Abstract
Every daughter cell inherits two things from its mother: genetic information and a spatially organized complement of pre-existing macromolecular components. Many macromolecular complexes can self-organize, but the extent to which de novo self-organization, as opposed to maintenance of an already-organized state, can suffice to yield functional cells is uncertain. Using Xenopus laevis egg extracts, here we show that homogenized cytoplasm self-organizes into 300-400 micrometer length scale compartments that resemble cells. Formation of these cell-like compartments requires microtubule polymerization, but does not require added demembranated sperm nuclei with their accompanying centrosomes. The compartments are capable of replicative division, with a mother compartment giving rise to two daughters at the end of the mitotic cycle. These results suggest that the cytoplasm can generate much of the spatial organization and reproductive function of a cell.
One Sentence Summary:
Homogenized cytoplasm self-organizes into cell-like compartments that can carry out self-replication.
If an organism were homogenized, probably most of us would predict that it would not spontaneously re-form its original structure and return to life (1). Indeed, organismal development unfolds the body plan in a specific sequence that is not likely to be circumvented by a one-pot reaction of its underlying chemical ingredients. However, it is not immediately clear whether the same is to be expected on the level of cells. If a population of cells of the same type is mechanically homogenized, will the resulting pooled cytoplasm remain in the homogenized state?
We chose undiluted Xenopus laevis egg extracts (Fig. 1A) as our experimental system to address this question. Interphase-arrested Xenopus laevis egg extracts were prepared by standard methods (2) and supplemented with demembranated Xenopus laevis sperm nuclei plus one or more of the following probes: green fluorescent protein or mCherry with a nuclear localization signal (GFP-NLS or mCherry-NLS, fluorescently labeled tubulin, and ER-Tracker dye or MitoTracker dye, to allow visualization of the nucleus, microtubules, endoplasmic reticulum (ER) and mitochondria. The resulting extracts were mixed thoroughly and imaged over time in a fluorinated ethylene propylene (FEP)-clad chamber using bright-field and fluorescence microscopy (Fig. 1B).
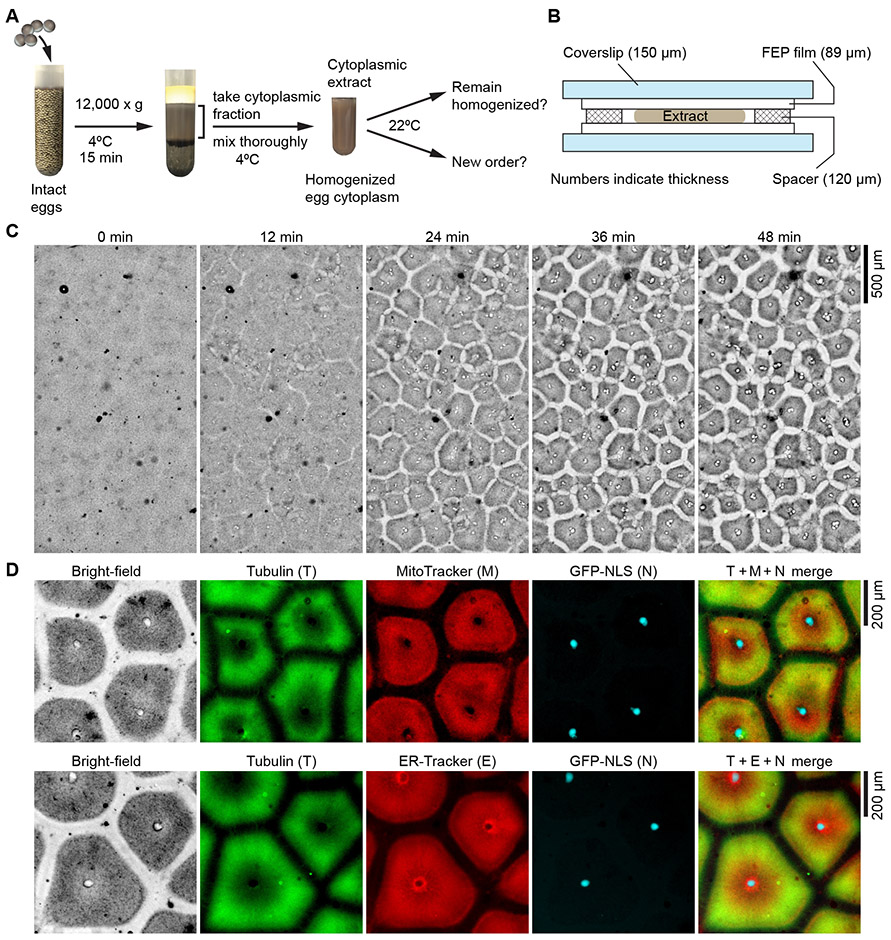
Fig. 1. Homogenized Xenopus laevis egg extracts self-organize into cell-like compartments.
(A) Schematic diagram of experimental procedures. (B) The design of the chamber used to image extracts. (C) Bright-field microscopy showing that homogenized Xenopus laevis egg cytoplasmic extracts spontaneously organized into cell-like compartments in a multi-millimeter scale field. Pattern formation dynamics in bright-field, tubulin and ER channels are presented in movie S1 and S2. (D) Spatial organization of microtubules, endoplasmic reticulum, nuclei and mitochondria in the cell-like compartments, visualized by added HiLyte 647 labeled porcine tubulin, ER-Tracker Red, GFP-NLS, and MitoTracker Red CMXRos. Pattern formation dynamics are presented in movie S3. The still images shown in (D) are from the last frame of movie S3, at 60.6 min after imaging began.
The homogenized extracts self-organized into a sheet of 300-400 μm compartments that resembled cells (Fig. 1C; movie S1 and S2). This self-organization was largely complete by ~30 min at room temperature. The added sperm nuclei migrated during compartment formation, and their initial localization did not entirely determine the final pattern (movie S2). Pattern formation did not appear to be coordinated by a propagating spatial signal, as the emergence of compartments across a large 2.6 mm field was approximately synchronous (movie S1). In addition, since interphase-arrested extracts contain cycloheximide, which inhibits protein translation, the pattern formation was independent of any possible genomic input from the added nuclei or translation of pre-existing maternal mRNA.
The localization of the nuclei, microtubules, mitochondria and ER was reminiscent of a typical interphase cell (3) (Fig. 1D). The dark gray regions observed in bright-field contained microtubules, ER, and mitochondria (Fig. 1D). The center of the compartment was populated by a single nucleus or a cluster of nuclei, as indicated by the GFP-NLS or mCherry-NLS signal. The border zones between the cell-like compartments were largely free of microtubules, ER, and mitochondria (Fig. 1D; movie S2 and S3). Typically, the microtubules were denser around the periphery of the compartments, and the ER and mitochondria were denser near the center (Figs. 1D, 2, A-C, 3A, 4, movie S2). Sometimes a dark boundary between compartments could be seen in the bright-field images (Fig. 1D and figure S1). This boundary weakly stained with ER-Tracker and MitoTracker (figure S1, movie S4).
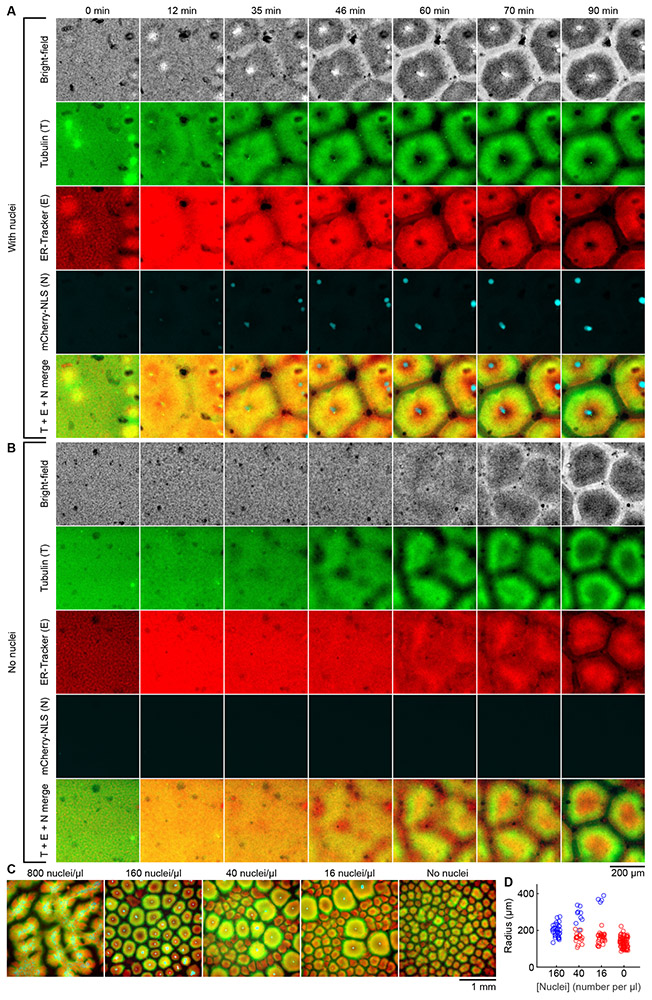
Fig. 2. The formation of cell-like compartments in egg extracts does not require added demembranated sperm nuclei.
(A) Time lapse montage of cell-like compartment formation in an interphase egg extract with approximately 160 demembranated Xenopus laevis sperm nuclei added per microliter of extract. (B) Time lapse montage of cell-like compartment formation in an extract from the same experiment as (A), but with no sperm nuclei added. Dynamics of pattern formation in (A) and (B) are presented in movie S4. (A) and (B) share the scale bar located at the bottom of (B). (C) Interphase egg extracts supplemented with different concentrations of sperm nuclei, viewed at a lower magnification. Microtubules are shown in green, ER in red, and nuclei in cyan. Dynamics of pattern formation are presented in movie S5. (D) Effective radii of cell-like compartments from the same experiment as (C). Blue symbols indicate radii of nucleated compartments and red symbols non-nucleated ones. For each compartment, its area A, volume V and effective radius r are interconvertible by A = π·r2 and V = A·d, where d = 120 μm is the thickness of the spacers (Fig. 1B). For example, an effective radius of 200 μm corresponds to a cross-sectional area of 0.125 mm2 and a volume of 15 nL.
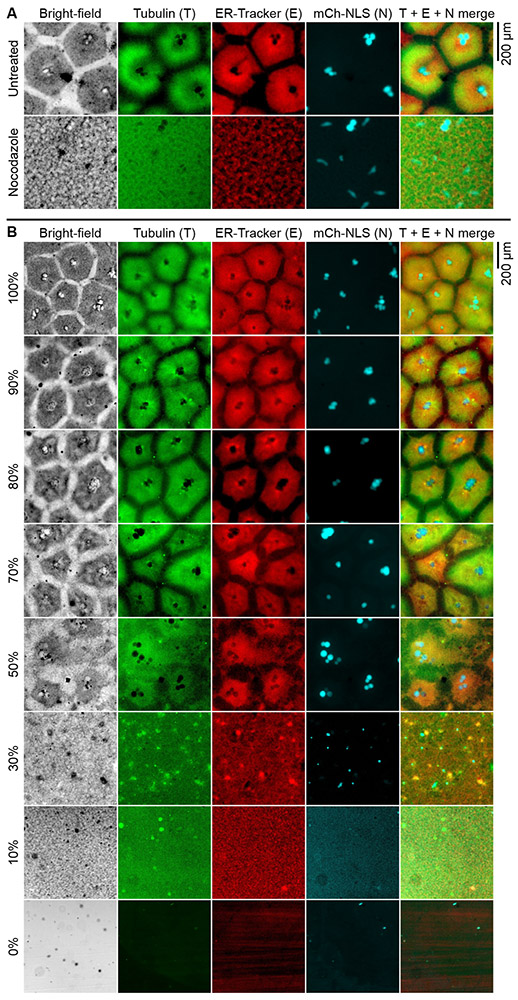
Fig. 3. Formation of cell-like compartments requires microtubule polymerization and a threshold concentration of cytoplasm.
(A) Nocodazole, a microtubule polymerization inhibitor, abolished the formation of cell-like compartments. Dynamics of the process is shown in movie S6. (B) Effect of dilution on pattern formation. Sperm nuclei, fluorescently labeled tubulin, ER-Tracker and mCherry-NLS were added after the indicated dilutions of extracts were performed, thus the final concentrations of these supplements were the same across all extracts. mCh-NLS indicates mCherry-NLS.
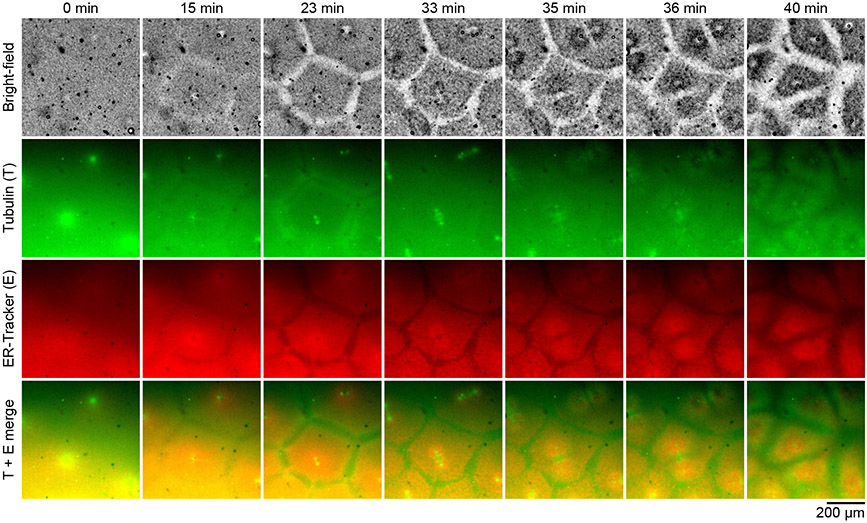
Fig. 4. Cell-like compartments are capable of self-replication.
Time lapse images of cell-like compartments performing a complete mitotic cell cycle in a cycling Xenopus laevis egg extract. Formation of the mother compartments became apparent at 15 min, and was completed by 23 min, when duplicated centrosomes could also be seen. A mitotic spindle subsequently formed at 33 min. The mother compartments completed their divisions at 40 min, each giving rise to two daughter compartments. Detailed dynamics of the process are shown in movie S7.
The self-organization of the extract raised the question of what cytoplasmic constituents mediate the process. The earliest step in the self-organization appeared to be the formation of microtubule asters at the centrosomes, which are associated with the added sperm nuclei (movie S2). The asters were always detectable by the first frame of the video, 5–10 min after the extracts were taken off ice. We therefore tested whether the added sperm, with their accompanying centrosomes, were required for pattern formation. To our surprise, grossly normal cell-like compartments still formed in the absence of sperm (Fig. 2A and B and movie S4). Compared to the sperm-supplemented extract, the time it took to form cell-like compartments was longer, and the initial dynamics of pattern formation were distinctly different in the sperm-free extract—no aster-like structures were ever seen. Nevertheless, the final patterns and compartment appearances in these two types of extract were almost the same (Fig. 2A and B; movie S4). Therefore, the self-organization phenomenon does not require the presence of a centrosome or chromatin.
At a high sperm concentration (800 nuclei/μl), some patterning still occurred, but cell-like compartments failed to form (Fig. 2C, movie S5). At an intermediate concentration of sperm (160 nuclei/μl), almost all of the compartments contained nuclei and appeared uniform in size, with an average effective radius of 199 ± 34 μm (mean ± S.D.). However, at lower sperm concentrations (16 to 40 nuclei/μl), the global patterns were a mix of nucleated and non-nucleated cell-like compartments of different sizes. For each sperm concentration in this range, the nucleated compartments were larger than non-nucleated ones in the final pattern, possibly because they formed earlier and were allowed more time to grow (Fig. 2C and D, movie S5). In the complete absence of nuclei, the compartments were fairly uniform in size, and were somewhat smaller than those seen in the presence of nuclei, with an average effective radius of 135 ± 28 μm (Fig. 2D, movie S5). These findings suggest that competition may be at play in determining the final length scale of the compartments.
Since both nucleated and non-nucleated cell-like compartments contained organized microtubules, we treated interphase extracts with the microtubule depolymerizer nocodazole and examined its effect on pattern formation. Extracts treated with 10 μg/ml nocodazole no longer formed cell-like compartments; self-organization appeared to be completely abolished (Fig. 3A; movie S6). Therefore, the formation of cell-like compartments requires microtubule polymerization. Note that these extracts did not contain actin filaments; cytochalasin B was added, as per standard Xenopus extract protocols (2, 4). Thus, microfilaments are not required for the formation of cell-like compartments.
A special feature of Xenopus extracts is that they can be prepared with essentially no dilution. We asked how much dilution could be tolerated by the self-organizing cytoplasm. We mixed undiluted interphase extracts with increasing amounts of egg lysis buffer, and monitored pattern formation in the diluted extracts. Nearly normal compartments still formed in extracts diluted to 70% of the original concentration (Fig. 3B). When cytoplasmic concentration was 50%, the pattern was noticeably abnormal, and when reduced to 30%, the compartments failed to form. Therefore, cell-like compartment formation requires a threshold concentration of the cytoplasm.
Finally, we wondered whether the cell-like compartments can carry out biological functions expected of a cell. One of the hallmark functions of cellular life is self-replication. We therefore asked whether the cell-like compartments can replicate themselves if the extracts are allowed to carry out the cell cycle. We prepared a cycling egg extract (4) in the absence of cycloheximide, which permits cyclin synthesis and allows Cdk1 to be activated and then inactivated. The extract was supplemented with demembranated sperm nuclei, ER-Tracker, and fluorescently labeled tubulin, and the dynamics were tracked by microscopy (Fig. 4 and movie S7).
Initially the cycling extract was in the first interphase after meiotic exit, and it appeared relatively homogeneous. By 15 min, cell-like compartments had begun to form, and by 23 min, they were indistinguishable from those seen in interphase-arrested extracts. The centrosomes separated at about this time, and shortly thereafter the texture of the ER changed, becoming coarser in appearance. At 33 min, well-defined mitotic spindles were visible. At this time, the ER-Tracker signal concentrated around the spindle poles, and a corresponding darker gray region was visible in the bright-field image. At 35 min, the division of the cell-like compartments began. Large microtubule asters emanated from the two daughter centrosomes, now pulled further away from each other, and a microtubule-enriched zone emerged near the bisecting point. ER appeared to be depleted from the same zone. By 40 min, the mother compartment had been completely divided into two daughter compartments, each with the same patterns of microtubules and ER as had been present in their mother prior to mitosis. These results demonstrate that the cell-like compartments are not only morphologically similar to cells, but can also perform one of the most important functions of a living cell.
In conclusion, these experiments show that homogenized Xenopus laevis egg cytoplasm can self-organize into spatially distinct compartments that resemble cells. The formation of these cell-like compartments does not require the presence of nuclei or centrosomes, but requires microtubule polymerization and a threshold concentration of the cytoplasm. The cell-like compartment is capable of self-replication. These results suggest that the cytoplasm can robustly generate the basic spatial organization of the cell and may retain much of its distinctive functions.
Cytoplasmic ingredients self-organize on many different scales. Minimal sets of purified cytoplasmic macromolecules can self-organize into molecular assemblies such as the apoptosome (5) and the ribosome (6), and subcellular structures such as the centrosome (7) and endoplasmic reticulum (8). Cell-free extracts can autonomously assemble larger subcellular structures like the bipolar meiotic spindle (9) and the machinery of cytokinesis (10). The phenomenon described here shows that self-organization of the cytoplasm can occur on the cellular level both in terms of scale and complexity, underscoring the importance of non-genetic information inherent in the components of the cytoplasm.
Supplementary Material
Acknowledgments:
We thank Hiro Funabiki and Mary Dasso for providing the GST-GFP-NLS construct, Tobias Meyer, Suzanne Pfeffer, Tim Stearns, and Julie Theriot for helpful discussions, and Oshri Afanzar, Yuxin Chen, Julia Kamenz, and Connie Phong for comments on the manuscript.
Funding: This work was supported by grants from the National Institutes of Health (R01 GM110564 and P50 GM107615).
Footnotes
Competing interests: None declared.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Weiss P, in Dynamics of Development: Experiments and Inferences, Weiss PA, Ed. (Academic Press, 1968), pp. 24–95. [Google Scholar]
- 2.Deming P, Kornbluth S, Study of apoptosis in vitro using the Xenopus egg extract reconstitution system. Methods Mol Biol 322, 379–393 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Alberts B et al. , Molecular Biology of the Cell. (Garland Science, New York, NY, ed. 6th, 2015), p. 24. [Google Scholar]
- 4.Murray AW, Cell cycle extracts. Meth Cell Biol 36, 581–605 (1991). [PubMed] [Google Scholar]
- 5.Kim HE, Du F, Fang M, Wang X, Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci U S A 102, 17545–17550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangiarotti G, Chiaberge S, Reconstitution of functional eukaryotic ribosomes from Dictyostelium discoideum ribosomal proteins and RNA. J Biol Chem 272, 19682–19687 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Woodruff JB et al. , The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077 e1010 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Powers RE, Wang S, Liu TY, Rapoport TA, Reconstitution of the tubular endoplasmic reticulum network with purified components. Nature 543, 257–260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heald R et al. , Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PA et al. , Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science 346, 244–247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JB, Ferrell JE Jr., Mitotic trigger waves and the spatial coordination of the Xenopus cell cycle. Nature 500, 603–607 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang JB, Ferrell JE Jr., Robustly cycling Xenopus laevis cell-free extracts in Teflon chambers. Cold Spring Harb Protoc 2018, pdb prot097212 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.