Abstract
Glycaemic index (GI) testing provides a useful point of comparison between carbohydrate sources. For this comparison to be meaningful, the methods used to determine GI values need to be rigorous and consistent between testing events. This requirement has led to increasing standardization of the GI methodology, with an international standard developed in joint consultation with FAO/WHO (ISO 26642:2010) currently the most up to date document. The purpose of this review is to compare the international standard to methods of published studies claiming to have performed a GI test. This analysis revealed that the international standard permits a wide range of choices for researchers when designing a GI testing plan, rather than a single standardized protocol. It has also been revealed that the literature contains significant variation, both between studies and from the international standard for critical aspects of GI testing methodology. The primary areas of variation include; what glucose specification is used, which reference food is used, how much reference food is given, what drink is given during testing, the blood sampling site chosen and what assay and equipment is used to measure blood glucose concentration. For each of these aspects we have explored some of the methodological and physiological implications of these variations. These insights suggest that whilst the international standard has assisted with framing the general parameters of GI testing, further stan-dardization to testing procedures is still required to ensure the continued relevance of the GI to clinical nutrition.
Keywords: Glycaemic index, Blood glucose, Nutritional guidelines, Food classifications, ISO 26642:2010
Core Tip: This review highlights major areas of variation that are currently permitted under the international glycaemic index (GI) testing standard and explores the significant influence these choices may have on the final GI value attributed to a food. This review also reports a summary of the range of choices researchers have made across a large set of published GI papers.
INTRODUCTION
The glycaemic index (GI) has been a major feature of nutritional science over the past 40 years. Low GI diets have been suggested to protect against diabetes, heart disease, cancer[1], acne[2] and improve cognition[3], endurance exercise[4], weight loss[5] and eye function[6]. It is clear from this list that the GI has been assessed by a diverse group of researchers to address some of the most significant challenges and chronic diseases faced by the global community today.
Typical of a concept that has caught the attention of a large number of scientists, regulators and consumers; the GI has attracted a range of critique. There is an on-going debate around the ability of the GI to be applied to mixed meals[7-9] and whether the GI of a food and the glycaemic response of an individual can be differentiated[10-12]. However, the most consistent criticism of GI relates to the reproducibility of GI testing results as a consequence of human participant variability, and in testing methods[13]. The criticism around mathematical variability has been addressed on several occasions with three large scale inter-laboratory studies reporting statistically non-significant differences between GI testing facilities[14-16]. This is in addition to the development of an international standard for the methods used for the determination of GI values, with the most recent edition being “ISO 26642:2010 food products - determination of the glycaemic index and recommendation for food classification” by the Food and Agriculture Organisation of the United Nations[17]. This international testing standard forms the foundation and structure for this review.
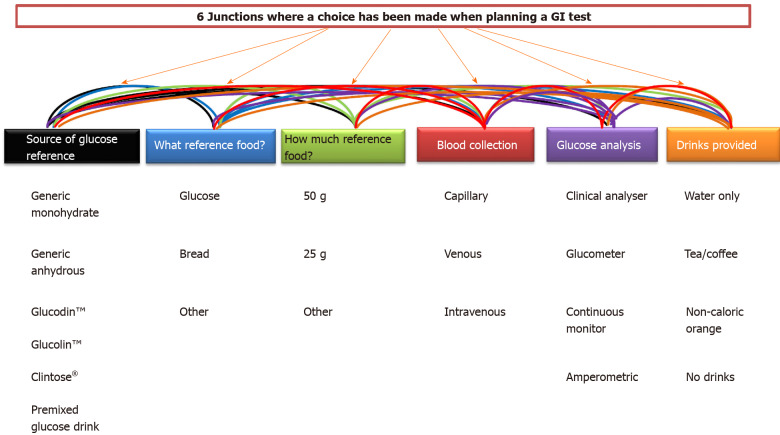
Commitment to the International standard has helped to reduce the variability involved in GI testing. A recent study compared 3 labs, all following the ISO 2010 method and found no significant difference in GI values for the foods tested[16]. However, what is interesting about the international standard is that in many areas of the testing methodology, researchers are given choices in how to carry out tests. We have identified 6 major junctions in the ISO standard where researchers are given a choice. Those areas include: (1) What glucose specification is used? (2) Which reference food is used? (3) How much reference food is given? (4) What drink is given during testing? (5) What the blood sampling site is? And (6) What assay and equipment is used to measure blood glucose concentration?
This review has two key purposes. The first is to identify what choices researchers have made across the published literature. This review collected 155 papers, some of which pre-date the current ISO 2010 standard. The GI has been a developing science and it is not expected that all of the papers that predate 2010 would comply with the standard. However, many of these papers serve as references for dieticians and clinical trial planning, so it is important to assess papers from the early years of the subject through to the present day, but through the lens of the current standard.
The second purpose of this review is to assess to what extent these different choices could influence final GI values. From these 6 broad categories, we identified 23 separate and more specific choice categories used in the published literature (Figure 1).
Figure 1.
Six separate decisions that must be made when planning a glycaemic index test and the 23 potential trial design variation each decision creates. GI: Glycaemic index.
Whilst, this number of possible options is quite large, this is not of any significance unless the different options chosen impact on the final result. Therefore, we have critically evaluated the potential choices to understand what impact they may have on final GI values. The GI is a powerful tool for guiding dietary choices that promote a healthy lifestyle. As a result, GI values are important for how individual products are marketed and these values are evidence used to support the health claims made on foods. This review attempts to summarise the past direction of the clinical research. This is primarily intended as a reference for researchers, but also to help sharpen the dependability and precision of GI results and testing methods into the future.
SOURCE OF GLUCOSE REFERENCE
The 50-g serve of glucose is the cornerstone of GI testing, because it is the reference that all foods are compared to. Even when bread is used as reference food, the GI value is corrected, so the final score is in reference to glucose. Because the classifications of low, medium and high GI are enforced as cut-off values, these differences can have significant implications for which category a food is classified into and perceived health status of a food.
Section 5.4.1 of the ISO:2010 standard states “The acceptable reference foods shall be as follows: (1) Anhydrous glucose powder (50 g); (2) Dextrose (glucose monohydrate, 55 g); and (3) Commercial solution used for the oral glucose tolerance test (OGTT) containing glucose (50 g). This appears as if there are 3 possible options resulting if glucose is used as reference food. However, further variability can be added to this due to the use of trademarked products, such as Glucodin™, Glucolin™ and Clintose® which are all monohydrated glucose varieties. Our analysis of the literature revealed that whilst these forms of glucose are monohydrated and are required by the standard to be corrected to 55 g, this fact is overlooked by many papers resulting in 10% error in the amount of glucose provided as a reference food[18-38].
The most common observation was that glucose source was not reported on 57 occasions. This was followed by Glucolin™ used 23 times, generic anhydrous glucose 17 times, commercial glucose drink 11 times, with both Glucodin™ and generic monohydrate used 10 times and Clintose® used once (Table 1).
Table 1.
Summary of glucose sources from 129 glycaemic index studies that used glucose as a reference standard
|
|
Generic monohydrate
|
Generic anhydrous
|
Glucodin™
|
Glucolin™
|
Clintose®
|
Pre-mixed glucose drink
|
Does not specify
|
| Source of glucose reference | 10 | 17 | 10 | 23 | 1 | 11 | 57 |
The choice of glucose source should not affect the GI value if it is an equivalent carbohydrate dosage to the test food. If the chemical composition is known and the standardisation is performed on the basis of a monohydrated glucose source, the methodology would be compliant. However, trademarked products such as Glucodin™, Glucolin™ and Clintose® appear to have added a layer of confusion to experimental design. These are monohydrated glucose products, but they have consistently been used in the literature at a dosage that suggests they have been assumed to be anhydrous.
Glucodin™ was used on 10 occasions[18-20,23,24,27,34-37]. None of these papers stated that a correction for monohydrate was made to 55 g. One paper stated that 50 g of the product was used[19], whilst, others make a correction to 51.4 g[34,35,37]. This 51.4 g amount is based on the manufacture’s recommendation that the average purity of each batch is 97% glucose, requiring a higher dosage to cover the discrepancy. However, given that this is a monohydrated form of glucose, more than 55 g is required to account for lower purity.
Aside from papers that did not report a specific glucose source, Glucolin™ was the most commonly observed choice taken by researchers, identified 23 times [21,22,25,26,28,30-33,39-49]. Many specifically noted that 50 g of the product was used[21,22,28,32,33] or 25 g[28,29,31], two papers identify that Glucolin™ is monohydrate, but continue to use 25 g or 50 g, rather than correcting by 10%[26,30] and occasionally the correction to 55 g for monohydrated glucose is made[44-46].
Another variety of dextrose, known as Clintose® was used in only one paper and was added at 54.6 g[50]. However, the reason for choosing this dosage is not addressed in this paper, but it is a likely a correction for monohydrated glucose.
The range of glucose source options identified demonstrates this is an area open to methodological variability. The major finding of this analysis is many reported GI values will not have been assessed on equivalent quantities of carbohydrate between test and reference food. This may seem like a minor oversight, however the concept of GI requires comparison between reference and test food to be equal to have any biological relevance.
This is not to suggest all previous GI values are now irrelevant. The OGTT had a similar challenge in the 1980’s. It was debated whether 75 g of monohydrated or anhydrous glucose should be used and during this time accurate diabetes diagnoses were still made[51,52]. The issue was that using monohydrated compared to anhydrous glucose may misclassify individuals as either diabetic or non-diabetic based on whether monohydrated or anhydrous glucose was given. This is analogous to how a food could be classified as either low GI or high GI following an inappropriate reference food calculation. Measured values may be clearly within category thresholds, so classification of diabetic or high GI would still be valid. In this example there is little opportunity for glucose source to be the deciding factor in the classification applied. However, for people being tested for diabetes, or for foods being tested for their GI that are on the boarder of categories there is a potential for misclassification. The amount of glucose could be the deciding factor on what category is given and the implications of each category are realised as a direct result of glucose dosage applied. In the example of diabetes testing with OGTT, the prevailing view is that it does not matter if anhydrous or monohydrated glucose is given. If either choice delivers equivalent carbohydrate then GI would not be different[52].
The ISO standard already has provisions for this correction, with 50 g of anhydrous glucose and 55 g for monohydrated glucose used to deliver equivalent carbohydrates. However, various glucose products bearing commercial trademarks have added a layer of confusion to the issue. It would not be unreasonable to specify a single, food grade and analytical specification of glucose that is to be used by each lab. A unified approach to glucose source could be a possible solution in an updated ISO standard.
DRINKS OFFERED DURING TESTING
Water was the most prevalent choice for beverage consumed during GI testing (Table 2, n = 77). However, given that 63 papers did not report beverage choices, what researchers provide for drinks with GI testing is largely unknown (Table 2). The international standard states that glucose should be weighed into 250 mL of water. However, we did observe some variability in water volume. Aside from the papers where 250 mL of water was used to dissolve the glucose, some researchers dissolved the reference in 100 mL to 750 mL of water[21,22,31,53-79]. In one paper[71], 500 mL of water was used to dissolve the reference glucose, but during the 2 h where the glycaemic response was being measured, water was given ad libitum, which further adjusts the volume and variation of water delivered.
Table 2.
Summary of drink information reported across 155 glycaemic index papers
|
Not reported
|
Water
|
Tea/coffee allowed
|
No beverages allowed with meals
|
Non-caloric orange drink
|
| 63 | 77 | 13 | 1 | 1 |
Not reported: No mention of beverages consumed during testing; Water: Some mention of water being consumed during testing, but no other options; Tea/coffee allowed: If tea or coffee was an option for participants, even if water could also be selected; No beverages allowed with meals: Drinking anything was specifically mentioned as not permitted; Non-caloric orange drink: Non-caloric orange drink as an option instead of water during testing.
Whilst many of these papers balance the water by providing the same volume of water with the test food, the range of water volumes used demonstrates that there is inconsistency between studies and from the ISO 2010 standard. However, further analysis is required to understand what impact this variation of water volume may have on final GI values.
This issue was addressed in a study into the impact of beverage choice and volume on glycaemic response[80]. There were differences in the blood glucose response curve when different volumes of water were given[80]. However, there was not a significant difference in the mean as a result of different volumes. Whilst statistical difference was not reached in this trial, it is unknown whether this change in gastric emptying would have a more pronounced effect on other test foods. This is a reminder that decisions on GI experimental design need to be made with the goal of producing a consistent biological challenge to participants, rather than reducing statistical variability alone.
A review on GI methodology recommended the use of 250 mL to avoid potential differences in the amount of liquid given to subjects and this recommendation has been accepted into the ISO 2010 standard[81]. It concluded that there is not enough data on the effect of water volume to disregard the possible effect it may have. The standardization of water volume was recommended as a result. Regardless, the ISO standard already stipulates 250 mL of water to be used to dissolve the glucose reference food, however the present review demonstrates that on many occasions this recommendation is not followed.
Despite the recommendation to deliver the reference glucose with 250 mL in the ISO 2010 standard, the water dosage allowed to be consumed over the 2 h of testing is less precisely restricted. The ISO standard does stipulate that either 250 mL or 500 mL of water is to be given during the test meal. The same beverage volume is given in each visit to control for intra-individual variation between tests due to changing volumes. However, the effect on inter-individual variation is less clear, due to a difference in choice between 250 mL and 500 mL.
Sievenpiper et al[82] demonstrated that the dilution of the 75 g OGTT test created significant differences in the glycaemic response of individuals. The pattern of response to a particular dilution was also different if the participant was lean, normal or obese. Here clear causation is difficult to establish, given the number of variables at play in a clinical environment. Because the relationship between water volume chosen and the glycaemic response appears to be non-linear, allowing variability in the amount of water participants choose may add further divergence to the GI values determined. If there is an interaction between the specific volume of water given to participants during testing and the final GI values observed, it is possible that a reduction in GI could be caused by the majority of participants choosing the optimal water volume by chance and not by the nature of the test food.
The limited studies on the effect of water volume are not sufficient to understand how the variety of food matrices will react to different water volumes. Allowing a range of water volume choices may lead to further challenges to the GI concept, but this could be overcome with a prescriptive volume to be consumed during testing. Therefore, consensus of the volume of water for both reference and test food should be reached.
There is another on-going debate about whether coffee and tea should be allowed during GI testing. It has been suggested that coffee may have an influence on insulin sensitivity[83], glucose tolerance[84,85] and gastric emptying[86]. This review only identified 13 studies where this is recorded to occur. However, because of the high number of trials where drink choices offered is not reported, it is difficult to know how commonly tea or coffee is provided during GI testing.
The 2008 inter-laboratory study explored any potential effect of choosing tea and coffee compared with choosing water. Wolever et al’s[15] 2008 study did not find a statistical difference between drink choices. It is recommended by the authors of the inter-laboratory trial that there may be no need to control for drink chosen during testing. However, the major shortfall of this trial was that only two test foods were analyzed. It is uncertain whether this result is specific to these test foods or is consistent across the full spectrum of foods. The potential differences in the action coffee and/or tea may have on digestive enzymes that would be involved with the reference food, compared to the test food should be considered.
Coffee and tea have inhibitory capabilities against carbohydrate inhibiting enzymes such as α-glucosidase and α-amylase[87-92] and as a result alter glycaemic responses[93]. The level of inhibition from coffee or tea will vary for α-glucosidase and α-amylase respectively and the effect on glycaemia may be different. Polyphenols are compounds in coffee and tea shown to inhibit these enzymes, however their influence on glycaemic activity may be broader than enzyme inhibition alone[94]. Polyphenols are present in more than double the quantity in coffee compared to black tea. Also, the specific varieties of phenolic compounds would be vastly different between the beverages[95]. If α-glucosidase and α-amylase are inhibited differently there are consequences for the GI testing method. If a participant chooses a beverage that strongly inhibits α-amylase, the amylase inhibition is unlikely to alter the iAUC of a reference glucose test, but may influence a high starch test food. This issue of salivary amylase inhibition is further complicated due to the variation in AMY1 gene copy number across the population[34]. The combined effect on GI values of someone with low AMY1 copy number choosing tea, compared to water has not been explored.
Beyond enzyme inhibition coffee has an effect on both high and low GI test meals, but each meal type is affected to a different extent[96]. A 2009 paper found that coffee and tea did not affect the GI and also reduced the statistical variability[97]. However, 5 participants for coffee and 5 participants for tea lacks the statistical power of a normal GI test of a minimum 10 participants per variable and would not meet the ISO 2010 standard for testing. The authors acknowledge the study was performed using solid reference foods and the effect may be different on liquid solutions. This suggests there may be interactions between specific choices. Without testing all of the combinations of these variables separately, it is difficult to know which of the choices interact with each other and to what extent.
In one example, dates increased from a low GI score of 55 ± 6 to 63 ± 5 correlating with coffee drinking[98]. There was not a statistically significant difference between these two values however it changes the GI category given. Other studies have found interesting results through GI testing, but are challenged on the basis that coffee and tea were permitted during testing[99].
A further consideration on this topic is that permission of coffee and tea also includes allowance for 30 mL of milk and non-nutritive sweeteners. Whilst milk is known to have an effect on GI, it may not have a significant impact on glycaemic response at this volume[100]. There have been analysis on the differing effects of sweeteners[101] and whilst they can alter the glycaemic response the amount added into a tea or coffee is unlikely to be significant based on current evidence.
The evidence surrounding the effect of coffee and tea on GI is conflicting. There is an argument to restrict coffee and tea during testing, as a safeguard against the possibility of an effect. There are examples already in the ISO 2010 standard such as restriction of alcohol the night before, even though there have been studies suggesting this does not influence results. Since there is not a strong benefit to justify the risk that coffee and tea do have an influence, it would be advised to be excluded. GI standards used in specific jurisdictions such as the Australian Standard (AS-4694-2007) do not allow coffee and tea and the international standards could incorporate this. In light of the data and evidence presented a uniform amount of water could be prescribed for both reference and test meals to minimise variation in the nature of GI testing conditions.
BLOOD GLUCOSE EQUIPMENT AND ASSAY CHOICE
Past studies have identified blood glucose assays as an area that should be controlled to aid in the precision of GI testing[15,102]. Table 3 shows the majority of papers use assays run by automated clinical chemistry analysers or the same assays in spectrophotometer/plate reader format (n = 121). Glucometers are still permitted in the ISO 2010 standard, provided that published CVs are < 3.6%. It is possible that some of the glucometers reported in these trials would not comply with this standard and different commercial glucometers have been shown to differ significantly in their performance and biases[103]. It is helpful that only 8 studies did not report a method of blood glucose analysis, allowing a fairly complete understanding in the trends present.
Table 3.
Summary of glucose analysis methods from 155 papers glycaemic index studies using glucose as a reference standard
|
|
Clinical chemistry analyser/plate reader
|
Glucometer (single reading or continuous)
|
Clinical enzymatic amperometric glucose detection
|
Not specified
|
|||
| Papers | 121 | 24 | 2 | 8 | |||
| Specific method | Glucose oxidase | Glucose hexokinase | Glucose dehydrogenase | Unknown | |||
| 66 | 19 | 3 | 33 | ||||
Glucose oxidase was the most common method of analysis reported (Table 3, n = 66). Glucose hexokinase was reported less often (n = 19) but may be a better option for blood glucose measurement. A recent paper comparing glucose oxidase and hexokinase used for diagnosis of gestational diabetes reported only 34% of positive diagnosis were common to both methods[104]. This indicates that there are fundamental differences in the glucose values detected by each measure. GI testing is different to diabetes testing because discrepancy is controlled for because each participant will have the same method for glucose detection for test and reference food. There are biological considerations that could affect values detected by glucose oxidase method, which could fluctuate in the time between reference and test foods values and vary results.
The glucose hexokinase method is known to be more specific than the glucose oxidase method[105]. Both will return statistically accurate values, with low CV% on a stock solution of glucose and therefore qualify for the international standard. However, substances such as uric acid, ascorbic acid, bilirubin, tetracycline, and glutathione may cause falsely low glucose results measured by the glucose oxidase method[105]. Using the example of ascorbic acid, the concentration of ascorbic acid in a test food may have an impact on the final blood glucose volume using a glucose oxidase method that would not be present in a glucose hexokinase assay. Other extremely precise methods have been suggested for the analysis of glucose in blood, such as isotope dilution gas chromatography–mass spectrometry (IDGC-MS)[106]. However, this level of instrumentation and technical capability is beyond the vast majority of GI laboratories and therefore glucose hexokinase is a practical compromise.
WHAT IS AN APPROPRIATE AMOUNT OF CARBOHYDRATES?
The data presented in Table 4. demonstrates that the most common choice for the amount of carbohydrates provided in GI testing is 50 g (n = 126). Two papers did not specify amount and more creative choices included matching 1000 kJ of glucose with 1000 kJ of a reference food[48] and another study where different carbohydrate quantities were based on the body weight of participants[107]. The typical carbohydrate amount chosen was 50 g, but the ISO 2010 standard for GI testing includes the clause that the test should be performed using 50 g, unless the amount required for a participant to consume is “unreasonably large”. The definition of unreasonably large is open to interpretation, which has some interesting implications if explored further.
Table 4.
Amount of carbohydrates delivered in test and reference foods
|
50 g
|
25 g
|
Other amount
|
Doesn’t specify
|
| 126 | 33 | 2 | 2 |
There is an argument that 50 g of any added carbohydrate is unreasonably large. Choosing to feed 50 g of pure glucose, when there is an option to use 25 g, raises an ethical question that researches should be prepared to address. A meta-analysis found that the relative risk increasing for type 2 diabetes is 0.97 per 50 g of carbohydrates consumed[108]. Potentially of greater concern is the observation that relative risk increased by 1.08 for every 5 points on the GI. Considering by definition glucose receives a GI value of 100 the relative risk increase would be the highest possible for pure glucose on the basis of risk added by GI values alone. The Greenwood et al[108] 2013 meta-analysis is targeted at the effect of consuming those amounts daily, compared to the sporadic increase that a GI test would cause. However, considering the combined relative risk of an additional 50 g GI = 100 carbohydrates, it should be sufficient to encourage researchers to only decide to give 50 g of glucose to participants if necessary to produce precise GI values.
An argument could also be made that if 50 g of added carbohydrates has been observed to increase the relative risk of disease, a 50 g portion size of any carbohydrate is too large to make meaningful correlations to a healthy dietary choice. Accordingly, some researchers have suggested a glycaemic measure based on the total food serving size, rather than a fixed amount of carbohydrates[109].
If there is sufficient evidence to support reduced carbohydrate serve being used to determine GI values, then there is no reason that a researcher should ever choose 50 g carbohydrates. In this scenario, 50 g would be more expensive and a greater stress to human participants well-being and therefore difficult to justify. This serving size of glucose has also been reported to induce nausea in GI trial participants[81]. Alternatively, if there is not sufficient evidence that a reduced carbohydrate serving size is comparable to 50 g, then reduced serving sizes such as 25 g should not be permitted for any GI testing. A brief summary of the literature addressing the amount of carbohydrate required and analysis of the degree to which different carbohydrate quantities can be compared will help inform the best direction to be chosen for GI testing.
One criteria for 25 g or 50 g to be used interchangeably would be that this decision does not influence the final GI value. Ideally, there would be a linear relationship between the amount of glucose given and the iAUC. However, performance of test foods appears to vary dependent on type of food. 25 g of barley produced an iAUC 52% of 50 g and other test foods such as potato at 25 g gave 75% of the response to 50 g of carbohydrates[110]. The iAUC of glucose at 25 g has been reported at 66% of the response at 50 g[111]. In the Lee et al[111] paper the difference between the values for 25 g and 50 g were not statistically significant. However, this study only had 8 participants, so it is difficult to determine if these values are a product of the small sample size or are close enough to support 25 g and 50 g being used interchangeably.
A subsequent study found a linear relationship between glucose and the iAUC[112]. Whilst the reference food response was linear, different test foods varied in response patterns based on the amount of food given. This observation that blood glucose levels differed, lead researchers to suggest that using a test food at the serving size likely to be consumed would be a good recommendation. They did acknowledge that further work on a greater variety of test foods would be warranted to support these suggestions. There have been studies published previous to the Wallace et al[112] 2009 paper that suggested that serving size did not influence final GI values[113]. However, this observation was made specifically in reference to the effect of serving size when bread was consumed and some adjustments to the typical method such as addition of margarine were made. Therefore, the recommendation for effect of serving size to be investigated on more varieties of food is still valid.
A review on GI methodology recommended that 50 g of carbohydrates was an optimal dosage to assess GI values and then 25 g when the meal size was unreasonable[81]. This recommendation is reflected in the ISO 2010 recommendations which come to a similar conclusion. From the data collected in the present review the majority of papers adopt the 50 g serve size. The question remains about the validity of a 25 g size in the International standard. If the 50 g carbohydrate serve for a GI test cannot be reasonably consumed for a certain food due to low carbohydrate concentration, a suggestion to exclude that food from testing could be made. This argument is not satisfactory however, because the purpose of GI is to have a methodology that allows comparison of carbohydrate quality. This should not be determined by the quantity of carbohydrates in a product. Therefore, the requirements for the quantity used when testing should be standardized to give meaning to the GI values, no matter if they are derived from a high carbohydrate food or low carbohydrate food.
One solution is to standardise the requirements for a maximum allowable portion size, before a 25 g serve size is required. This approach is more likely to group foods of comparable macronutrient profiles together in the way they are tested. However, there is insufficient evidence to recommend a carbohydrate level where a lower dosage should be used.
BREAD OR GLUCOSE REFERENCE FOOD?
Glucose or bread is chosen as reference food in the majority of cases (Table 5, n = 127 for glucose and n = 40 for bread). Despite the standard making provisions for other reference foods with a well characterised GI to be used, it was only observed on 5 occasions. Four of these studies used rice[32,33,114,115] and in one study the reference food used appeared to be a control drink that would be unlikely to qualify as an acceptable reference food due to limited data on the control drink[116].
Table 5.
Reference standards used across the 1551 glycaemic index papers
|
Bread
|
Glucose
|
Other
|
| 40 | 127 | 5 |
Because some papers compared mixed methods and compared reference standards combined total of table entries is > 155.
The choice between feeding a glucose or bread reference food has been a debated aspect of GI testing. Bread has been suggested as a reference standard that reflects a more typical and physiologically relevant reference compared to glucose and produces a similar amount of statistical variation[14,81]. A complication of using bread as a reference food is that a final back calculation is required by the standard, so that reported values are still expressed relative to glucose. The standard states that the GI value of white bread is 71 when expressed as a percentage of the glucose at a value of 100. Therefore, the GI value produced when bread is used as a reference food, needs to be multiplied by 0.71 to give the final result relative to glucose. A central dogma of GI theory is that the GI value is a property of the food and by extension this value of 71 is robust and reproducible enough for this calculation to be accurate. However, there are a number of methodological considerations have the potential to impact on this rigid value of 71.
One consideration with the GI value used to correlate a bread reference food is whether there is variability in response depending on where the bread is from. The 2003 inter-laboratory study compared an anhydrous glucose reference to a bread reference sourced from their local area[14]. This study reported no significant difference between the locally sourced bread and the common glucose reference. Some papers included in the present review use a bread maker to prepare homemade bread as reference food and it is not known whether this adds further variability compared to commercial products. The online database of GI values maintained by Sydney University provides a resource to assess how fixed the 71 value for white bread is. Here it is recorded that white bread, made with wheat flour can vary in GI values from 59 to 89[117]. These values demonstrate that there is enough variability in the response of participants to white bread to warrant further investigation.
The 2008 inter-laboratory study on reproducibility of GI values demonstrated similarity of glucose to white bread, with an average GI on glucose reference of 58.5 ± 1.6 and 62.9 ± 5.6 on white bread[15]. This data suggests that bread could be interchanged with glucose as a reference food. However, similar to other factors discussed in this review it is not clear whether this close result between bread and glucose is applicable to a wide variety of foods or only to the specific two foods tested in this paper. For example, other studies have shown that bread correlates quite closely with glucose when capillary blood is used to determine GI values, but this relationship differs depending on the test food challenge provided[118]. It is not feasible to repeat the inter-laboratory study and include the full spectrum of potential test foods that may be included in a GI test. Therefore, one option is to analyse if there are any biological factors which could influence the outcome and exclude as many of these factors as possible.
One physiological consideration in this area is the influence of AMY1 gene copy number on bread vs a glucose solution. It has been hypothesised that the number of AMY1 copy numbers may influence the rate of starch digestion and the resulting GI values[34]. This provides evidence that bread may be a more appropriate reference food for starchy foods as high AMY1 copy number was associated with higher glycaemic responses. However, other studies have found AMY1 to be correlated with lower glycaemic responses[119]. Demonstrating the role of amylase copy number on GI is still being explored. The benefit of using bread as a reference food to compare with a starchy test food is that influence of these physiological differences will be controlled to some extent within the individual. The comparison between a food primarily composed of starch and a glucose reference may not correlate so closely and requires further exploration.
The glycaemic response to bread has also been shown to be closely linked to the specific microbiome composition of individuals and may be a source of variability[120]. This study was able to predict what the glycaemic response to each variety of bread would be, based on the composition of the microbiome and demonstrated large inter-personal variability. This was not a GI study, but a week long intervention trial and it is difficult to know how applicable these results are to the GI setting. A variety of factors can alter the microbiome composition of an individual and therefore could vary throughout the course of testing, or even as a direct result of the testing schedule. The variability of response to bread mediated by the microbiome does challenge the unchanging conversion factor of 0.71 from a bread glycaemic response to one that is in reference to glucose. Removing this option from the ISO standard would ensure that all future tests involve the same nutritional challenge.
CAPILLARY VS VENOUS BLOOD
In the majority of studies capillary blood is used to analyse blood glucose (Table 6, n = 123). This was followed by venous blood listed 24 times, intravenous catheter 7 times, continuous glucose monitors once and 7 papers did not specify how blood was sampled.
Table 6.
Blood sampling across the 1551 glycaemic index papers
|
Capillary
|
Venous
|
Intravenous catheter
|
Continuous glucose monitor
|
Not specified
|
| 123 | 24 | 7 | 1 | 7 |
Because some papers compared mixed methods and compared reference standards combined total of table entries is > 155.
The popularity of capillary blood is likely due to a number of reasons. Firstly, this option is the least intrusive for participants, compared to other methods. This is beneficial from an ethics point of view and is also useful in reducing participant dropout. The ISO2010 standard recommends capillary blood due to improved precision and reduced variation[14,118,121]. Capillary blood returns a significantly higher iAUC than venous blood, but once a test food is compared to glucose in methods such as GI[118] or glyceamic glucose equivalence[121] there is no significant difference in the calculated values.
An interesting observation in the Hätönen et al[118] paper is that the choice of capillary or venous blood may have interactions with other choices described in this review. For example, capillary blood returned comparable CV% values between bread and glucose. The CV percentages for glucose were 45, 48, 49 and for bread were 46, 39, 39. When venous blood was used, variation was less predictable with CV% for glucose were 121, 21, 22 and for bread 83, 121, 63. This observation supports the view that the combination of methodological decisions made is important, not just the validity of measures in isolation. Venous blood may be comparable to capillary blood under a defined set of parameters, but under others it may not be. There have been reports that choice of blood glucose assay may be more accurate when using venous blood, compared to capillary for blood sampling[122]. On the balance of evidence, it seems that recommending capillary sampling of blood is quite a reasonable choice for the ISO 2010 standard. However, it must be acknowledged that this choice may have implications depending on the other variables chosen in a set of GI testing experiments. This supports the continued standardisation of GI testing, to reduce the number of possible experimental designs and therefore the possibility of differing outcomes.
CONCLUSION
The inter-laboratory studies have reported a lack of statistical difference between the GI values between laboratories. However, the findings of the present review demonstrate that there is further standardisation required. Wolever et al[123] 2017 quite accurately point out that the margin of error for GI testing at the current stage of evolution is ± 17%, which is more accurate than what is permitted for macronutrient composition on a nutrition information panel. However, GI is unique to other nutrition information with the rigid thresholds of low, medium and high GI regulated by many jurisdictions. A mean of 17% SEM could easily misidentify foods by either under or overestimation. Much has been written discussing statistical variation in the GI and it is beyond the scope of this review to add further to this discussion. However, constant analysis of these methodological differences should be a fundamental component of a concept such as GI based on the principals of scientific rigour.
This review has identified a number of methodological variations present in GI testing and evaluated these against the biological implications different choices may initiate. The fundamental purpose of the GI is not as a mathematical exercise, but as a tool to compare and understand the biological action of different carbohydrates. This review has identified methodological variation that cannot be corrected for by statistics. This is because there is currently significant variety in the chemical and biological nature of GI tests conducted around the world. This variety currently exists despite an international standard being well accepted. For this reason, it is important that the methodology between individual research centres is replicated as closely as possible and a more prescriptive international standard is required.
It is hoped that the insights collated in this review contribute the foundation for further discussion into factors that may be introducing biological variation or inconsistency to testing methodology, so the credibility of the GI can continue to improve.
Footnotes
Conflict-of-interest statement: Flavel M and Kitchen B are both employees of The Product Makers Pty. Ltd., the manufacturer of patented glycaemic index lowering plant extracts.
Manuscript source: Invited manuscript
Peer-review started: October 28, 2020
First decision: November 16, 2020
Article in press: December 23, 2020
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang J S-Editor: Huang P L-Editor: A P-Editor: Ma YJ
Contributor Information
Matthew Flavel, Bioactive Division, The Product Makers, Keysborough 3173, Victoria, Australia; School of Life Sciences, La Trobe University, Bundoora 3086, Australia. mflavel@tpm.com.au.
Markandeya Jois, School of Life Sciences, La Trobe University, Bundoora 3086, Australia.
Barry Kitchen, Bioactive Division, The Product Makers, Keysborough 3173, Victoria, Australia; School of Life Sciences, La Trobe University, Bundoora 3086, Australia.
References
- 1.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 2.Smith RN, Braue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41–52. doi: 10.1016/j.jdermsci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Ingwersen J, Defeyter MA, Kennedy DO, Wesnes KA, Scholey AB. A low glycaemic index breakfast cereal preferentially prevents children's cognitive performance from declining throughout the morning. Appetite. 2007;49:240–244. doi: 10.1016/j.appet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Moore LJ, Midgley AW, Thurlow S, Thomas G, Mc Naughton LR. Effect of the glycaemic index of a pre-exercise meal on metabolism and cycling time trial performance. J Sci Med Sport. 2010;13:182–188. doi: 10.1016/j.jsams.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WH, Astrup A Diet; Obesity; and Genes (Diogenes) Project. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88:1104–1110. doi: 10.1093/ajcn/88.4.1104. [DOI] [PubMed] [Google Scholar]
- 7.Alfenas RC, Mattes RD. Influence of glycemic index/load on glycemic response, appetite, and food intake in healthy humans. Diabetes Care. 2005;28:2123–2129. doi: 10.2337/diacare.28.9.2123. [DOI] [PubMed] [Google Scholar]
- 8.Wolever TM, Brand-Miller JC. Influence of glycemic index/load on glycemic response, appetite, and food intake in healthy humans. Diabetes Care. 2006;29:474–475. doi: 10.2337/diacare.29.02.06.dc05-1912. [DOI] [PubMed] [Google Scholar]
- 9.Wolever TM. Is glycaemic index (GI) a valid measure of carbohydrate quality? Eur J Clin Nutr . 2013;67:522–531. doi: 10.1038/ejcn.2013.27. [DOI] [PubMed] [Google Scholar]
- 10.Barclay AW, Brand-Miller JC, Wolever TM. Glycemic index, glycemic load, and glycemic response are not the same. Diabetes Care. 2005;28:1839–1840. doi: 10.2337/diacare.28.7.1839. [DOI] [PubMed] [Google Scholar]
- 11.Whelan WJ, Hollar D, Agatston A, Dodson HJ, Tahal DS. The glycemic response is a personal attribute. IUBMB Life. 2010;62:637–641. doi: 10.1002/iub.365. [DOI] [PubMed] [Google Scholar]
- 12.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials. 2010;31:5–11. doi: 10.1016/j.cct.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Wolever TM, Vorster HH, Björck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL, Venter C, Xiaomei Wu. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475–482. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- 15.Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Björck I, Brighenti F, Brown R, Brynes A, Casiraghi MC, Cazaubiel M, Dahlqvist L, Delport E, Denyer GS, Erba D, Frost G, Granfeldt Y, Hampton S, Hart VA, Hätönen KA, Henry CJ, Hertzler S, Hull S, Jerling J, Johnston KL, Lightowler H, Mann N, Morgan L, Panlasigui LN, Pelkman C, Perry T, Pfeiffer AF, Pieters M, Ramdath DD, Ramsingh RT, Robert SD, Robinson C, Sarkkinen E, Scazzina F, Sison DC, Sloth B, Staniforth J, Tapola N, Valsta LM, Verkooijen I, Weickert MO, Weseler AR, Wilkie P, Zhang J. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr. 2008;87:247S–257S. doi: 10.1093/ajcn/87.1.247S. [DOI] [PubMed] [Google Scholar]
- 16.Wolever TMS, Meynier A, Jenkins AL, Brand-Miller JC, Atkinson FS, Gendre D, Leuillet S, Cazaubiel M, Housez B, Vinoy S. Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method. Nutrients. 2019;11:2218. doi: 10.3390/nu11092218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Standards Organization. ISO 26642: Food products - Determination of the glycaemic index (GI) and recommendation for food classification. Geneva (Switzerland): ISO; 2010 Available from: https://www.iso.org/standard/43633.html .
- 18.Chan HM, Brand-Miller JC, Holt SH, Wilson D, Rozman M, Petocz P. The glycaemic index values of Vietnamese foods. Eur J Clin Nutr. 2001;55:1076–1083. doi: 10.1038/sj.ejcn.1601265. [DOI] [PubMed] [Google Scholar]
- 19.MacIntosh CG, Holt SH, Brand-Miller JC. The degree of fat saturation does not alter glycemic, insulinemic or satiety responses to a starchy staple in healthy men. J Nutr. 2003;133:2577–2580. doi: 10.1093/jn/133.8.2577. [DOI] [PubMed] [Google Scholar]
- 20.Blair RM, Henley EC, Tabor A. Soy foods have low glycemic and insulin response indices in normal weight subjects. Nutr J. 2006;5:35. doi: 10.1186/1475-2891-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert SD, Ismail AA, Winn T, Wolever TM. Glycemic index of common Malaysian fruits. Asia Pac J Clin Nutr. 2008;17:35–39. [PubMed] [Google Scholar]
- 22.Robert SD, Ismail AA. Two varieties of honey that are available in Malaysia gave intermediate glycemic index values when tested among healthy individuals. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:145–147. doi: 10.5507/bp.2009.024. [DOI] [PubMed] [Google Scholar]
- 23.Bao J, Atkinson F, Petocz P, Willett WC, Brand-Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr. 2011;93:984–996. doi: 10.3945/ajcn.110.005033. [DOI] [PubMed] [Google Scholar]
- 24.Brand-Miller J, Atkinson F, Rowan A. Effect of added carbohydrates on glycemic and insulin responses to children’s milk products. Nutrients. 2013;5:23–31. doi: 10.3390/nu5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeevetha S, Barakatun-Nisak MY, Ngan HB, Ismail A, Azlan A. Relationship between amylose content and glycemic index of commonly consumed white rice. IOSR J Agric Vet Sci. 2014;7:2319–2372. [Google Scholar]
- 26.Zin CA, Robert SD, Ishak WR. Effect of biscuits and muffins added with cornlettes powder on the glycemic responses of healthy individuals. Food Nutr Sci. 2014;5:2195–2202. [Google Scholar]
- 27.Sluik D, Atkinson FS, Brand-Miller JC, Fogelholm M, Raben A, Feskens EJ. Contributors to dietary glycaemic index and glycaemic load in the Netherlands: the role of beer. Br J Nutr. 2016;115:1218–1225. doi: 10.1017/S0007114516000052. [DOI] [PubMed] [Google Scholar]
- 28.Robert SD, Ismail AA, Rosli WI. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. Eur J Nutr. 2016;55:2275–2280. doi: 10.1007/s00394-015-1037-4. [DOI] [PubMed] [Google Scholar]
- 29.Chapagai MK, Bakar NA, Jalil RA, Muda WA, Karrila T, Ishak WR, Siwaporn P. Glycaemic index values and physicochemical properties of five brown rice varieties cooked by different domestic cooking methods. FFHD . 2016;6:506–518. [Google Scholar]
- 30.Se CH, Chuah KA, Mishra A, Wickneswari R, Karupaiah T. Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties. Nutrients. 2016;8:308. doi: 10.3390/nu8050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osman NM, Mohd-Yusof BN, Ismail A. Estimating glycemic index of rice-based mixed meals by using predicted and adjusted formulae. Rice Sci . 2017;24:274–282. [Google Scholar]
- 32.Zhu R, Liu M, Han Y, Wang L, Ye T, Lu J, Fan Z. Acute effects of non-homogenised and homogenised vegetables added to rice-based meals on postprandial glycaemic responses and in vitro carbohydrate digestion. Br J Nutr. 2018;120:1023–1033. doi: 10.1017/S0007114518002489. [DOI] [PubMed] [Google Scholar]
- 33.Zhu R, Fan Z, Dong Y, Liu M, Wang L, Pan H. Postprandial Glycaemic Responses of Dried Fruit-Containing Meals in Healthy Adults: Results from a Randomised Trial. Nutrients. 2018;10:694. doi: 10.3390/nu10060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson FS, Hancock D, Petocz P, Brand-Miller JC. The physiologic and phenotypic significance of variation in human amylase gene copy number. Am J Clin Nutr. 2018;108:737–748. doi: 10.1093/ajcn/nqy164. [DOI] [PubMed] [Google Scholar]
- 35.Luke DR, Lee KKY, Rausch CW, Cheng C. Phase 1 Study of the Pharmacology of BTI320 Before High-Glycemic Meals. Clin Pharmacol Drug Dev. 2019;8:395–403. doi: 10.1002/cpdd.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph J. Nutritional, Glycemic and Ecological Assessment of Green Jackfruit for Diabetes in Kerala. Int J Diab July. 2019:14–18. [Google Scholar]
- 37.Atkinson FS, Villar A, Mulà A, Zangara A, Risco E, Smidt CR, Hontecillas R, Leber A, Bassaganya-Riera J. Abscisic Acid Standardized Fig (Ficus carica) Extracts Ameliorate Postprandial Glycemic and Insulinemic Responses in Healthy Adults. Nutrients. 2019;11:1757. doi: 10.3390/nu11081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusof BN, Talib RA, Karim NA. A study of blood glucose response following temperate and tropical fruit ingestion in healthy adults. Malay J Nutr. 2005;11:47–57. [Google Scholar]
- 39.Nisak B, Talib RA, Karim NA, Kamarudin NA, Arshad F. The Effect of Low Glycemic Index Bread Eaten with Different Fillings on Blood Glucose Response in Healthy Individuals. MJHS. 2008;6 [Google Scholar]
- 40. Hettiaratchi UP, Ekanayake S, Welihinda J. Glycaemic indices of three Sri Lankan wheat bread varieties and a bread-lentil meal. Int J Food Sci Nutr . 2009;60 Suppl 4:21–30. doi: 10.1080/09637480802360392. [DOI] [PubMed] [Google Scholar]
- 41. Yusof BN, Abd Talib R, Karim NA, Kamarudin NA, Arshad F. Glycaemic index of four commercially available breads in Malaysia. Int J Food Sci Nutr. 2009;60:487–496. doi: 10.1080/09637480701804268. [DOI] [PubMed] [Google Scholar]
- 42. Robert SD, Ismail AA. Glycemic responses of patients with type 2 diabetes to individual carbohydrate-rich foods and mixed meals. Ann Nutr Metab. 2012;60:27–32. doi: 10.1159/000335224. [DOI] [PubMed] [Google Scholar]
- 43. Ishak WR, Zin CA, Robert SD. Young Corn Ear Addition Improves Some Nutrients and Lowering Glycemic Index of Chiffon Cake. Food Nutr Sci. 2014;5:1545. [Google Scholar]
- 44. Gunathilaka MD, Ekanayake S. Do Different Cooking Methods Affect Glycaemic Index Of Rice. In: Young Scientist Forum Symposium. 2015: 5-8. doi: 10.4038/cmj.v60i2.7545. [DOI] [PubMed] [Google Scholar]
- 45. Gunathilaka MD, Ekanayake S. Effect of different cooking methods on glycaemic index of Indian and Pakistani basmati rice varieties. Ceylon Med J. 2015;60:57–61. doi: 10.4038/cmj.v60i2.7545. [DOI] [PubMed] [Google Scholar]
- 46. Barling PM, Shyam S, Selvathevan MD, Misra S. Anomalous association of salivary amylase secretion with the postprandial glycaemic response to starch. BMC Nutr. 2016;2:50. [Google Scholar]
- 47. Ng SH, Robert SD, Wan Ahmad WA, Wan Ishak WR. Incorporation of dietary fibre-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017;227:358–368. doi: 10.1016/j.foodchem.2017.01.108. [DOI] [PubMed] [Google Scholar]
- 48. Mahilrajan S, Balakumar S, Arasaratnam V, Kumanan T, Kailayalinkam R. Glycemic Index and Insulin Index of Palmyrah Based Edible Products Commonly Consumed in Jaffna. IOSR-JBB . 2017;3:37–42. [Google Scholar]
- 49.Nounmusig J, Kongkachuichai R, Sirichakwal PP, Yamborisut U, Charoensiri R, Vanavichit A. The effect of low and high glycemic index based rice varieties in test meals on postprandial blood glucose, insulin and incretin hormones response in prediabetic subjects. IFRJ. 2018;25:835–841. [Google Scholar]
- 50. Gourineni V, Stewart ML, Skorge R, Wolever T. Glycemic Index of Slowly Digestible Carbohydrate Alone and in Powdered Drink-Mix. Nutrients . 2019;11:1228. doi: 10.3390/nu11061228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garcia-Webb P, Bonser AM. Glucose or glucose monohydrate for glucose tolerance tests? Diabetologia. 1981;21:580. doi: 10.1007/BF00281554. [DOI] [PubMed] [Google Scholar]
- 52. Wiener K. What is 75 g of glucose? Ann Clin Biochem. 1990;27 (Pt 4):283–284. doi: 10.1177/000456329002700401. [DOI] [PubMed] [Google Scholar]
- 53. Soh NL, Brand-Miller J. The glycaemic index of potatoes: the effect of variety, cooking method and maturity. Eur J Clin Nutr. 1999;53:249–254. doi: 10.1038/sj.ejcn.1600713. [DOI] [PubMed] [Google Scholar]
- 54. Schenk S, Davidson CJ, Zderic TW, Byerley LO, Coyle EF. Different glycemic indexes of breakfast cereals are not due to glucose entry into blood but to glucose removal by tissue. Am J Clin Nutr. 2003;78:742–748. doi: 10.1093/ajcn/78.4.742. [DOI] [PubMed] [Google Scholar]
- 55. Hall RS, Thomas SJ, Johnson SK. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac J Clin Nutr. 2005;14:91–97. [PubMed] [Google Scholar]
- 56. Yusof BNM, Talib RA, Karim NA. Glycaemic index of eight types of commercial rice in Malaysia. Malay J Nutr . 2005;11:151–163. [Google Scholar]
- 57. Hofman Z, De Van Drunen J, Kuipers H. The Glycemic Index of standard and diabetes-specific enteral formulas. Asia Pac J Clin Nutr. 2006;15:412–417. [PubMed] [Google Scholar]
- 58. Henry CJ, Lightowler HJ, Kendall FL, Storey M. The impact of the addition of toppings/fillings on the glycaemic response to commonly consumed carbohydrate foods. Eur J Clin Nutr. 2006;60:763–769. doi: 10.1038/sj.ejcn.1602380. [DOI] [PubMed] [Google Scholar]
- 59.Mäkeläinen H, Anttila H, Sihvonen J, Hietanen RM, Tahvonen R, Salminen E, Mikola M, Sontag-Strohm T. The effect of beta-glucan on the glycemic and insulin index. Eur J Clin Nutr. 2007;61:779–785. doi: 10.1038/sj.ejcn.1602561. [DOI] [PubMed] [Google Scholar]
- 60.Mettler S, Lamprecht-Rusca F, Stoffel-Kurt N, Wenk C, Colombani PC. The influence of the subjects' training state on the glycemic index. Eur J Clin Nutr . 2007;61:19–24. doi: 10.1038/sj.ejcn.1602480. [DOI] [PubMed] [Google Scholar]
- 61. Ranawana DV, Henry CJ, Lightowler HJ, Wang D. Glycaemic index of some commercially available rice and rice products in Great Britain. Int J Food Sci Nutr. 2009;60 Suppl 4:99–110. doi: 10.1080/09637480802516191. [DOI] [PubMed] [Google Scholar]
- 62. Godley R, Brown RC, Williams SM, Green TJ. Moderate alcohol consumption the night before glycaemic index testing has no effect on glycaemic response. Eur J Clin Nutr. 2009;63:692–694. doi: 10.1038/ejcn.2008.27. [DOI] [PubMed] [Google Scholar]
- 63. Pen JJ, Khorosheva G, Van de Velde U, Debroye C, Huyghebaert A, Rottiers R, Keymeulen B. Zùsto: A new sweetening agent with low glycemic index. Clin Nutr ESPEN . 2018;23:103–106. doi: 10.1016/j.clnesp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 64. Thondre PS, Henry CJ. High-molecular-weight barley beta-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr Res. 2009;29:480–486. doi: 10.1016/j.nutres.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 65. Jenkins AL, Kacinik V, Lyon MR, Wolever TM. Reduction of postprandial glycemia by the novel viscous polysaccharide PGX, in a dose-dependent manner, independent of food form. J Am Coll Nutr. 2010;29:92–98. doi: 10.1080/07315724.2010.10719821. [DOI] [PubMed] [Google Scholar]
- 66. Monro JA, Wallace A, Mishra S, Eady S, Willis JA, Scott RS, Hedderley D. Relative glycaemic impact of customarily consumed portions of eighty-three foods measured by digesting in vitro and adjusting for food mass and apparent glucose disposal. Br J Nutr . 2010;104:407–417. doi: 10.1017/S0007114510000589. [DOI] [PubMed] [Google Scholar]
- 67. Chlup R, Peterson K, Zapletalová J, Kudlová P, Seckar P. Extended prandial glycemic profiles of foods as assessed using continuous glucose monitoring enhance the power of the 120-minute glycemic index. J Diabetes Sci Technol. 2010;4:615–624. doi: 10.1177/193229681000400316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ning B, Brown RC, Venn BJ, Williams SM, Green TJ. The effect of the fat and carbohydrate contents in the evening meal preceding GI testing on GI. Eur J Clin Nutr . 2010;64:224–226. doi: 10.1038/ejcn.2009.133. [DOI] [PubMed] [Google Scholar]
- 69.Thondre PS, Wang K, Rosenthal AJ, Henry CJ. Glycaemic response to barley porridge varying in dietary fibre content. Br J Nutr. 2012;107:719–724. doi: 10.1017/S0007114511003461. [DOI] [PubMed] [Google Scholar]
- 70.Shobana S, Kokila A, Lakshmipriya N, Subhashini S, Ramya Bai M, Mohan V, Malleshi NG, Anjana RM, Henry CJ, Sudha V. Glycaemic index of three Indian rice varieties. Int J Food Sci Nutr. 2012;63:178–183. doi: 10.3109/09637486.2011.615300. [DOI] [PubMed] [Google Scholar]
- 71. Vega-López S, Ausman LM, Matthan NR, Lichtenstein AH. Postprandial lipid responses to standard carbohydrates used to determine glycaemic index values. Br J Nutr. 2013;110:1782–1788. doi: 10.1017/S000711451300130X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gonzalez-Anton C, Rico MC, Sanchez-Rodriguez E, Ruiz-Lopez MD, Gil A, Mesa MD. Glycemic responses, appetite ratings and gastrointestinal hormone responses of most common breads consumed in Spain. A randomized control trial in healthy humans. Nutrients. 2015;7:4033–4053. doi: 10.3390/nu7064033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mohan V, Anjana RM, Gayathri R, Ramya Bai M, Lakshmipriya N, Ruchi V, Balasubramaniyam KK, Jakir MM, Shobana S, Unnikrishnan R, Krishnaswamy K, Henry JK, Sudha V. Glycemic Index of a Novel High-Fiber White Rice Variety Developed in India--A Randomized Control Trial Study. Diabetes Technol Ther. 2016;18:164–170. doi: 10.1089/dia.2015.0313. [DOI] [PubMed] [Google Scholar]
- 74. Kristensen M, Jensen MG, Riboldi G, Petronio M, Bügel S, Toubro S, Tetens I, Astrup A. Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite . 2010;54:163–169. doi: 10.1016/j.appet.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 75. Meng H, Matthan NR, Ausman LM, Lichtenstein AH. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am J Clin Nutr. 2017;105:842–853. doi: 10.3945/ajcn.116.144162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meng H, Matthan NR, Ausman LM, Lichtenstein AH. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am J Clin Nutr. 2017;106:1246–1256. doi: 10.3945/ajcn.117.162727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthan NR, Ausman LM, Meng H, Tighiouart H, Lichtenstein AH. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104:1004–1013. doi: 10.3945/ajcn.116.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang R, Li Y, Mu W, Li Z, Sun J, Wang B, Zhong Z, Luo X, Xie C, Huang Y. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine (Baltimore) 2018;97:e11996. doi: 10.1097/MD.0000000000011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emaleku SA, Omueti OD, Emaleku GO. Talinum triangulare Whole wheat meal fortified with soy flour consumed with Talinum triangulare (gbure) soup glycemic index and the test human subjects' lipid profiles. Diabetes Metab Syndr. 2018;12:831–837. doi: 10.1016/j.dsx.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 80. Young KWH, Wolever TMS. Effect of volume and type of beverage consumed with a standard test meal on postprandial blood glucose responses. Nutr Res. 1998;18:1857–1863. [Google Scholar]
- 81. Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 82. Sievenpiper JL, Jenkins DJ, Josse RG, Vuksan V. Dilution of the 75-g oral glucose tolerance test improves overall tolerability but not reproducibility in subjects with different body compositions. Diabetes Res Clin Pract. 2001;51:87–95. doi: 10.1016/s0168-8227(00)00209-6. [DOI] [PubMed] [Google Scholar]
- 83. Greer F, Hudson R, Ross R, Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes. 2001;50:2349–2354. doi: 10.2337/diabetes.50.10.2349. [DOI] [PubMed] [Google Scholar]
- 84. Feinberg LJ, Sandberg H, De Castro O, Bellet S. Effects of coffee ingestion on oral glucose tolerance curves in normal human subjects. Metabolism. 1968;17:916–922. doi: 10.1016/0026-0495(68)90158-3. [DOI] [PubMed] [Google Scholar]
- 85. Battram DS, Arthur R, Weekes A, Graham TE. The glucose intolerance induced by caffeinated coffee ingestion is less pronounced than that due to alkaloid caffeine in men. J Nutr. 2006;136:1276–1280. doi: 10.1093/jn/136.5.1276. [DOI] [PubMed] [Google Scholar]
- 86. Lien HC, Chen GH, Chang CS, Kao CH, Wang SJ. The effect of coffee on gastric emptying. Nucl Med Commun. 1995;16:923–926. doi: 10.1097/00006231-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 87. Hara Y, Honda M. The inhibition of α-amylase by tea polyphenols. Agr Bio Chem. 1990;54:1939–1945. [Google Scholar]
- 88. Zhang J, Kashket S. Inhibition of salivary amylase by black and green teas and their effects on the intraoral hydrolysis of starch. Caries Res. 1998;32:233–238. doi: 10.1159/000016458. [DOI] [PubMed] [Google Scholar]
- 89.Honda M, Hara Y. Inhibition of Rat Small Intestinal Sucrase and α-Glucosidase Activities by Tea Polyphenols. Biosci Biotechnol Biochem. 1993;57:123–124. doi: 10.1271/bbb.57.123. [DOI] [PubMed] [Google Scholar]
- 90. Murthy PS, Manjunatha MR, Sulochannama G, Naidu MM. Extraction, characterization and bioactivity of coffee anthocyanins. Eur J of Bio Sci. 2012;4:13–19. [Google Scholar]
- 91. Murase T, Yokoi Y, Misawa K, Ominami H, Suzuki Y, Shibuya Y, Hase T. Coffee polyphenols modulate whole-body substrate oxidation and suppress postprandial hyperglycaemia, hyperinsulinaemia and hyperlipidaemia. Br J Nutr. 2012;107:1757–1765. doi: 10.1017/S0007114511005083. [DOI] [PubMed] [Google Scholar]
- 92. Kim SD. Α-glucosidase inhibitor isolated from coffee. J Microbiol Biotechnol. 2015;25:174–177. doi: 10.4014/jmb.1411.11057. [DOI] [PubMed] [Google Scholar]
- 93. Bryans JA, Judd PA, Ellis PR. The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans. J Am Coll Nutr. 2007;26:471–477. doi: 10.1080/07315724.2007.10719638. [DOI] [PubMed] [Google Scholar]
- 94. Oboh G, Agunloye OM, Adefegha SA, Akinyemi AJ, Ademiluyi AO. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J Basic Clin Physiol Pharmacol. 2015;26:165–170. doi: 10.1515/jbcpp-2013-0141. [DOI] [PubMed] [Google Scholar]
- 95. Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res. 2013;57:48–57. doi: 10.1002/mnfr.201200511. [DOI] [PubMed] [Google Scholar]
- 96. Moisey LL, Kacker S, Bickerton AC, Robinson LE, Graham TE. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr. 2008;87:1254–1261. doi: 10.1093/ajcn/87.5.1254. [DOI] [PubMed] [Google Scholar]
- 97. Aldughpassi A, Wolever TM. Effect of coffee and tea on the glycaemic index of foods: no effect on mean but reduced variability. Br J Nutr. 2009;101:1282–1285. doi: 10.1017/s0007114508079610. [DOI] [PubMed] [Google Scholar]
- 98. Al-Mssallem MQ, Brown JE. Arabic coffee increases the glycemic index but not insulinemic index of dates. Saudi Med J. 2013;34:923–928. [PubMed] [Google Scholar]
- 99.Wolever TM, Jenkins AL, Vuksan V, Campbell J. The glycaemic index values of foods containing fructose are affected by metabolic differences between subjects. Eur J Clin Nutr. 2009;63:1106–1114. doi: 10.1038/ejcn.2009.30. [DOI] [PubMed] [Google Scholar]
- 100.Walsh JH. Gastric Secretion. In: The Role of the Gastrointestinal Tract in Nutrient Delivery. Green M, Greene HL. Orlando: Academic Press, 1984: 110-112 [Google Scholar]
- 101. Wiebe N, Padwal R, Field C, Marks S, Jacobs R, Tonelli M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011;9:123. doi: 10.1186/1741-7015-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Velangi A, Fernandes G, Wolever TM. Evaluation of a glucose meter for determining the glycemic responses of foods. Clin Chim Acta. 2005;356:191–198. doi: 10.1016/j.cccn.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 103. Klonoff DC, Parkes JL, Kovatchev BP, Kerr D, Bevier WC, Brazg RL, Christiansen M, Bailey TS, Nichols JH, Kohn MA. Investigation of the Accuracy of 18 Marketed Blood Glucose Monitors. Diabetes Care . 2018;41:1681–1688. doi: 10.2337/dc17-1960. [DOI] [PubMed] [Google Scholar]
- 104. Dickson LM, Buchmann EJ, Janse Van Rensburg C, Norris SA. The impact of differences in plasma glucose between glucose oxidase and hexokinase methods on estimated gestational diabetes mellitus prevalence. Sci Rep. 2019;9:7238. doi: 10.1038/s41598-019-43665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Moodley N, Ngxamngxa U, Turzyniecka MJ, Pillay TS. Historical perspectives in clinical pathology: a history of glucose measurement. J Clin Pathol. 2015;68:258–264. doi: 10.1136/jclinpath-2014-202672. [DOI] [PubMed] [Google Scholar]
- 106. Hagvik J. Glucose measurement: time for a gold standard. J Diabetes Sci Technol . 2007;1:169–172. doi: 10.1177/193229680700100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Abdulrhman M, El Hefnawy M, Ali R, Abdel Hamid I, Abou El-Goud A, Refai D. Effects of honey, sucrose and glucose on blood glucose and C-peptide in patients with type 1 diabetes mellitus. Complement Ther Clin Pract. 2013;19:15–19. doi: 10.1016/j.ctcp.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 108. Greenwood DC, Threapleton DE, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Burley VJ. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. 2013;36:4166–4171. doi: 10.2337/dc13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Monro JA. Glycaemic glucose equivalent: combining carbohydrate content, quantity and glycaemic index of foods for precision in glycaemia management. Asia Pac J Clin Nutr. 2002;11:217–225. doi: 10.1046/j.1440-6047.2002.00295.x. [DOI] [PubMed] [Google Scholar]
- 110. Wolever TM, Bolognesi C. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J Nutr. 1996;126:2798–2806. doi: 10.1093/jn/126.11.2798. [DOI] [PubMed] [Google Scholar]
- 111. Lee BM, Wolever TM. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur J Clin Nutr. 1998;52:924–928. doi: 10.1038/sj.ejcn.1600666. [DOI] [PubMed] [Google Scholar]
- 112. Wallace AJ, Eady SL, Willis JA, Scott RS, Monro JA, Frampton CM. Variability in measurements of blood glucose response to foods in human subjects is not reduced after a standard breakfast. Nutr Res. 2009;29:238–243. doi: 10.1016/j.nutres.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 113. Aston LM, Gambell JM, Lee DM, Bryant SP, Jebb SA. Determination of the glycaemic index of various staple carbohydrate-rich foods in the UK diet. Eur J Clin Nutr. 2008;62:279–285. doi: 10.1038/sj.ejcn.1602723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sugiyama M, Tang AC, Wakaki Y, Koyama W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr . 2003;57:743–752. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 115. Venn BJ, Kataoka M, Mann J. The use of different reference foods in determining the glycemic index of starchy and non-starchy test foods. Nutr J. 2014;13:50. doi: 10.1186/1475-2891-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Amiruddin NE, Zahary MN, Bhaskar R, Mhd Jalil AM. Glycaemic index, palatability and acceptability of energy drinks prepared with β-glucan and whey protein. Food Res . 2020;4:421–430. [Google Scholar]
- 117.Brand-Miller J. The International Glycaemic Index Database . Available from: http://www.glycemicindex.com/accessed 3/12/2019 . [Google Scholar]
- 118. Hätönen KA, Similä ME, Virtamo JR, Eriksson JG, Hannila ML, Sinkko HK, Sundvall JE, Mykkänen HM, Valsta LM. Methodologic considerations in the measurement of glycemic index: glycemic response to rye bread, oatmeal porridge, and mashed potato. Am J Clin Nutr . 2006;84:1055–1061. doi: 10.1093/ajcn/84.5.1055. [DOI] [PubMed] [Google Scholar]
- 119. Mandel AL, Breslin PA. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J Nutr. 2012;142:853–858. doi: 10.3945/jn.111.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Korem T, Zeevi D, Zmora N, Weissbrod O, Bar N, Lotan-Pompan M, Avnit-Sagi T, Kosower N, Malka G, Rein M, Suez J, Goldberg BZ, Weinberger A, Levy AA, Elinav E, Segal E. Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses. Cell Metab 2017; 25: 1243-1253. :e5. doi: 10.1016/j.cmet.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 121. Wallace AJ, Willis JA, Monro JA, Frampton CM, Hedderley DI, Scott RS. No difference between venous and capillary blood sampling and the Minimed continuous glucose monitoring system for determining the blood glucose response to food. Nutrition Res . 2006;26:403–408. [Google Scholar]
- 122. McNamara PJ, Sharief N. Comparison of EML 105 and advantage analysers measuring capillary versus venous whole blood glucose in neonates. Acta Paediatr. 2001;90:1033–1041. doi: 10.1080/080352501316978129. [DOI] [PubMed] [Google Scholar]
- 123. Wolever TM, Augustin LS, Brand-Miller JC, Delport E, Livesey G, Ludwig DS, Sievenpiper JL. Glycemic index is as reliable as macronutrients on food labels. Am J Clin Nutr. 2017;105:768–769. doi: 10.3945/ajcn.116.146092. [DOI] [PMC free article] [PubMed] [Google Scholar]