ABSTRACT
Sebaceous carcinoma is a rare malignant cutaneous neoplasm that is most commonly arises in the ocular region. Although it can occur in extraocular sites, sebaceous carcinoma is rarely encountered in the vulva. The use of immunohistochemical staining is very crucial to exclude other differential diagnoses including primary cutaneous and metastatic neoplasms. Unlike ocular sebaceous carcinoma, little is known about the clinical behavior and the prognostic factors in vulvar sebaceous carcinoma. Herein, we present a case of vulvar sebaceous carcinoma in a 27-year-old female, who presented with a labial tumor with lung metastases. To the best of our knowledge, only 11 similar cases were previously reported in the literature.
Key words: Vulva, vulvar carcinoma, vulvar sebaceous carcinoma
INTRODUCTION
Carcinoma of the sebaceous glands is a rare malignant cutaneous neoplasm.[1] The potential risk factors include female gender, Asian race, and previous exposure to head and neck radiation. Sebaceous carcinomas can be divided into two groups: ocular and extraocular. The latter is less common and can involve head and neck and genitals. Sebaceous carcinoma is an aggressive tumor that may spread to the local lymph nodes.[1] Patients usually present with a gradually growing painless nodule.
Sebaceous carcinoma of the female genital tract is very uncommon. We are presenting an unusual case of sebaceous carcinoma of the vulva that metastasized to the lung.
CASE REPORT
A 27-year-old, Filipino lady presented to the obstetric and gynecological clinic with painful vaginal swelling for 4 weeks which increased in size over 2 weeks. She also complained of painful micturition. The patient had no family history of any malignancy.
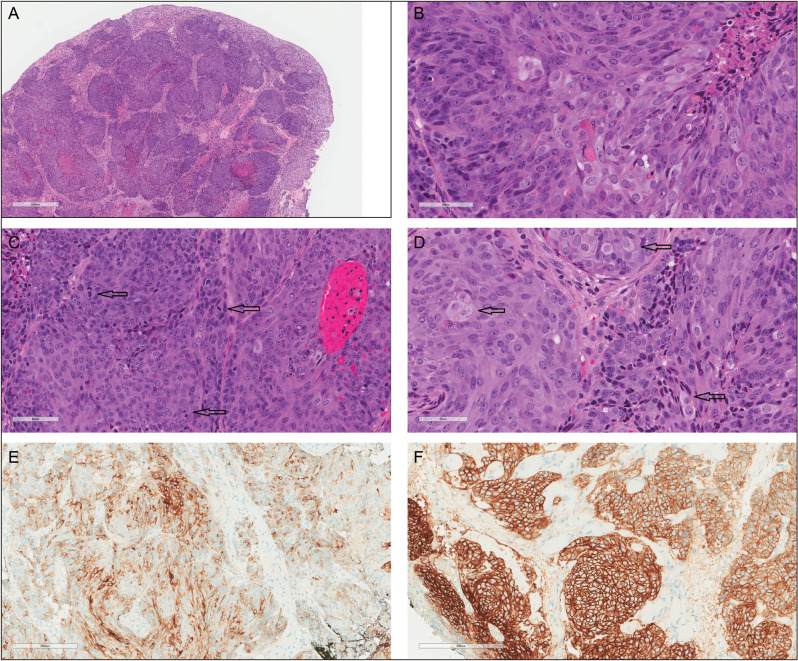
Pelvic examination revealed a 1 cm red tender firm lesion in the left labia majora. A punch biopsy was taken. Microscopically, the biopsy showed a high-grade neoplasm involving the dermis with focal ulceration of the overlying epidermis. The tumor was composed of lobules/nests of cells separated by fibrovascular stroma [Figure 1A]. The nuclei were large, with prominent nucleoli and scattered mitoses [Figure 1B and C]. Tumor nests focally exhibited comedo-type necrosis. Scattered cells showed sebaceous differentiation, manifested by finely vacuolated or foamy cytoplasm [Figure 1D].
Figure 1.
The tumor is composed of lobules/nests of cells separated by a fibrovascular stroma. Comedo-type necrosis is present (A). Tumor cells have large nuclei with prominent nucleoli (B) and scattered mitoses (C). Scattered cells show sebaceous differentiation manifested by finely vacuolated or foamy cytoplasm (D). Tumor cells are positive EMA (E) and BER-EP4 (F) immunostains
By immunohistochemistry, the neoplastic cells were positive for EMA, BER-EP4 [Figure 1E and F], CK8/18, and focally positive for CK20. They were negative for androgen receptor (AR), P16, S100, P63, CK5/6, CK7, and Pax-8. Ki-67 immunostain showed a high proliferation index (approximately 50–60%). Based on the morphology and the immunoprofile, the diagnosis of vulvar sebaceous carcinoma was favored.
An endometrial biopsy was performed and showed complex endometrial hyperplasia without atypia, associated with extensive squamous metaplasia (morules) and chronic endometritis. A cervical Pap smear was also obtained which yielded normal findings.
The subsequent radiological work-up, including abdomen and pelvis computed tomography (CT) scan and magnetic resonance imaging, revealed a left adnexal solid mass measuring 8.2 cm × 6.8 cm × 6.6 cm associated with retroperitoneal lymphadenopathy. These findings were worrisome for malignancy. A chest CT scan showed multiple bilateral suspicious lung nodules.
Positron emission tomography (PET)/CT was performed and it demonstrated an intensely hypermetabolic mass extending from the vulva through the vagina which was consistent with the biopsy-proven malignancy. Another hypermetabolic pelvic mass abutting the uterus is also noted. Multiple hypermetabolic lung nodules were observed. A left ischium lesion, as well as para-aortic and inguinal lymph nodes, was suspicious of metastasis.
CT-guided core biopsy of the lung nodules was obtained and it revealed a small focus of metastatic poorly differentiated carcinoma, morphologically, and immunohistochemically consistent with the patient’s previous diagnosis of labial sebaceous carcinoma.
Based on the radiological and histopathological findings, the patient was diagnosed with stage IV sebaceous carcinoma. She was started on a chemotherapy regimen of Carboplatin and Paclitaxel.
The patient completed 8 weeks of chemotherapy. A follow-up CT scan showed a partial response in the lungs and stable disease in the abdomen and pelvis. Next-generation sequencing performed on the vulvar biopsy revealed a microsatellite stable tumor with low mutational burden. There were no official druggable mutations; however, PIK3CA mutation was detected. Accordingly, Everolimus was offered as an additional treatment. Due to her pain, she was given 20 Gy in five fractions to the left lytic ischial lesion. Despite the optimal medical treatment, her disease progressed and she subsequently passed away 8 months after the initial diagnosis.
DISCUSSION
Sebaceous carcinoma is a rare, yet aggressive, cutaneous appendageal tumor that most commonly arises in the periocular area. Rarely, sebaceous carcinoma occurs in extraocular sites such as the face, scalp, trunk, limbs, and external genitalia.[2]
Sebaceous carcinoma may occur sporadically or in association with a genetic predisposition to Muir–Torre syndrome, an autosomal dominant disease characterized by the concurrent or sequential development of sebaceous gland tumors and at least one internal malignancy.[3,4] Muir–Torre syndrome is caused by germline mutations in the DNA mismatch repair (MMR) genes and is considered a phenotypic variant of hereditary nonpolyposis colorectal carcinoma syndrome (HNPCC, Lynch syndrome).[3] Internal malignancies most frequently associated with this syndrome include colorectal, endometrial, ovarian, and urothelial carcinomas.[5,6]
Sebaceous carcinoma of the vulva is exceptionally rare with only 11 cases reported previously [Table 1]. It was first reported in 1974 by Rulon et al.[7] The median age at the time of the diagnosis, including the present case, is 60 years. The tumor size varies from 0.5 cm to 4.0 cm in maximum dimension. The clinical appearance of the lesion is extremely variable with no specific shape or color.
Table 1.
Clinicopathological features of published cases of vulvar sebaceous carcinoma
| Author | Age | Site | Size | Appearance | Duration of symptoms | Management | Metastasis | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Present case | 27 | Left labia majora | 1 cm (4.3 × 3 × 4.5 cm) by MRI | Red swelling | 4 weeks | Chemotherapy and immunotherapy | Lung (confirmed by biopsy) Left ischium lesion, para-aortic and inguinal lymph nodes and possibly left ovary (by PET/CT) |
CT scan which showed a partial response in the lungs and stable disease in the abdomen and pelvis. Tumor resisted chemo and immunotherapy. Died of disease after 8 months of follow-up. |
| Thakur et al.[13] | 55 | Right labia majora and minora | Two lesions 2.5 × 2 cm and 1 × 1.5 cm | Ulcerative nodule | 4 months | Radiotherapy | Right inguinal lymph node | 8 months of follow-up, alive, no evidence of recurrence |
| Sullivan et al.[14] | 76 | Vulva, not otherwise specified. | 0.5 cm | Visible papule | Not stated | Resection and left inguinal lymphadenectomy | No metastasis | 10 months of follow-up, alive, no evidence of recurrence |
| Yam et al.[15] | 64 | Right labia minora | 2 × 1.5 cm | Exophytic tumor | 12 months | Excision | No metastasis | Regular follow-ups, alive, no evidence of recurrence. |
| Pusiol et al.[8] | 51 | Left labia majora | 2.5 × 1.5 cm | Exophytic red and white tumor | 6 months | Hemivulvectomy | No metastasis | 18 months of follow-up, alive, no evidence of recurrence |
| Khan et al.[2] | 49 | Right labia majora | 0.5 cm | Papilloma | Not stated | Excision of the right vulva, bilateral inguinal lymphadenectomy, and external beam radiotherapy to both groins | Left inguinal lymph node | Recurrence after 7 months. Excision was performed followed by palliative chemotherapy |
| Escalonilla et al.[16] | 76 | Right labia majora | 4 cm × 3 cm | Red white tumor and small papule | 4 months | Left radical hemivulvectomy | No metastasis | 12 months of follow-up, alive, no evidence of recurrence |
| Carlson et al.[12] | 46 | Left labia majora | Not stated | Cyst | Not stated | Left radical hemivulvectomy with left Inguinal lymphadenectomy | No metastasis | 31 months of follow-up, alive, no evidence of recurrence |
| Kawamoto et al.[17] | 78 | Left labia minora | 2.5 × 1.5 cm | Yellow white nodule | 6 months | Simple vulvectomy, dissection of the left inguinal lymph nodes, and radiation therapy for the left inguinal area | Left inguinal lymph node | 17 months of follow-up, alive, no evidence of recurrence |
| Jacobs et al.[18] | 89 | Left labia minora | Two lesion: 3.0 × 1.4 cm and 1.0 × 0.8 cm. | Pink white plaque | 1 year | Left radical hemivulvectomy | No metastasis | Not stated |
| Ikuse et al.[19] | 75 | Labia majora | Not available | Red ulcer | 2 years | Not available | Lung | Dead |
| Rulon et al.[7] | 31 | Left labia minora | 2.0 cm × 1.1 cm | Raw yellow slightly indurated plaque | 6 months | Excision | Not stated | 13 years and 7 months of follow-up, alive, no evidence of recurrence |
Due to its rarity, other differential diagnoses must be excluded including primary cutaneous and metastatic neoplasms. These include basal cell carcinoma with sebaceous differentiation, squamous cell carcinoma with clear cell features, melanoma, and metastatic carcinoma from other sites (i.e. metastatic serous carcinoma in our case). The use of ancillary studies can help in differentiating between these entities. Like in our case, the negative staining for CK5/6 and P63 rules out squamous cell carcinoma. Both sebaceous carcinoma and basal cell carcinoma can express BerEp4; however, these two tumors can be differentiated using EMA immunostain which is usually positive in sebaceous carcinoma and negative in basal cell carcinoma. S100 immunostain would be helpful in melanocytic tumors. Metastatic serous carcinoma can be excluded by the negative staining for CK7, P16, and PAX-8.
The overall clinical characteristics of the previously reported cases are favorable. However, unlike ocular sebaceous carcinoma, little is known about prognostic factors in vulvar sebaceous carcinoma. Older age, higher-grade tumors, and distant and/or inguinal lymph node metastasis have been described as poor prognostic factors.[8,9] Of the 12 cases, metastasis to inguinal lymph nodes was observed in four cases, while two cases, including our patient, have lung involvement at the time of presentation. Additionally, it is reported that the loss of AR expression (like in our case) is considered as an adverse prognostic factor in sebaceous carcinoma and it may represent a lack of differentiation or dedifferentiation of the tumor.[10]
Due to the limited number of reported cases, the optimal treatment of sebaceous carcinoma of the vulva has not been well established.[8] Although there is limited supportive data, surgical excision with or without lymphadenectomy appears to be the appropriate first intervention with a goal of complete excision of disease.[2,9,11] Cases that showed metastasis were either followed by chemotherapy, radiotherapy, or both. Radiotherapy alone was used successfully in some cases of recurrence and inguinal lymph node metastases.[8,12]
In summary, sebaceous carcinoma of the vulva is an exceptionally uncommon neoplasm. Due to its rarity, the clinical behavior, prognosis, and optimal treatment are yet to be further assessed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009;115:158–65. doi: 10.1002/cncr.23952. [DOI] [PubMed] [Google Scholar]
- 2.Khan Z, Misra G, Fiander AN, Dallimore NS. Sebaceous carcinoma of the vulva. Bjog. 2003;110:227–8. [PubMed] [Google Scholar]
- 3.Gay JT, Gross GP. Muir-Torre Syndrome. Treasure Island (FL): StatPearls; 2019. [PubMed] [Google Scholar]
- 4.Harwood CA, Swale VJ, Bataille VA, Quinn AG, Ghali L, Patel SV, et al. An association between sebaceous carcinoma and microsatellite instability in immunosuppressed organ transplant recipients. J Invest Dermatol. 2001;116:246–53. doi: 10.1046/j.1523-1747.2001.01233.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen PR, Kohn SR, Davis DA, Kurzrock R. Muir-Torre syndrome. Dermatol Clin. 1995;13:79–89. [PubMed] [Google Scholar]
- 6.John AM, Schwartz RA. Muir-Torre syndrome (MTS): An update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558–66. doi: 10.1016/j.jaad.2015.09.074. [DOI] [PubMed] [Google Scholar]
- 7.Rulon DB, Helwig EB. Cutaneous sebaceous neoplasms. Cancer. 1974;33:82–102. doi: 10.1002/1097-0142(197401)33:1<82::aid-cncr2820330115>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Pusiol T, Morichetti D, Zorzi MG. Sebaceous carcinoma of the vulva: Critical approach to grading and review of the literature. Pathologica. 2011;103:64–7. [PubMed] [Google Scholar]
- 9.Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29:545–52. [PubMed] [Google Scholar]
- 10.Na HY, Choe JY, Shin SA, Choung HK, Oh S, Chung JH, et al. Proposal of a provisional classification of sebaceous carcinoma based on hormone receptor expression and HER2 status. Am J Surg Pathol. 2016;40:1622–30. doi: 10.1097/PAS.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 11.Audisio RA, Lodeville D, Quagliuolo V, Clemente C. Sebaceous carcinoma arising from the eyelid and from extra-ocular sites. Tumori. 1987;73:531–5. doi: 10.1177/030089168707300519. [DOI] [PubMed] [Google Scholar]
- 12.Carlson JW, McGlennen RC, Gomez R, Longbella C, Carter J, Carson LF. Sebaceous carcinoma of the vulva: A case report and review of the literature. Gynecol Oncol. 1996;60:489–91. doi: 10.1006/gyno.1996.0079. [DOI] [PubMed] [Google Scholar]
- 13.Thakur BK, Verma S, Khonglah Y, Jitani A. Multifocal sebaceous carcinoma of the vulva. Indian J Dermatol Venereol Leprol. 2017;83:221–4. doi: 10.4103/0378-6323.198436. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan SA, Tran AQ, O’Connor S, Gehrig PA. Sebaceous carcinoma of the vulva: A case report and review of the literature. Gynecol Oncol Rep. 2016;18:40–1. doi: 10.1016/j.gore.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yam P, Namuduri R, Chia Y, Kuei T. Sebaceous carcinoma of the vulva – A rare tumor of vulva. Int J Gynecol Obstet. 2012;119S3:S531–867. [Google Scholar]
- 16.Escalonilla P, Grilli R, Cañamero M, Soriano ML, Fariña MC, Manzarbeitia F, et al. Sebaceous carcinoma of the vulva. Am J Dermatopathol. 1999;21:468–72. doi: 10.1097/00000372-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto M, Fukuda Y, Kamoi S, Sugisaki Y, Yamanaka N. Sebaceous carcinoma of the vulva. Pathol Int. 1995;45:767–73. doi: 10.1111/j.1440-1827.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs DM, Sandles LG, Leboit PE. Sebaceous carcinoma arising from Bowen’s disease of the vulva. Arch Dermatol. 1986;122:1191–3. [PubMed] [Google Scholar]
- 19.Ikuse S, Jinbou A, Matsushima I. A case of sebaceous carcinoma. Jpn J Dermatol. 1976;86:783. [Google Scholar]