several laboratory data, including triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total bilirubin, aspartate aminotransferase and alanine aminotransferase concentrations, and the risk of NPDR or PDR were considered, it was found that lower total bilirubin was associated with the risk of NPDR and PDR compared with the control and NDR groups (P = 0.002).
Association between plasma melatonin and DR risk
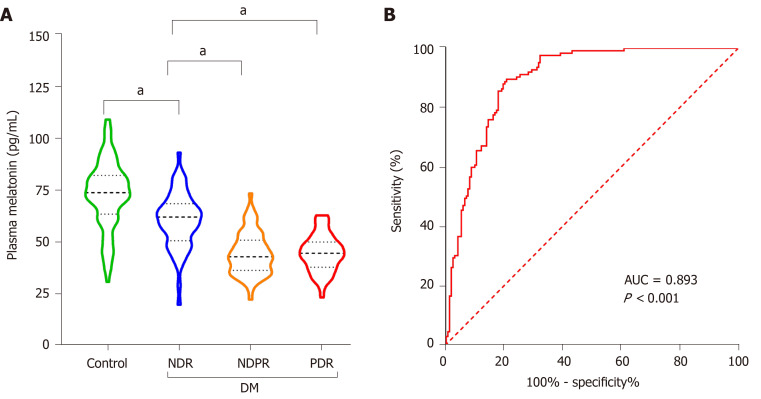
When assessing the association between plasma melatonin and the presence and severity of DR, it was found that plasma melatonin was 72.83 ± 16.25, 60.38 ± 13.43, 44.48 ± 10.30 and 44.69 ± 8.95 pg/mL in the healthy controls, NDR group, NPDR group and PDR group, respectively. As shown in Figure 2A, it was found that low melatonin was detected in the NDR group compared with the healthy controls (P < 0.001). Lower melatonin concentrations were also found in the NPDR and PDR groups compared with the NDR group (P < 0.001). As shown in Figure 2B, the diagnostic role of plasma melatonin in DR cases was detected using the ROC curve and it was found that plasma melatonin can be used as a potential diagnostic biomarker for DR (AUC = 0.893, P < 0.001).
Figure 2.
Plasma melatonin concentrations in healthy controls and diabetic retinopathy patients. A: Plasma melatonin in the healthy controls, without diabetic retinopathy (NDR), non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) groups; B: The diagnostic effect of plasma melatonin on diabetic retinopathy was discovered using the receiver operating characteristic curve. aP < 0.001. NDR: Without diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; DM: Diabetes mellitus; AUC: Area under the receiver operating characteristic curve.
Clinical and laboratory risk factors for NPDR and PDR
To detect the clinical and laboratory risk factors for DR at different stages, the association between age, diabetes duration, hypertension, BMI, smoking, drinking, fasting blood glucose (FBG) concentration, HbA1C content, total bilirubin, melatonin concentration and both NPDR and PDR risk were evaluated. As shown in Table 2, longer diabetes duration, higher FBG and lower melatonin concentration were associated with increased NPDR risk (P < 0.001). In advanced analyses of PDR risk, it was found that hypertension status and higher HbA1C content were risk factors for PDR (P < 0.001).
Table 2.
Multiple logistic regression analysis of the association between clinical characteristics and diabetic retinopathy/proliferative diabetic retinopathy risk
|
DR
|
PDR
|
|||
|
Multivariate OR (95%CI)
|
P
value
|
Multivariate OR (95%CI)
|
P
value
|
|
| Age (10 yr) | 0.03 (0.008 to 0.063) | 0.134 | 0.939 (0.003 to 0.011) | 0.005 |
| Diabetes duration(1 yr) | 0.032 (0.025 to 0.039) | < 0.001 | 0.007 (0.007 to 0.023) | 0.002 |
| Hypertension | -0.023 (-0.105 to 0.059) | 0.577 | 0.015 (-0.129 to 0.022) | < 0.001 |
| BMI | -0.008 (-0.019 to 0.001) | 0.090 | -0.053 (-0.023 to -0.001) | 0.166 |
| Smoking | -0.033 (-0.109 to 0.044) | 0.402 | -0.012 (-0.098 to 0.075) | 0.032 |
| Drinking | -0.023 (-0.096 to 0.050) | 0.532 | -0.012 (-0.143 to 0.021) | 0.791 |
| FBG (mg/dL) | 0.002 (0.001 to 0.003) | < 0.001 | -0.061 (0.001 to 0.004) | 0.143 |
| HbA1C (%) | 0.017 (-0.002 to 0.036) | 0.084 | 0.002 (-0.006 to 0.037) | < 0.001 |
| Total bilirubin (mmol/L) | 0.004 (-0.006 to 0.014) | 0.428 | 0.015 (-0.016 to 0.006) | 0.158 |
| Melatonin (pg/mL) | -0.008 (-0.010 to -0.005) | < 0.001 | -0.005 (-0.004 to 0.002) | 0.412 |
FBG: Fasting blood glucose; BMI: body mass index; HbA1C: Hemoglobin A1C.
Factors related to plasma melatonin concentrations
Considering the potential diagnostic role of plasma melatonin in DR, it could provide more information on the relationship between the baseline information and laboratory data and plasma melatonin concentrations. It was found that diabetes duration, systolic blood pressure, mean arterial blood pressure, FBG and HbA1C contents were negatively associated with melatonin concentration (P < 0.001). A significant positive relationship was observed between total bilirubin and melatonin content (P < 0.001) (Table 3).
Table 3.
Correlations between baseline information and laboratory data and plasma melatonin concentrations
|
Baseline information and laboratory data
|
Plasma melatonin concentrations
|
|
|
r
|
P
value
|
|
| Age | -0.1018 | 0.078 |
| Gender | -0.1428 | 0.013 |
| Diabetes duration | -0.5623 | < 0.001 |
| SBP | -0.2224 | < 0.001 |
| DBP | -0.1326 | 0.022 |
| MAP | -0.2146 | < 0.001 |
| Hypertension | 0.1429 | 0.013 |
| BMI | -0.09046 | 0.118 |
| Smoking | -0.0543 | 0.349 |
| Drinking | -0.06159 | 0.288 |
| FBG (mg/dL) | -0.464 | < 0.001 |
| HbA1C (%) | -0.481 | < 0.001 |
| Triglycerides (mmol/L) | 0.04224 | 0.466 |
| Total cholesterol (mmol/L) | 0.01544 | 0.790 |
| LDL cholesterol (mmol/L) | 0.02813 | 0.628 |
| HDL cholesterol (mmol/L | -0.01587 | 0.784 |
| Total bilirubin (mmol/L) | 0.4386 | < 0.001 |
| AST (U/L) | -0.06536 | 0.259 |
| ALT (U/L) | -0.06815 | 0.239 |
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; MAP: Mean arterial pressure; FBG: Fasting blood glucose; HbA1C: Hemoglobin A1C; BMI: Body mass index; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; AST: Aspartate transaminase; ALT: Alanine aminotransferase.
The association between total bilirubin and melatonin concentration
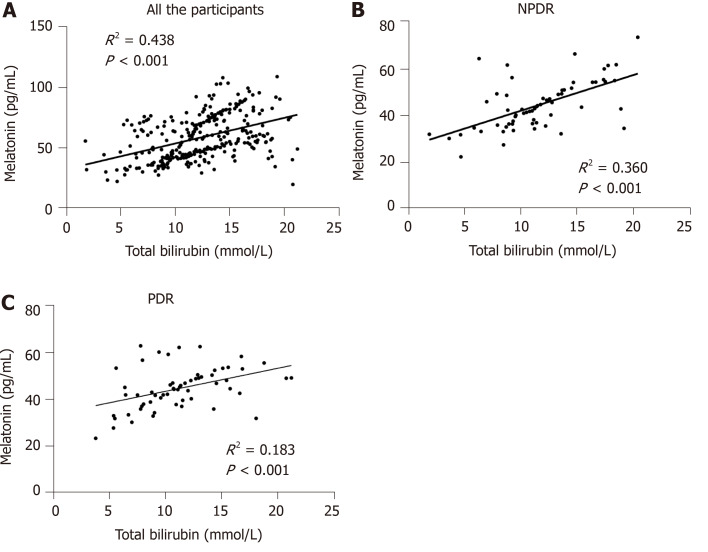
As there was a significant positive association between total bilirubin and melatonin concentration, more advanced studies on this relationship in the different groups were conducted. Among the healthy controls, NDR, NPDR as well as PDR cases, there was a significant linear association between total bilirubin and melatonin concentration (R2 = 0.438, P < 0.001). When the different DR stages were considered, significant associations between total bilirubin and melatonin content were detected in the NPDR (R2 = 0.360, P < 0.001) and PDR (R2 = 0.183, P < 0.001) groups (Figure 3).
Figure 3.
Linear relationship between total bilirubin and melatonin concentration in the different groups. A: The linear relationship in all the participants; B: The linear relationship in the non-proliferative diabetic retinopathy (NPDR) group; C: The linear relationship in the proliferative diabetic retinopathy (PDR) group. NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
DISCUSSION
To date, limited information on circulating biomarkers of DR is available. In the current study, we detected the expression of plasma melatonin in DR cases with different disease severity. The results showed that plasma melatonin was decreased in NPDR and PDR patients. Thus, plasma melatonin could be used as a diagnostic marker for DR. The correlation analyses demonstrated that total bilirubin was positively related to melatonin concentration in all NPDR and PDR cases.
Retinal fundus examination, including fundus ophthalmoscopy and fundus photography, was used in the diagnosis of DR. An optimized retinal fundus examination strategy could be used in DR screening and is important in the primary prevention of DR[13]. However, retinal fundus examination can detect pathological changes when a microaneurysm and small bleeding spots occur in the retina. However, this examination shows relatively late changes, as these pathological signs are associated with microvascular damage due to long-term hyperglycemic toxicity. Thus, the development of early diagnostic biomarkers would have important preventive value in DR. In an in silico and in vivo study on retinal and circulating miRNA expression patterns in DR, a group of miRNAs were identified for the potential diagnosis of DR[14]. In a hospital-based cross-sectional study of 2696 participants, it was reported that serum CA125 level was associated with the presence and severity of DR in Chinese patients with type 2 DM[15]. These previous observational studies have provided the feasibility for developing novel circulating biomarkers for DR.
Melatonin is an endogenous regulatory factor and is synthesized by the pineal gland and part of the retina in humans[8]. Melatonin has been reported to have important regulatory effects and prevents a variety of disorders, including cardio-vascular diseases, neurovascular diseases and diabetes, in addition to the complications secondary to diabetes[16]. As DR has been confirmed to be related to oxidative stress and inflammation, melatonin, which is a powerful antioxidant, may provide important protective effects in the development of DR. Melatonin can prevent oxidative damage of retinal nerve tissue, and protect retinal vascular endothelial cells by inhibiting the expression of inducible nitric oxide synthase (iNOS). In addition, it could play a role in the prevention and treatment of DR from various aspects, and is expected to become a major therapeutic drug[17,18]. It was also demonstrated that the secretion of melatonin in patients with PDR was reduced, because melatonin, as an antioxidant/mitochondrial protector and inflammatory factor, may lead to the progression of DR[19]. Inconsistent with a previous study[20], plasma melatonin in the NPDR group was decreased compared with the healthy controls and the NDR group in the current study. A relatively larger sample size was included in this study and the ELISA detection method may explain the differences between these two studies.
In this study, a significant positive correlation was observed between total bilirubin and melatonin concentration. Bilirubin is produced by the catabolism of heme and abnormal bilirubin concentration is related to liver damage. In recent years, the beneficial effects of higher but physiological levels of circulating bilirubin is regarded as an area of ongoing research in different diseases, including DR. In a cross-sectional study of 1637 individuals, it was found that serum bilirubin was significantly decreased in DR cases compared with type 2 DM cases[21]. Another study enrolled a total of 490 patients with type 2 DM lasting for ≥ 10 years and it was reported that higher total bilirubin could be regarded as a protective factor for DR[22]. Bilirubin, which demonstrates strong antioxidant and anti-inflammatory effects on the microvascular system, is a strong endogenous antioxidant and has a potential mechanism in the development of DR[23]. The similar physiological roles of bilirubin and melatonin in the progression of DR may be the reason for the positive relationship between these two factors. The significant positive correlation of these two factors highlighted the potential regulatory effect between them.
There were several limitations in the current study. Firstly, the relatively small number of participants included in this study weakened the reliability of the conclusions. Advanced observational studies with more cases and a long-term follow-up would provide further information on the effect of melatonin on the development and progression of DR. Secondly, the secretion of melatonin demonstrated a circadian rhythm pattern; however, only one time point was chosen in this study. Although we chose a standard time point for all the participants, more time points in future studies would help to measure the circadian rhythm of melatonin secretion.
The data obtained in this study demonstrated that plasma melatonin concentration was decreased in DR cases and may help in the management of DR. Based on the sensitivity and specificity assay, it was identified that plasma melatonin could be used as a sensitive and specific marker for the detection of DR. A significant positive relationship between total bilirubin and melatonin was detected. However, more clinical studies with reasonable study designs as well as functional research on animal or cellular models would help in understanding the role of melatonin in DR.
CONCLUSION
The data obtained in this study demonstrated that plasma melatonin concentration was decreased in DR cases and could be used as a sensitive and specific marker for the diagnosis of DR. A significant positive relationship between total bilirubin and melatonin was detected. More related studies are required to understand the role of melatonin in DR.
ARTICLE HIGHLIGHTS
Research background
Melatonin is reported to be related to diabetes mellitus (DM) risk; however, the effect of melatonin on diabetic retinopathy (DR) risk remains unclear.
Research motivation
To determine the effect of melatonin on the risk of diabetic retinopathy (DR).
Research objectives
To assess the effect of melatonin on diabetic retinopathy (DR) risk, and determine whether plasma melatonin can be used as a sensitive and specific marker for detecting DR.
Research methods
A hospital-based case-control study was conducted from January 2020 to June 2020. The participants were divided into the DM cases without DR group, non-proliferative DR group and proliferative DR group. Plasma melatonin concentration was detected using an enzyme-linked immunosorbent assay kit.
Research results
Plasma melatonin concentration was decreased in DR cases, and a significant positive relationship between total bilirubin and melatonin was observed.
Research conclusions
Plasma melatonin concentration may help in the management of DR. Plasma mela-tonin could be used as a sensitive and specific marker for the detection of DR.
Research perspectives
More studies on animal or cellular models would help in understanding the role of melatonin in DR.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethical Committee of the Changshu No. 2 People’s Hospital (Approval No. 2020-KY-003).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors report no conflicts of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: August 2, 2020
First decision: November 18, 2020
Article in press: December 10, 2020
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sulkowski S S-Editor: Gao CC L-Editor: Webster JR P-Editor: Yuan YY
Contributor Information
Wen-Cui Wan, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Yang Long, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Wei-Wei Wan, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Hong-Zhuo Liu, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Hao-Hao Zhang, Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Wei Zhu, Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China. shzhuwei0722@163.com; Department of Ophthalmology, Changshu No. 2 People's Hospital, Changshu 215000, Jiangsu Province, China.
Data sharing statement
No additional data are available.
References
- 1.Philipson LH. Harnessing heterogeneity in type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:79–80. doi: 10.1038/s41574-019-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, Simó R. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8:337–347. doi: 10.1016/S2213-8587(19)30411-5. [DOI] [PubMed] [Google Scholar]
- 3.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, Mitchell P, Klein R, Cheung CMG, Wong TY. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 4.Adki KM, Kulkarni YA. Potential Biomarkers in Diabetic Retinopathy. Curr Diabetes Rev. 2020;16:971–983. doi: 10.2174/1573399816666200217092022. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Kwon JS, Hsu SM, Chuang HS. Sensitive tear screening of diabetic retinopathy with dual biomarkers enabled using a rapid electrokinetic patterning platform. Lab Chip. 2020;20:356–362. doi: 10.1039/c9lc00975b. [DOI] [PubMed] [Google Scholar]
- 6.Fan W, Nittala MG, Fleming A, Robertson G, Uji A, Wykoff CC, Brown DM, van Hemert J, Ip M, Wang K, Falavarjani KG, Singer M, Sagong M, Sadda SR. Relationship Between Retinal Fractal Dimension and Nonperfusion in Diabetic Retinopathy on Ultrawide-Field Fluorescein Angiography. Am J Ophthalmol. 2020;209:99–106. doi: 10.1016/j.ajo.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Dantas da Costa E Silva ME, Polina ER, Crispim D, Sbruzzi RC, Lavinsky D, Mallmann F, Martinelli NC, Canani LH, Dos Santos KG. Plasma levels of miR-29b and miR-200b in type 2 diabetic retinopathy. J Cell Mol Med. 2019;23:1280–1287. doi: 10.1111/jcmm.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutin JA, Witt-Enderby PA, Sotriffer C, Zlotos DP. Melatonin receptor ligands: A pharmaco-chemical perspective. J Pineal Res. 2020;69:e12672. doi: 10.1111/jpi.12672. [DOI] [PubMed] [Google Scholar]
- 9.D'Angelo G, Chimenz R, Reiter RJ, Gitto E. Use of Melatonin in Oxidative Stress Related Neonatal Diseases. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Zhao Z, Ma J, Zheng J, Huang S, Hu S, Gu Q, Chen S. Elevated Plasma Melatonin Levels Are Correlated With the Non-motor Symptoms in Parkinson's Disease: A Cross-Sectional Study. Front Neurosci. 2020;14:505. doi: 10.3389/fnins.2020.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu Y, Zhu M, Wang Z, Wang K, Chen L, Liu W, Shi Q, Zhao Q, Sun Y, Wang X, Song E, Liu X. Melatonin inhibits Müller cell activation and pro-inflammatory cytokine production via upregulating the MEG3/miR-204/Sirt1 axis in experimental diabetic retinopathy. J Cell Physiol. 2020;235:8724–8735. doi: 10.1002/jcp.29716. [DOI] [PubMed] [Google Scholar]
- 12.Niroomand M, Afsar J, Hosseinpanah F, Afrakhteh M, Farzaneh F, Serahati S. Comparison of the International Association of Diabetes in Pregnancy Study Group Criteria with the Old American Diabetes Association Criteria for Diagnosis of Gestational Diabetes Mellitus. Int J Endocrinol Metab. 2019;17:e88343. doi: 10.5812/ijem.88343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muqit MMK, Kourgialis N, Jackson-deGraffenried M, Talukder Z, Khetran ER, Rahman A, Chan WO, Chowdury FA, Nag D, Ahmad J, Friedman DS. Trends in Diabetic Retinopathy, Visual Acuity, and Treatment Outcomes for Patients Living With Diabetes in a Fundus Photograph-Based Diabetic Retinopathy Screening Program in Bangladesh. JAMA Netw Open. 2019;2:e1916285. doi: 10.1001/jamanetworkopen.2019.16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platania CBM, Maisto R, Trotta MC, D'Amico M, Rossi S, Gesualdo C, D'Amico G, Balta C, Herman H, Hermenean A, Ferraraccio F, Panarese I, Drago F, Bucolo C. Retinal and circulating miRNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br J Pharmacol. 2019;176:2179–2194. doi: 10.1111/bph.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao L, Zhong Y, He L, Wang Y, Wu J, Geng J, Zhou Y, Zhang J, Chen J, Shan Z, Teng W, Xu Y, Chen L, Liu L. Serum CA125 Level Is Associated with Diabetic Retinopathy in Chinese Patients with Type 2 Diabetes. Diabetes Metab Syndr Obes. 2020;13:1803–1812. doi: 10.2147/DMSO.S250928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Wu J, Qin Z, Fu W, Zhao B, Li X, Wang W, Sha T, Sun M, Li J, Zeng Z, Chen Z. Melatonin and its analogues for the prevention of postoperative delirium: A systematic review and meta-analysis. J Pineal Res. 2020;68:e12644. doi: 10.1111/jpi.12644. [DOI] [PubMed] [Google Scholar]
- 17.Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S. Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol Metab Syndr. 2020;12:30. doi: 10.1186/s13098-020-00537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JY, Yu F, Shi L, Ko ML, Ko GY. Melatonin Affects Mitochondrial Fission/Fusion Dynamics in the Diabetic Retina. J Diabetes Res. 2019;2019:8463125. doi: 10.1155/2019/8463125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crooke A, Huete-Toral F, Colligris B, Pintor J. The role and therapeutic potential of melatonin in age-related ocular diseases. J Pineal Res. 2017;63 doi: 10.1111/jpi.12430. [DOI] [PubMed] [Google Scholar]
- 20.Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:655–660. doi: 10.2147/OPTH.S19559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Cai X, Liu Y, Hu M, Zhou L, Liu W, Wu J, Zhang R, Gao X, Yang W, Zhang S, Gong S, Luo Y, Li M, Gao L, Chen L, Chen J, Huang X, Ren Q, Zhang X, Zhou X, Han X, Ji L. Serum Albumin, but not Bilirubin, is Associated with Diabetic Chronic Vascular Complications in a Chinese Type 2 Diabetic Population. Sci Rep. 2019;9:12086. doi: 10.1038/s41598-019-48486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Duan C, Fang D, Liu Y, Xu H, Zheng Y, Xuan Y, Wang L, Ye L, Su R, An M. Protective factors for diabetic retinopathy in Type 2 diabetes mellitus patients: Long duration of no less than 10 years. J Diabetes Complications. 2019;33:107383. doi: 10.1016/j.jdiacomp.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Li Y, Li J, Lv X, He Y. Elevated serum total bilirubin levels are negatively associated with major diabetic complications among Chinese senile diabetic patients. J Diabetes Complications. 2017;31:213–217. doi: 10.1016/j.jdiacomp.2016.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.