Abstract
Introduction: Although collagen is widely used in various forms as a functional ingredient in skin care products, the effect of oral supplementation of collagen tripeptides (CTPs) on human skin is unclear. Moreover, the majority of the positive outcomes of CTP reported so far have not considered the effect of weather conditions. Therefore, we tested the effect of CTP and adjusting for climate change on skin properties in middle-aged women.
Materials and Methods: A randomized controlled trial was conducted with 84 women between 40 and 60 years of age. Participants were randomized to receive placebo or 1,000 mg CTP daily for 12 weeks. CTP was prepared from the skin of Nile Tilapia by the digestion method using collagenase from non-pathogenic bacteria of the genus Bacillus. Skin hydration, wrinkling, and elasticity were assessed at baseline and after 6 and 12 weeks with adjustments for temperature, humidity, and ultraviolet A exposure during the evaluation time using weather data from the regional meteorological office.
Results: Of the 82 participants, 74 completed the trial without adverse effects. Compared with the control group, trans-epidermal water loss was reduced more in the CTP group after 12 weeks (P < 0.05). At 12 weeks, even after adjustment for humidity, temperature, and UVA in the region, the difference of the two groups in TEWL remained statistically significant (adjusted for humidity and temperature, P = 0.024; adjusted for UVA, P = 0.032; adjusted for temperature, high temperature, and ultraviolet A, P = 0.031). In terms of skin hydration, more improvement was evident in the CTP group than in the control group. In the subgroup analysis, subjects under 50 years of age showed a significant improvement in total score and moisture in the subjective skin improvement questionnaire after taking CTP for 12 weeks. Application of CTP was well-tolerated, and no notable adverse effect was reported from both groups.
Discussion: Our findings suggest that oral ingestion of CTP from the Skin of Nile Tilapia (Oreochromis niloticus) is well-tolerated and helps reduce water loss in in middle-aged women.
Clinical Trial Registration: www.clinicaltrials.gov/, Identifier: NCT03505684.
Keywords: collagen tripeptide, skin hydration, skin elasticity, skin wrinkling, middle aged women, climate change
Introduction
In the natural process of aging, elastin fibers and collagen in the skin decrease, which eventually results in skin aging, fine lines, and deep wrinkles (1). The amount of glycosaminoglycans (GAGs) in the epidermis and dermis decline with age, leading to a decrease in the capacity to retain moisture within the skin and an increase in skin dryness (2). Collagen is the major structural constituent of the dermal extracellular matrix (ECM), which accounts for more than 70% of the dry weight of the normal human skin dermis (3).
New insights into the effects of the oral intake of biologically active compounds on skin properties have led to the development of nutritional supplements to benefit human skin (4). For instance, some dietary supplements with antioxidant properties reportedly influence the skin via secondary messengers. Alternatively, in the process of digestion, they can traverse the gastrointestinal tract, cross the intestinal barrier, and reach the skin, potentially in an active form, via the blood stream. Blood containing these bioactive compounds continuously replenishes the skin and all skin compartments, including the epidermis, dermis, subcutaneous fat, and sebum (5). Among these compounds, collagen has been widely used as a material in food, cosmetic, and pharmaceutical industries due to its biological and functional properties (6). In particular, collagen peptides derived from sea fish have been recognized as a dietary supplement that is useful for treating high blood pressure (7), muscle damage (8), lipid and glucose control (7, 9), and weight management in overweight individuals (10).
Collagen has a triple helix configuration (Gly-X-Y)n in which X and Y are mostly proline (Pro) and hydroxyproline (Hyp) (11). After consumed, Gly-Pro-Hyp is partially hydrolyzed by the brush-border membrane of the intestinal epithelium-bound aminopeptidase N to eliminate Gly (12). Thereafter, Pro-Hyp, a major active constituent of collagen-derived peptides, can be transported into small intestinal epithelial cells via the H+-coupled oligopeptide transporter (PEPT-1) (13). A higher level of Gly-Pro-Hyp in plasma was detected after the oral intake of low molecular weight collagen hydrolysates or collagen tripeptide (CTP) compared to high molecular weight collagen peptide (14). In addition, previous studies showed that Gly-Pro-Hyp and Pro-Hyp were stable in gastrointestinal fluid and plasma without being decomposed by gastric acid and enzymes, pancreatin, or plasma peptidases (14). These results indicate that oral intake of CTP can be an efficient approach to taking bioactive peptides owing to the enzymatic stability and intestinal permeability of Gly-Pro-Hyp and Pro-Hyp.
Evidence regarding the benefits of CTP continues to accumulate. In a recent randomized controlled trial, the oral intake of a low-molecular-weight collagen peptide (LMWCP) improved hydration, elasticity, and wrinkling in the skin of middle-aged women (15). However, the effect of LMWCP, evaluated by measuring various skin properties, was assessed without considering the influence of the temperature, humidity, and ultraviolet A (UVA) at the time of testing. Given that climatic factors can affect the overall skin texture, it is prudent to evaluate the effect of CTP on skin health by analyzing skin parameters after adjusting for the temperature, humidity, and UVA of the region and the season that the subjects were tested in. This randomized, double-blind clinical trial assessed whether the oral administration of CTP derived from fish skin of Nile Tilapia could still improve skin condition among middle-aged women after adjustments for the weather conditions.
Materials and Methods
Study Design and Ethical Aspects
The study was designed as a randomized, placebo-controlled, double-blind clinical trial. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Institutional Review Board at Pusan National University Yangsan Hospital (IRB No. 02-2017-033, November 6, 2017). This trial is registered with ClinicalTrials.gov (NCT03505684, April 23, 2018).
Study Participants
Apparently healthy candidates were recruited through an advertisement at a tertiary hospital in Yangsan-si, South Korea. Eligible subjects included those between the ages of 40 and 60 years with a transepidermal water loss (TEWL) score ≥4 measured using a Tewameter. Additionally, the candidates were required to apply the same skin care products (topical and oral supplements) as those used 4 weeks before the enrollment and did not receive any other skin care procedures during the 12-week study period. The main exclusion criteria were as follows: use within the 3 months preceding enrollment of any medication or supplements that could change in skin properties, including retinoids, steroids, and any other hormonal products, history of skin hypersensitivity and allergic reaction to any ingredients and sunlight, any skin improvement procedure within the prior 6 months, alcohol abuse, smoking cessation within 3 months of enrollment, uncontrolled blood pressure, blood glucose, or gastrointestinal symptoms, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) serum level >80 mg/dL, or creatinine (Cr) level >1.5 mg/dL, pregnancy or breastfeeding, allergy to any study ingredient, and intention to move away during the period of the study. In addition, for safety reasons, candidates diagnosed with cardiovascular diseases or any cancer during the 6 months prior to study commencement were also excluded. The subjects were considered to drop out or discontinue participation in the trial and were excluded from the per protocol analysis according to the following criteria: not taking the test product or placebo for 5 consecutive days, failure to attend follow-up assessments, poor compliance (<80%), excessive alcohol consumption or sunlight exposure, and relocation elsewhere during the period of the study. Compliance was assessed by counting the remaining capsules at every visit. Eleven participants met the exclusion criteria and three participants declined to participate.
Randomization
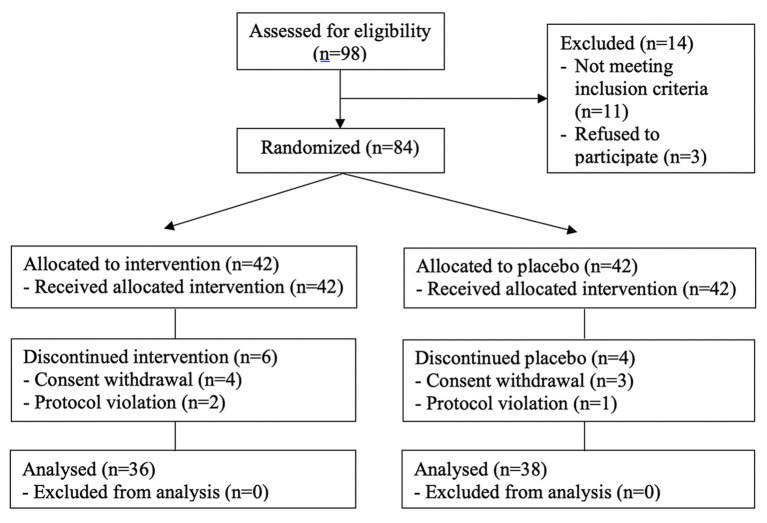
Ninety-eight participants were recruited for screening and 84 (85.7%) participants were finally enrolled after undergoing baseline measurements. They were randomly assigned to one of the intervention group with CTP (IG) and the control group with placebo (CG) through block randomization using randomized numbers and given identification numbers on recruitment. The IG (n = 42) received 1,000 mg of CTP supplement (250 mg each capsule) per day. The CG (n = 42) received a placebo (Figure 1). Randomization codes were created by an expert in statistics using nQuery Advisor 7.0. Those who were responsible for deciding on study eligibility and conducting the measurements were kept unaware of the results of the randomization throughout the whole study process. Every participant was asked to visit the center four times in total (visit 1; for screening, visit 2; randomization and start taking supplements, visit 3; 6 weeks after intervention, visit 4; 12 weeks later).
Figure 1.
CONSORT flow diagram.
Test Product and Placebo
CTP was prepared from the skin of Nile Tilapia (Oreochromis niloticus) by collagenase digestion (Collagen-Tripep20S; Amicogen Inc., Jinju, Gyeongsangnam-do, South Korea) from non-pathogenic bacteria of the genus Bacillus. The average molecular weight of the total CTPs was 500 Da with a 20% CTP content including 3.2% Gly–Pro–Hyp. Gly–Pro–Hyp was proven to be stable and bioavailable in vivo (14, 15). Additionally, its cutaneous hydration effect was demonstrated histologically by the enhanced gene and protein expressions in mice (16). The dosage of CTP applied to the subjects had been determined based on the results from previous animal studies where mice fed CTP daily (333 mg/kg) had shown a significant improvement in skin hydration and wrinkling without any adverse events (17, 18). Thus, 1,000 mg of collagen hydrolysate was considered appropriate for humans with an average body weight of 60 kg according to the guidance for estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers (19). In addition, in studies similar to our study, subjects were given a dose of between 1.0 and 3.0 g/day (15, 20–22). Two capsules (250 mg per capsule) of CTP were taken twice a day in the morning and evening by the IG subjects (total of four capsules each day) for 12 weeks. CG subjects were given the placebos with the same protocol and duration. Placebo consisted of 170 mg (68.2%) of maltodextrin and 80 mg (31.8%) of dextrin. In our preliminary study, plasma titer level of tripeptide and kinetics of the product were demonstrated (14).
Measurements of Efficacy
Prior to assessment for skin properties at every visit, the participants stayed for 30 min in the same room with controlled temperature (23 ± 1°C) and humidity (45 ± 5%). The primary efficacy variable was the change from the baseline in TEWL at 6 and 12 weeks of CTP or placebo use. The secondary outcome parameters involving skin hydration, elasticity, wrinkling and the participants' self-assessments were assessed at every visit.
Skin Transepidermal Water Loss
Three measurements were performed on the skin of the 10 cm frontal area from the antecubital fossa using an evaporimeter (Tewameter TM300; Courage + Khazaha Electronic GmbH, Köln, Germany) (23). The average value was reported in the analysis.
Skin Hydration
Hydration of the external layer of the epidermis (stratum corneum) was measured on the forehead capacitively with a corneometer (Corneometer CM 825; Courage + Khazaha Electronic GmbH, Köln, Germany) (24). For each measurement time, at least five measurements at different locations in the test area on the forehead were performed, then the average of three values excluding maximum and minimum readings were used for the analysis.
Skin Elasticity
For the assessment of skin elasticity, a cutometer (Cutometer MPA 580; Courage + Khazaha Electronic GmbH, Köln, Germany) was used (24). For each measurement, skin elasticity at three different places of the test area (forearm) was assessed and the values were averaged.
Skin Wrinkling
Measurement of skin wrinkling was evaluated on both sides of the crow's-feet area using a visiometer (Skin-Visiometer SV 600; Courage + Khazaka electronic GmbH, Köln, Germany) (25). The visual grade was evaluated on a scale of global photodamage scoring system (25) by a blinded dermatologist.
Questionnaire
After 6 and 12 weeks, the study subjects completed a questionnaire and subjectively assessed their perception of improvement in skin properties with time. Their responses were based on a 5-point Likert scale (1, much worse; 2, worse; 3, unchanged; 4, improved; 5, much improved). In addition, any unpleasant experience related to the study supplement was also reported.
Safety Evaluation
Blood pressure and heart rate were tested three times in the sitting position after a 10-min rest using a model BP-203 RV II device (Colin Corp., Aichi, Japan). The average measurement was recorded. Body weight and height were measured using a digital scale and stadiometer (BSM370; Biospace Co. Ltd., Seoul, South Korea), with patients wearing a light gown without shoes. Following a 4 h fast, blood samples were collected at baseline and at 6 and 12 weeks after start of the study. Serum AST, ALT, total bilirubin, glucose, and Cr were measured using a model TBA200FR biochemical analyzer (Toshiba Co. Ltd., Tokyo, Japan). Reports of any other adverse events or unpredicted allergic reactions were collected throughout the study.
Climate Data
The data on the temperature, humidity, and UVA of Kyungsang province and Busan, which were the regions where all participants resided during the entire study period (from January through October) were obtained from the local meteorological office.
Statistical Analyses
nQuery Advisor software version 7.0 (Cork, Ireland) was used to calculate sample size. The sample size of the study was calculated based on Choi et al. (21), who tested the effects of CTP supplement on skin properties. The estimated sample size was 34 subjects per group for 80% power to detect a difference of 3.53 in the mean TEWL levels, assuming a standard deviation of 5.0 in the primary outcome variable and an alpha error of 5%. Then, the sample size was adjusted to 42 participants per group to allow for 20% dropouts. Intent to treat (ITT) was the primary analysis for comparisons of outcomes between the two groups with multiple imputation of missing data (n = 84). A per-protocol (PP) analysis was also performed (n = 71) to assess effectiveness of the supplementation. The Shapiro–Wilk's test was performed to test the normality assumption. Intergroup comparisons of baseline characteristics and their changes at weeks 6 and 12 of the trial were performed using the two-sample t-test for continuous variables (or Mann-Whitney's U test in case of valuables showing non-normal distributions) or the Chi-square test for categorical variables. Intragroup comparisons were conducted using the paired t-test for continuous variables (or Wilcoxon signed rank test in case of valuables showing non-normal distributions). In addition, the subgroup analysis was performed based on the age. A repeated measures analysis of covariance was performed to compare intergroup differences in outcomes after adjustment for temperature, humidity and UVA of the day when parameters were checked. A P < 0.05 was considered statistically significant. SPSS Statistics for Windows Version 22.0 (IBM Corp., Armonk, NY) was used for the analysis.
Results
CONSORT (Consolidated Standards of Reporting Trials) Flow Diagram and Baseline Characteristics of the Subjects
The flow of subjects through the controlled interventional trial is depicted in a CONSORT conform diagram (26) (Figure 1). Eighty-four subjects aged 40–60 years were statistically analyzed. The mean age in the IG (n = 42) was 48.0 ± 5.9 years and in the CG (n = 42), 49.9 ± 6.5 years. Four participants in the IG and three in the CG withdrew consent for personal reasons that were not considered associated with the trial. Additionally, three subjects (on two in the IG and one in the CG) were excluded due to the protocol violation. The characteristics of these 10 participants were similar to those of the others who completed the study. Compliance exceeded 88% in both the IG and CG. Hence, ITT population and PP population were 84 and 71, respectively. No statistically significant intergroup differences were observed for age, body weight, alcohol drinking, and smoking at baseline (Table 1). The double-blind requirement was well-maintained throughout the study.
Table 1.
Baseline characteristics of the study subjects.
| Intention to treat population | Per protocol population | |||||
|---|---|---|---|---|---|---|
| CG (n = 41) | IG (n = 41) | P | CG (n = 38) | IG (n = 36) | P | |
| Age (years) | 49.9 ± 6.5 | 48.0 ± 5.9 | 0.176 | 49.5 ± 6.4 | 47.9 ± 6.0 | 0.303 |
| Weight (kg) | 56.7 ± 6.8 | 57.4 ± 8.9 | 0.813 | 56.4 ± 7.0 | 57.1 ± 9.2 | 0.864 |
| Height (cm) | 157.9 ± 5.7 | 157.6 ± 4.9 | 0.807 | 158.1 ± 5.7 | 157.5 ± 4.9 | 0.614 |
| Alcohol drinking (%) | 2 (4.8) | 4 (9.8) | 0.396 | 2 (5.3) | 3 (8.3) | 0.599 |
| Smoker (%) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
CG, control group with placebo; IG, intervention group with collagen tripeptide. Alcohol drinking was defined as consumption of alcohol with an average of five cups or more, more than two times a week. Data are presented as mean ± standard deviation or number (%). Shapiro–Wilk's test was employed for test of normality assumption. Mann–Whitney's U test for age, weight, two-sample t-test for height, Chi-square test for alcohol drinking.
Changes in Skin Properties
Skin Parameters
After 6 and 12 weeks of the trial, both groups showed decreased water loss with a greater reduction in TEWL in the IG than in the CG (Table 2). At 12 weeks, even after adjustment for humidity, temperature, and UVA in the region, the difference of the two groups in TEWL remained statistically significant (adjusted for humidity and temperature, P = 0.024; adjusted for UVA, P = 0.032; adjusted for temperature, high temperature, and ultraviolet A, P = 0.031) (Figure 2). In terms of skin hydration, more improvement was evident in the IG than in the CG, although there were no statistical significances in the differences of the change from the baseline between the two groups (Figure 2). After adjustment for humidity, temperature, and UVA in the region, TEWL was significantly different between the subjects over 50 years of age in both the CG and IG (Table 3).
Table 2.
Comparison of changes in skin properties in the full analysis set population.
| Variable | Observed values | Changes from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTP (n = 36) | CG (n = 38) | CTP (n = 36) | Pa | CG (n = 38) | Pa | Pb | Pc | Pd | Pe | |
| Transepidermal water loss (g/m2/h) | ||||||||||
| Baseline | 6.08 ± 2.1 | 5.64 ± 1.4 | ||||||||
| At 6 weeks | 4.43 ± 1.6 | 4.25 ± 1.1 | −1.65 ± 1.9 | <0.001 | −1.39 ± 1.9 | <0.001 | 0.577 | 0.675 | 0.411 | 0.445 |
| At 12 weeks | 5.14 ± 1.5 | 5.31 ± 1.5 | −0.94 ± 1.8 | 0.004 | −0.33 ± 1.9 | 0.297 | 0.168 | 0.024 | 0.032 | 0.031 |
| Skin hydration (A.U.) | ||||||||||
| Baseline | 192.5 ± 21.4 | 199.7 ± 21.9 | ||||||||
| At 6 weeks | 199.2 ± 19.6 | 200.1 ± 24.0 | 6.73 ± 22.0 | 0.076 | 0.45 ± 21.9 | 0.900 | 0.223 | 0.243 | 0.204 | 0.214 |
| At 12 weeks | 209.0 ± 18.7 | 212.1 ± 16.0 | 16.50 ± 21.7 | <0.001 | 12.44 ± 21.7 | <0.001 | 0.423 | 0.325 | 0.335 | 0.278 |
| Skin elasticity (mm) | ||||||||||
| Baseline | 0.40 ± 0.11 | 0.42 ± 0.10 | ||||||||
| At 6 weeks | 0.35 ± 0.10 | 0.36 ± 0.10 | −0.04 ± 0.1 | 0.050 | −0.06 ± 0.1 | 0.002 | 0.574 | 0.514 | 0.557 | 0.642 |
| At 12 weeks | 0.33 ± 0.10 | 0.36 ± 0.09 | −0.07 ± 0.1 | <0.001 | −0.05 ± 0.1 | 0.002 | 0.480 | 0.471 | 0.546 | 0.603 |
| Lt. crow's–feet visual score | ||||||||||
| Baseline | 2.25 ± 0.73 | 2.47 ± 0.98 | ||||||||
| At 6 weeks | 1.94 ± 0.63 | 1.97 ± 0.88 | −0.31 ± 0.6 | 0.003 | −0.50 ± 0.6 | <0.001 | 0.145 | 0.207 | 0.148 | 0.208 |
| At 12 weeks | 1.61 ± 0.60 | 1.71 ± 0.73 | −0.64 ± 0.6 | <0.001 | −0.76± 0.5 | <0.001 | 0.349 | 0.307 | 0.303 | 0.312 |
| Rt. crow's-feet visual score | ||||||||||
| Baseline | 2.11 ± 0.78 | 2.24 ± 0.94 | ||||||||
| At 6 weeks | 1.61 ± 0.64 | 1.82 ± 0.83 | −0.50 ± 0.6 | <0.001 | −0.42 ± 0.5 | <0.001 | 0.524 | 0.417 | 0.512 | 0.431 |
| At 12 weeks | 1.44 ± 0.56 | 1.47 ± 0.65 | −0.67 ± 0.6 | <0.001 | −0.76 ± 0.6 | <0.001 | 0.514 | 0.497 | 0.537 | 0.564 |
| Lt. wrinkles by vision meter | ||||||||||
| Baseline | 28.0 ± 6.2 | 25.8 ± 6.0 | ||||||||
| At 6 weeks | 31.4 ± 6.9 | 29.3 ± 5.4 | 3.33 ± 9.2 | 0.037 | 3.44 ± 8.3 | 0.011 | 0.960 | 0.797 | 0.985 | 0.897 |
| At 12 weeks | 29.8 ± 4.8 | 30.2 ± 6.1 | 1.82 ± 7.1 | 0.131 | 4.41 ± 7.9 | 0.002 | 0.141 | 0.181 | 0.241 | 0.286 |
| Rt. wrinkles by vision meter | ||||||||||
| Baseline | 27.2 ± 6.0 | 27.9 ± 6.8 | ||||||||
| At 6 weeks | 29.3 ± 5.4 | 28.4 ± 6.1 | 2.15 ± 7.9 | 0.117 | 0.57 ± 8.0 | 0.663 | 0.406 | 0.390 | 0.334 | 0.395 |
| At 12 weeks | 29.3 ± 5.0 | 30.5 ± 7.0 | 2.08 ± 7.4 | 0.100 | 2.66 ± 8.6 | 0.065 | 0.758 | 0.894 | 0.976 | 0.942 |
Values are presented as mean ± SD.
Paired t-test.
Two-sample t-test.
Repeated measures ANCOVA (adjusted for temperature and humidity).
ANCOVA (adjusted for ultraviolet A).
ANCOVA (adjusted for temperature, high temperature, and ultraviolet A).
Figure 2.
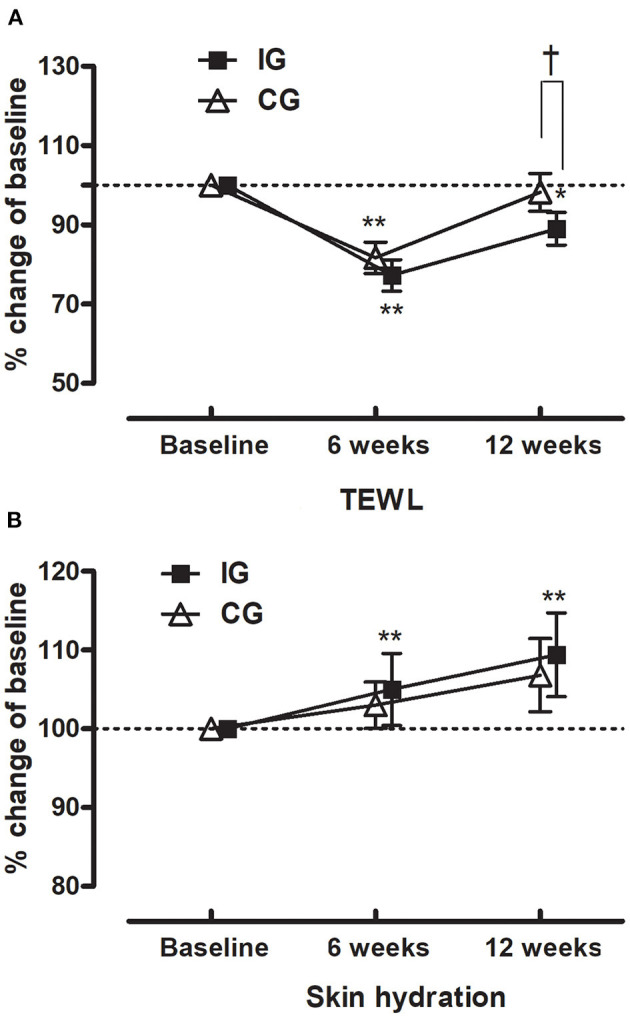
Changes in skin parameters in individuals receiving collagen tripeptide or placebo. (A) Changes in transepidermal water loss (TEWL). (B) Changes in skin hydration. *P < 0.05, **P < 0.01 by paired t-test comparisons with baseline values. P < 0.05 by two-sample t-test comparisons between values in the intervention group and the control group.
Table 3.
Comparison of changes in skin properties in over 50 years of age.
| Variable | Observed values | Changes from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IG (n = 14) | CG (n = 19) | IG (n = 14) | Pa,b | CG (n = 19) | Pa,b | Pc,d | Pe | Pf | |
| Transepidermal water loss (g/m2/h) | |||||||||
| Baseline | 6.60 ± 2.64 | 5.54 ± 1.61 | |||||||
| At 6 weeks | 4.53 ± 1.25 | 3.97 ± 0.97 | −2.07 ± 2.20 | 0.003a | −1.57 ± 2.19 | 0.002a | 0.413c | 0.152 | 0.294 |
| At 12 weeks | 5.39 ± 1.87 | 5.16 ± 1.12 | −1.21 ± 2.00 | 0.056a | −0.39 ± 1.89 | 0.657a | 0.209c | 0.034 | 0.040 |
| Skin hydration (A.U.) | |||||||||
| Baseline | 192.39 ± 24.09 | 195.89 ± 25.70 | |||||||
| At 6 weeks | 203.90 ± 21.82 | 192.92 ± 27.34 | 11.50 ± 19.57 | 0.047b | −2.97 ± 21.56 | 0.555b | 0.057d | 0.043 | 0.046 |
| At 12 weeks | 212.55 ± 19.33 | 212.42 ± 16.51 | 20.16 ± 21.55 | 0.004b | 16.53 ± 22.70 | 0.005b | 0.646d | 0.622 | 0.639 |
| Skin elasticity (mm) | |||||||||
| Baseline | 0.40 ± 0.11 | 0.39 ± 0.10 | |||||||
| At 6 weeks | 0.38 ± 0.12 | 0.37 ± 0.09 | −0.03 ± 0.13 | 0.485b | −0.03 ± 0.07 | 0.124b | 0.986d | 0.931 | 0.720 |
| At 12 weeks | 0.35 ± 0.11 | 0.37 ± 0.10 | −0.05 ± 0.10 | 0.079b | −0.02 ± 0.07 | 0.208b | 0.316d | 0.316 | 0.330 |
| Lt. crow's–feet visual score | |||||||||
| Baseline | 2.57 ± 0.65 | 3.00 ± 0.88 | |||||||
| At 6 weeks | 2.29 ± 0.47 | 2.47 ± 0.90 | −0.29 ± 0.61 | 0.219a | −0.53 ± 0.61 | 0.004a | 0.353c | 0.287 | 0.447 |
| At 12 weeks | 1.93 ± 0.62 | 2.00 ± 0.82 | −0.64 ± 0.63 | 0.012a | −1.00 ± 0.47 | <0.001a | 0.094c | 0.033 | 0.039 |
| Rt. crow's-feet visual score | |||||||||
| Baseline | 2.43 ± 0.76 | 2.68 ± 0.95 | |||||||
| At 6 weeks | 1.93 ± 0.73 | 2.26 ± 0.87 | −0.50 ± 0.52 | 0.016a | −0.42 ± 0.51 | 0.008a | 0.673c | 0.731 | 0.506 |
| At 12 weeks | 1.71 ± 0.61 | 1.79 ± 0.71 | −0.71 ± 0.61 | 0.004a | −0.89 ± 0.66 | 0.0001a | 0.446c | 0.414 | 0.471 |
| Lt. wrinkles by vision meter | |||||||||
| Baseline | 28.16 ± 7.87 | 26.58 ± 6.24 | |||||||
| At 6 weeks | 30.71 ± 5.56 | 28.59 ± 5.34 | 2.56 ± 10.12 | 0.330b | 2.01 ± 8.18 | 0.298b | 0.865d | 0.607 | 0.740 |
| At 12 weeks | 30.23 ± 4.06 | 30.04 ± 5.65 | 2.07 ± 7.42 | 0.315b | 3.46 ± 8.74 | 0.081b | 0.634d | 0.891 | 0.973 |
| Rt. wrinkles by vision meter | |||||||||
| Baseline | 28.33 ± 6.09 | 28.12 ± 6.92 | |||||||
| At 6 weeks | 29.03 ± 6.12 | 27.63 ± 6.60 | 0.70 ± 9.24 | 0.764b | −0.48 ± 8.71 | 0.811b | 0.709d | 0.536 | 0.548 |
| At 12 weeks | 30.16 ± 6.38 | 30.72 ± 7.47 | 1.83 ± 9.22 | 0.445b | 2.60 ± 9.21 | 0.418a | 0.814d | 0.955 | 0.870 |
Values are presented as mean ± SD.
Wilcoxon's signed rank test.
Paired t-test.
Mann–Whitney's U test.
Two-sample t-test.
Repeated measures ANCOVA (adjusted for ultraviolet A).
ANCOVA (adjusted for temperature, high temperature, ultraviolet A).
Self-Assessment by Participants
Subjects in both groups reported that their skin felt a bit better over time, with no significant difference between the groups (Table 4). They reported no unpleasant skin sensations after the 12-week oral consumption of the supplements (Table 4). But, in the subgroup analysis, subjects under 50 years of age showed a significant improvement in total score and moisture in the subjective skin improvement questionnaire after taking CTP for 12 weeks (P < 0.05, data not shown), and subjects over 50 years of age reported that the tightness was significantly improved compared to the control group after taking CTP for 6 weeks (P < 0.05, data not shown).
Table 4.
Self-assessment of skin texture in per protocol analysis.
| Variable | Intervention group (n = 36) | Control group (n = 38) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 week | 12 week | Δ | Pa | 6 week | 12 week | Δ | Pa | Pb | Pc | |
| Total score | 35.22 ± 3.4 | 35.75 ± 3.6 | 0.53 ± 2.7 | 0.256 | 34.87 ± 4.1 | 35.45 ± 4.4 | 0.58 ± 4.8 | 0.461 | 0.748 | 0.719 |
| Hydration | 3.42 ± 0.6 | 3.50 ± 0.6 | 0.08 ± 0.6 | 0.373 | 3.37 ± 0.6 | 3.50 ± 0.7 | 0.13 ± 0.8 | 0.303 | 1.000 | 0.969 |
| Smoothness | 3.36 ± 0.5 | 3.53 ± 0.6 | 0.17 ± 0.6 | 0.083 | 3.34 ± 0.7 | 3.53 ± 0.7 | 0.18 ± 0.8 | 0.147 | 0.992 | 0.911 |
| Roughness | 3.28 ± 0.6 | 3.47 ± 0.7 | 0.19 ± 0.6 | 0.070 | 3.42 ± 0.6 | 3.47 ± 0.7 | 0.05 ± 0.9 | 0.711 | 0.993 | 0.959 |
| Glowing | 3.36 ± 0.5 | 3.36 ± 0.5 | 0.00 ± 0.5 | 1.000 | 3.26 ± 0.7 | 3.32 ± 0.7 | 0.05 ± 0.7 | 0.644 | 0.766 | 0.843 |
| Elasticity | 3.39 ± 0.6 | 3.36 ± 0.5 | −0.03 ± 0.5 | 0.744 | 3.34 ± 0.6 | 3.32 ± 0.7 | −0.03 ± 0.8 | 0.845 | 0.749 | 0.756 |
| Wrinkles | 3.31 ± 0.5 | 3.25 ± 0.4 | −0.06 ± 0.5 | 0.487 | 3.16 ± 0.5 | 3.29 ± 0.6 | 0.13 ± 0.6 | 0.169 | 0.739 | 0.914 |
| Itching | 2.97 ± 0.3 | 2.97 ± 0.6 | 0.00 ± 0.6 | 1.000 | 2.97 ± 0.4 | 2.97 ± 0.6 | 0.00 ± 0.8 | 1.000 | 0.992 | 0.923 |
| Aching | 3.00 ± 0.0 | 3.00 ± 0.0 | 0.00 ± 0.0 | – | 3.00 ± 0.4 | 2.95 ± 0.4 | −0.05 ± 0.6 | 0.571 | 0.422 | 0.382 |
| Burning | 2.94 ± 0.3 | 3.00 ± 0.2 | 0.06 ± 0.2 | 0.160 | 3.00 ± 0.4 | 3.03 ± 0.4 | 0.03 ± 0.6 | 0.785 | 0.746 | 0.827 |
| Stinging | 2.97 ± 0.4 | 3.00 ± 0.4 | 0.03 ± 0.3 | 0.571 | 2.89 ± 0.3 | 2.92 ± 0.4 | 0.03 ± 0.5 | 0.734 | 0.383 | 0.366 |
| Tightness | 3.22 ± 0.6 | 3.31 ± 0.5 | 0.08 ± 0.4 | 0.263 | 3.11 ± 0.5 | 3.16 ± 0.6 | 0.05 ± 0.6 | 0.600 | 0.282 | 0.300 |
Values are presented as mean ± SD.
Paired t-test.
Two-sample t-test comparing the change between 6 and 12 weeks.
Repeated measures ANCOVA (adjusted for temperature, humidity and ultraviolet A).
Safety
Most of the subjects completed the protocol without adverse symptoms. One subject in the CG complained of mouth dryness. This symptom was not determined to be unrelated with taking CTP. No clinical changes in the levels of liver enzyme, creatinine, and glucose were observed in either group. No intergroup differences were found in these parameters during the study period.
Discussion
Although CTP has long been one of the most popular supplements for anti-skin aging, there is scarce evidence of its effect on humans. This clinical trial aimed to confirm the efficacy, safety, and tolerability of CTP on various skin parameters. Our results showed that daily oral intake of 1,000 mg CTP for 12 weeks decreased TEWL and was tolerated among middle-aged women. Skin condition is determined by a combination of factors that include age, sex, and measurement site, and also lifestyle and skin physiology (27). Skin moisturizing properties and UV exposure are higher in spring and summer seasons than in the dry autumn and winter seasons (28). UVA can penetrate deep into the skin and damage blood vessels and intradermal cell infiltration (29–31). Therefore, we adjusted for external weather factors to specifically assess the efficacy of CTP intake among subjects. After adjustment for humidity, temperature and UVA of the region in which the participants lived during the trial, the difference in TEWL between the two groups remained significant.
Aging is associated with a reduction in skin thickness and in the number of epithelial cells, with a concurrent decrease in stromal collagen (2, 3). Compared to younger women, the skin aging process in middle-aged women may intensify as estrogen deficiency is reported to have a direct effect on the epidermis (32). Also, many middle-aged women are reported to be very concerned about their facial appearance and visible aging phenomena indicated by increasing expenditures for cosmetic products and aesthetic procedures (33). In this context, our findings are clinically important as the study included middle-aged women who could be expected to benefit most from collagen treatment compared to men or younger women.
The biological underlying mechanism of efficacy of collagen in skin function can be explained by its action in the dermis (34). However, it is clinically challenging to make collagen bioavailable so that it reaches the target tissue and participates in various physiological functions, since collagen is a large molecule and is rarely cleaved in the gastrointestinal tract into a bioactive form (35). This is why there is relatively insufficient evidence of the effect of collagen on skin, even though it is one of the most commonly used ingredients of skin health supplements (4, 21, 36). In an effort to enhance the biological activity of collagen on skin, LMWCP has increasingly been manufactured. The smaller peptide is expected to better tolerate the action of gastrointestinal and plasma enzymes, and will pass across the membranes of intestinal epithelial cells (15).
The CTP used contained 3.2% Gly-Pro-Hyp. This sequence is a major active constituent of collagen-derived peptides (37). Pharmacological bioavailability research has revealed that the Pro-Hyp dipeptide that is derived from Gly-Pro-Hyp is available at high concentrations in the human blood stream for several hours after oral administration (35, 38). In an animal study, it was demonstrated that 14C-labeled Pro-Hyp reaches the skin and bone tissues rapidly after ingestion (39). A more recent animal study found that orally administered collagen peptide protects against UVB-induced skin aging through the absorption of Pro-Hyp (40). Moreover, a clinical study identified Pro-Hyp in urine after collagen hydrolysate intake, suggesting that these two types of collagen are relatively stable and resistant to peptidases in the blood stream, and can reach the skin tissues (13, 41). Additionally, some in vitro studies that investigated the physiological function of Pro-Hyp in skin dermal fibroblasts reported that Pro-Hyp enhanced cell proliferation activity and stimulating the chemotaxis of dermal fibroblasts (34). Additionally, Pro-Hyp facilitated the production of hyaluronic acid in dermal fibroblasts (42). These physiological roles of Pro-Hyp are important to improve the efficacy of collagen hydrolysates in maintaining skin health. Presently, the beneficial outcomes of the oral intake of collagen on the assessed skin conditions suggests that Gly-Pro-Hyp and Pro-Hyp contained in CTP may be efficiently absorbed and biologically active.
The majority of clinical studies of CTP have reported positive results concerning the overall improvement in skin texture after supplementation with CTP (4, 15, 36, 43–45). However, the collagen supplements used in these studies included other ingredients that boost collagen production or prevent its degradation, such as antioxidants containing vitamins or minerals (46). Therefore, the increase in skin elasticity might be attributed not only to the presence of the collagen bioactive peptides, but also to the presence of a mix of antioxidants. Since the beneficial properties of these ingredients are well-known and have been extensively studied (47), previous results are inconclusive concerning whether CTP itself has a favorable effect on skin properties and the degree of improvement. The present study used a supplement containing only CTP in the test group to specifically investigate the effect of CTP on skin.
With respect to the property of CTP used, a recent randomized control trial was comparable to our study (15). The trial compared the study group given 1,000 mg of LMWCP (containing more than >15% tripeptide content including 3% Gly-Pro-Hyp) and 100 mg of vitamin C with the placebo group that received 100 mg of vitamin C only. This study demonstrated that the 12-week oral intake of LMWCP improved hydration, elasticity, and wrinkling among females 40–60 years of age. However, the positive outcomes may have resulted from the different weather conditions in which parameters of skin properties of the participants were tested, considering that the study was conducted from February (winter with low humidity and cold temperatures in the country in which the study was carried out) through June (summer with high humidity and temperatures). Even though the authors tried to exclude the effect of atmosphere on skin texture by having the participants remain in a constant temperature (22–24°C) and humidity (40–60%) room for 30 min prior to the assessment, the skin conditions of the participants must have been affected by the overall weather conditions at the two different time points of the evaluation. Environmental humidity, temperature, and UVA affect skin barrier functions (31). Climatic factors, such as cold temperatures and low humidity, accelerate water loss from the skin and increase the risk of skin dryness and dermatitis (31).
In that sense, clinical studies related to changes in skin texture should include the seasonal variations of the region where the intervention were performed as a major covariate factor. In an effort to exclude the effect of the weather conditions on skin properties, we adjusted for humidity, temperature, and UVA of the region in which our study participants lived during the study period in the analysis of the changes in skin parameters after 12 weeks of oral intake of CTP. As the average humidity, temperature and UVA were obtained from the local weather office, the environmental data were reliable and accurate. This adjustment for the weather conditions might partially explain the reduced improvements in skin parameters in our study participants compared to the participants of the previous study (15). Additionally, we assumed that a significant improvement in skin conditions in our placebo group might be resulted from seasonal changes. Before our study, there have been a number of studies showing the positive outcomes of CTP application in skin health (4, 15, 36, 43–45). However, no one had considered climatic factors, which distinguishes our study from previous research.
Owing to the low temperature and/or high salt in the surrounding environment, fish are a good source of collagen compared with collagen peptide derived from land animals. The fish-derived collagen features unique molecular and biological properties of amino acid composition and antioxidant, anti-skin aging and anti-fat accumulating activities (10, 36, 48). Ohara et al. (49) compared quantity and structures of food-derived gelatin hydrolysate peptides in human blood from different sources (gelatin hydrolysis of fish and porcine) of type 1 collagen. In the case of free form Hyp, fish gelatin hydrolysate was more absorbed than porcine gelatin hydrolysis suggesting that low molecular collagen from fish gelatin can be greater at improving bioavailability and skin health. Moreover, in comparison with mammalian-based collagen, the utilization of marine-based collagen is growing fast due to its unique properties such as no risk of transmitting diseases, a lack of religious constraints, a cost-effective process, low molecular weight, biocompatibility, and its easy absorption by the human body.
To increase the bioavailability of bioactive peptides, the CTP used presently was prepared from fish skin of Tilapia by digestion method using collagenase from Bacillus, non-pathogenic bacteria of the genus Bacillus. The Gly-Pro-Hyp and Pro-Hyp constituents of the CTP were better absorbed and attained higher plasma levels after oral administration in rats compared to high molecular weight collagen peptide in an animal study; Gly-Pro-Hyp and Pro-Hyp were stable in gastrointestinal fluid and rat plasma for 2 h, and Gly-Pro-Hyp was able to be transported across the intestinal cell monolayer (14). Furthermore, a more recent histological study analyzed the expressions of biomarkers in mice to evaluate the cutaneous hydration effect of oral intake of this CTP product (28). Increased expression levels of ceramide kinase, hyaluronic acid, collagen 1A, and hyaluronan synthase-2 (HAS2), and decreased levels of hyaluronidase-1 (HYAL1) and CD44 were observed in human derma fibroblasts after application of CTP. Furthermore, significant reductions of TEWL, scratching behavior, HYAL1, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), and increased water content and HAS2 levels were observed. These results suggested that CTP product used in our study could enhance skin hydration and have potential as a skin hydration agent in humans. Fish is becoming a popular ingredient in both raw and cooked form because of its nutritional value and reduced caloric intake. As a result, a large amount of fish skin is currently disposed. From an environmental perspective, making better use of by-products such as skin is prudent.
The present study has several limitations. We conducted at a single center and included only females, which prevents generalization of the results. Due to small sample size and short study duration, the difference of the effect of collagen on skin properties between the two groups was modest although it was statistically significant. Also, it could be difficult to extend all of our considerations to climatic conditions. Lastly, we used collagen pills in order to offer the same flavor, color, and taste to the two groups as a double-blind RCT. However, it is known that liquid collagen is absorbed into the bloodstream more quickly and efficiently and shortens the digestion time of protein than do solid supplements. Despite these limitations, our study is considerably valuable owing to the following strengths. Firstly, to the best of the authors' knowledge, it is the first clinical study to examine the efficacy of CTP on skin properties after adjustment for the weather conditions of the area where the subjects lived during the period of the study using accurate data from the local meteorological office. This study was a rigorously conducted randomized controlled trial, with the appropriate criteria for inclusion/exclusion. Moreover, the measurements of skin parameters were checked objectively by using a reliable quantitative method.
In conclusion, this randomized, placebo-controlled clinical trial demonstrates that CTP ingestion is well-tolerated and helps reduce water loss in in middle-aged women. Our results also provide initial insight into the effects of the climate data on the temperature, humidity, and UVA of the region in which the participants lived on skin properties in humans, and suggest that, in particular, the weather effects of the skin intervention on human skin health should be considered in future clinical trials with a population, especially in areas with four seasons.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Institutional Review Board at Pusan National University Yangsan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YJT, YLL, H-CK, and SYL contributed to the conceptualization of the study. DKS, AHK, JIK, YLL, H-CK, and SYL designed the methodology of the work. YLL and SYL had an active role in the process of participants and data acquisition. YJT and SYL contributed to the validation of results. DKS, AHK, and JIK carried out the formal analysis of the data. YJT and AHK worked together for data curation. YJT, Y-WK, and SYL wrote the work's draft and reviewed the final document. SYL coordinated and supervised the entire project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
DKS, AHK, and JIK is currently employed by Amicogen Inc. Biotech R & D center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Sarbacher CA, Halper JT. Connective tissue and age-related diseases. Subcell Biochem. (2019) 91:281–310. 10.1007/978-981-13-3681-2_11 [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Oh JH, Chung JH. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. (2016) 83:174–81. 10.1016/j.jdermsci.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. (2015) 61:427–34. 10.1159/000371708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. (2017) 30:146–58. 10.1159/000464470 [DOI] [PubMed] [Google Scholar]
- 5.Shamloul N, Hashim PW, Nia JJ, Farberg AS, Goldenberg G. The role of vitamins and supplements on skin appearance. Cutis. (2019) 104:220–4. [PubMed] [Google Scholar]
- 6.Felician FF, Xia C, Qi W, Xu H. Collagen from marine biological sources and medical applications. Chem Biodivers. (2018) 15:e1700557. 10.1002/cbdv.201700557 [DOI] [PubMed] [Google Scholar]
- 7.Zhu CF, Li GZ, Peng HB, Zhang F, Chen Y, Li Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am J Med Sci. (2010) 340:360–6. 10.1097/MAJ.0b013e3181edfcf2 [DOI] [PubMed] [Google Scholar]
- 8.Clifford T, Ventress M, Allerton DM, Stansfield S, Tang JCY, Fraser WD, et al. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids. (2019) 51:691–704. 10.1007/s00726-019-02706-5 [DOI] [PubMed] [Google Scholar]
- 9.Zhu CF, Li GZ, Peng HB, Zhang F, Chen Y, Li Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl Physiol Nutr Metab. (2010) 35:797–804. 10.1139/H10-075 [DOI] [PubMed] [Google Scholar]
- 10.Tak YJ, Kim YJ, Lee JG, Yi YH, Cho YH, Kang GH, et al. Effect of oral ingestion of low-molecular collagen peptides derived from skate (raja kenojei) skin on body fat in overweight adults: a randomized, double-blind, placebo-controlled trial. Mar Drugs. (2019) 17:157. 10.3390/md17030157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkness MW, Lehmann K, Forde NR. Mechanics and structural stability of the collagen triple helix. Curr Opin Chem Biol. (2019) 53:98–105. 10.1016/j.cbpa.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 12.Aito-Inoue M, Lackeyram D, Fan MZ, Sato K, Mine Y. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J Pept Sci. (2007) 13:468–74. 10.1002/psc.870 [DOI] [PubMed] [Google Scholar]
- 13.Yazaki M, Ito Y, Yamada M, Goulas S, Teramoto S, Nakaya MA, et al. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J Agric Food Chem. (2017) 65:2315–22. 10.1021/acs.jafc.6b05679 [DOI] [PubMed] [Google Scholar]
- 14.Sontakke SB, Jung JH, Piao Z, Chung HJ. Orally available collagen tripeptide: enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J Agric Food Chem. (2016) 64:7127–33. 10.1021/acs.jafc.6b02955 [DOI] [PubMed] [Google Scholar]
- 15.Kim DU, Chung HC, Choi J, Sakai Y, Lee BY. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: a randomized, double-blind, placebo-controlled study. Nutrients. (2018) 10:826. 10.3390/nu10070826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AH, Kim YS, Zhe P, Shin YC, Ha MW. Cutaneous hydration effect of collagen hydrolysate containing collagen tripeptides. Korean J Food Sci Technol. (2018) 50:420–9. [Google Scholar]
- 17.Woo MJ, Song YO, Kang KH, Noh JS. Anti-obesity effects of collagen peptide derived from skate (raja kenojei) skin through regulation of lipid metabolism. Mar Drugs. (2018) 16:306–11. 10.3390/md16090306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Kim OK, Yoon HG, Park J, You Y, Kim K, et al. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr Res. (2016) 60:30428. 10.3402/fnr.v60.30428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. (2008) 22:659–61. 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 20.Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. (2014) 27:47–55. 10.1159/000351376 [DOI] [PubMed] [Google Scholar]
- 21.Choi SY, Ko EJ, Lee YH, Kim BG, Shin HJ, Seo DB, et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J Cosmet Laser Ther. (2014) 16:132–7. 10.3109/14764172.2013.854119 [DOI] [PubMed] [Google Scholar]
- 22.Inoue N, Sugihara F, Wang X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J Sci Food Agric. (2016) 96:4077–81. 10.1002/jsfa.7606 [DOI] [PubMed] [Google Scholar]
- 23.Alexander H, Brown S, Danby S, Flohr C. Research techniques made simple: transepidermal water loss measurement as a research tool. J Invest Dermatol. (2018) 138:2295–300. 10.1016/j.jid.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 24.Constantin MM, Poenaru E, Poenaru C, Constantin T. Skin hydration assessment through modern non-invasive bioengineering technologies. Maedica (Buchar). (2014) 9:33–38. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Yang H, Kim S, Kim M, Kang H, Kim N, et al. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int J Cosmet Sci. (2016) 38:375–81. 10.1111/ics.12299 [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. (2010) 8:18 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misery L, Myon E, Martin N, Consoli S, Boussetta S, Nocera T, et al. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol Venereol. (2017) 21:620–8. 10.1111/j.1468-3083.2006.02027.x [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Park JW, Yeon Y, Han JY, Kim E. Influence of exposure to summer environments on skin properties. J Eur Acad Dermatol Venereol. (2019) 33:2192–6. 10.1111/jdv.15745 [DOI] [PubMed] [Google Scholar]
- 29.Kim EO. Factors related the skin health practices among middle-aged women. Kor J Aesthet Cosmetol. (2006) 4:71–87. [Google Scholar]
- 30.Rhie SJ, Kim YC. A study on the oxidative damage induced by UVB irradiation to mouse skin. J Environ Toxicol. (2006) 21:165–182. [Google Scholar]
- 31.Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. (2016) 30:223–49. 10.1111/jdv.13301 [DOI] [PubMed] [Google Scholar]
- 32.Irrera N, Pizzino G, D'Anna R, Vaccaro M, Arcoraci V, Squadrito F, et al. Dietary management of skin health: the role of genistein. Nutrients. (2017) 9:622. 10.3390/nu9060622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twigg J, Majima S. Consumption and the constitution of age: expenditure patterns on clothing, hair and cosmetics among post-war “baby boomers”. J Aging Stud. (2014) 30:23–32. 10.1016/j.jaging.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 34.Ohara H, Ichikawa S, Matsumoto H, Akiyama M, Fujimoto N, Kobayashi T, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. (2010) 37:330–8. 10.1111/j.1346-8138.2010.00827.x [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int J Food Sci Nutr. (2010) 61:52–60. 10.3109/09637480903257711 [DOI] [PubMed] [Google Scholar]
- 36.Ito N, Seki S, Ueda F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels—a randomized, double-blind, placebo-controlled trial. Mar Drugs. (2018) 16:482. 10.3390/md16120482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuyama K, Miyama K, Mizuno K, Bächinger HP. Crystal structure of (Gly-Pro-Hyp)(9) : implications for the collagen molecular model. Biopolymers. (2012) 97:607–16. 10.1002/bip.22048 [DOI] [PubMed] [Google Scholar]
- 38.Shigemura Y, Akaba S, Kawashima E, Park EY, Nakamura Y, Sato K. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem. (2011) 129:1019–24. 10.1016/j.foodchem.2011.05.066 [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi T, Nanbu NP, Kurokawa M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol Pharm Bull. (2012) 35:422–7. 10.1248/bpb.35.422 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Jang HL, Ahn DK, Kim HJ, Jeon HY, Seo DB, et al. Orally administered collagen peptide protects against UVB-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp. Biosci Biotechnol Biochem. (2019) 83:1146–56. 10.1080/09168451.2019.1580559 [DOI] [PubMed] [Google Scholar]
- 41.Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C, et al. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. (2009) 57:444–9. 10.1021/jf802785h [DOI] [PubMed] [Google Scholar]
- 42.León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen-sources and applications. Molecules. (2019) 24:4031. 10.3390/molecules24224031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borumand M, Sibilla S. Daily consumption of the collagen supplement Pure Gold Collagen ® reduces visible signs of aging. Clin Interv Aging. (2014) 9:1747–58. 10.2147/CIA.S65939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borumand M, Sibilla S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J Med Nutr Nutraceutic. (2015) 4:47–53. 10.4103/2278-019X.146161 [DOI] [Google Scholar]
- 45.Proksch E, Schunck M, Zague V, Segger D, Degwert J, Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol Physiol. (2014) 27:113–9. 10.1159/000355523 [DOI] [PubMed] [Google Scholar]
- 46.Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, et al. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. (2007) 23:155–62. 10.1111/j.1600-0781.2007.00298.x [DOI] [PubMed] [Google Scholar]
- 47.Lademann J, Patzelt A, Schanzer S, Richter H, Meinke MC, Sterry W, et al. Uptake of antioxidants by natural nutrition and supplementation: pros and cons from the dermatological point of view. Skin Pharmacol Physiol. (2011) 24:269–73. 10.1159/000328725 [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Wang YM, Chi CF, Luo HY, Deng SG, Ma JY. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar Drugs. (2013) 11:4641–61. 10.3390/md11114641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. (2007) 55:1532–5. 10.1021/jf062834s [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.