Abstract
Objectives
We investigated the performance of soluble TNF Receptor 2 (sTNFR2) as a biomarker of renal activity, damage, treatment response, and long-term outcome in lupus nephritis (LN).
Methods
Serum sTNFR2 levels were assessed in 64 LN patients (52 proliferative, 12 membranous) before and after induction treatment, and in 314 non-lupus controls. In LN patients, renal biopsies were performed at baseline and post-treatment. Patients with ≥50% reduced proteinuria, normal or improved estimated glomerular filtration rate (eGFR) by ≥25% and inactive urinary sediment were considered clinical responders (CR). Patients with ≥50% improved renal Activity Index were considered histopathological responders (HR). Long-term renal outcome was determined using the Chronic Kidney Disease (CKD) stage after a median follow-up of 11.3 years.
Results
sTNFR2 levels were elevated in LN patients versus controls both at baseline (p<0.001) and post-treatment (p<0.001), and decreased following treatment (p<0.001). Baseline sTNFR2 correlated with Chronicity Index scores both in baseline (r=0.34, p=0.006) and post-treatment (r=0.43, p<0.001) biopsies. In membranous LN, baseline sTNFR2 levels were higher in CR (p=0.048) and HR (p=0.03) versus non-responders, and decreased only in CR (p=0.03). Both baseline (p=0.02) and post-treatment (p=0.03) sTNFR2 levels were associated with decreasing eGFR through long-term follow-up, and post-treatment levels were higher in patients with a long-term follow-up CKD stage ≥3 versus 1–2 (p=0.008).
Conclusions
Our data suggest serum sTNFR2 as a marker of kidney tissue damage and a predictor of long-term prognosis in LN, and merit further evaluation of sTNFR2 as a predictor of clinical and histopathological treatment outcomes in membranous LN.
Lupus nephritis (LN) affects a significant fraction of patients with systemic lupus erythematosus (SLE) and is a substantial cause of morbidity [1]. Renal biopsies remain the gold standard for the diagnosis and classification of LN, though reliable biomarkers for tracking renal disease activity and predicting treatment outcome are needed in order to improve the management and prognosis of LN.
Tumor Necrosis Factor α (TNF-α) is a multifunctional cytokine with a pivotal role in immune responses and autoimmunity [2]. Its biologic functions are mediated by binding to two cell surface receptors: (i) TNF receptor 1 (TNFR1), also known as TNFRSF1A, CD120a, and p55, and (ii) TNF receptor 2 (TNFR2), also known as TNFRSF1B, CD120b, and p75 [3].
Accumulating evidence indicates the involvement of TNFRs in kidney diseases [4–10], and in SLE [11–21]. In patients with diabetes, high soluble (s)TNFR levels predicted progression of chronic kidney disease (CKD) and development of end-stage renal disease (ESRD) [5, 6], and were associated with progression of albuminuria [9] and renal function deterioration [8]. In other cohorts, sTNFR levels correlated with renal function and albuminuria even in the absence of diabetes [7]. In immunoglobulin (Ig)A nephropathy, elevated sTNFR levels were associated with the severity of renal interstitial fibrosis [10]. Moreover, high sTNFR levels at initial diagnosis of idiopathic membranous nephropathy predicted poor renal outcome [22].
Although TNFR1 and TNFR2 are strongly correlated to each other, they have distinct roles in immune responses, apoptosis, and inflammatory renal injury [4, 23]. TNFR2 is expressed on cells within specific lymphocyte populations, including T-regulatory cells (Tregs) [24, 25], and has an important role in apoptotic cell death [26] and in thymocyte and cytotoxic T-cell proliferation [27, 28]. sTNFR2 is formed by proteolytic cleavage of its membrane-bound counterpart.
Genetic loci associated with SLE include loci encoding TNFR2 [20], and experiments have demonstrated associations of TNFR2 polymorphisms with SLE [12, 13, 16]. In SLE, sTNFR2 levels were higher in patients than in healthy controls [11, 15] and during active disease or prior to flare than during inactive disease [11, 17], and they correlated with disease activity, renal involvement and cardiovascular comorbidities [15, 18, 19].
In LN, sTNFR2 levels were elevated before treatment, and decreased six months after treatment [11]. In recent reports, sTNFR2 levels differentiated patients with active LN from patients with active non-renal or inactive SLE [29], and correlated strongly with renal function, as well as with activity and chronicity features in renal biopsies [30]. In this study, we aimed to further investigate the performance of sTNFR2 as a marker of renal activity and damage, and also as a predictor of response to treatment and long-term renal outcome in LN.
Material and methods
Sixty-four patients from the Karolinska SLE cohort were enrolled on the occasion of an active biopsy-proven LN between 1996 and 2011 and were followed prospectively. The 1982 revised criteria [31] and the Systemic Lupus International Collaborating Clinics criteria [32] for classification of SLE were met in all patients. Population-based individuals without SLE prior to enrolment (n=314) were recruited as controls for comparisons. These individuals were free from kidney diseases, except for one who was diagnosed with IgA nephropathy and one who had polycystic kidney disease. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics
| LN patients (n=64) | Controls (n=314) | |
|---|---|---|
| Age (years); M (R) | 31.7 (18.8–60.7) | 47.7 (18.0–84.5) |
| Sex; Female; n (%) | 55 (86%) | 289 (92%) |
| Ethnicity | ||
| Caucasian; n (%) | 56 (87.5%) | 307 (97.8%) |
| Asian; n (%) | 3 (4.7%) | 2 (0.006%) |
| Hispanic; n (%) | 3 (4.7%) | 4 (0.01%) |
| African; n (%) | 2 (3.1%) | 1 (0.003%) |
| SLE disease duration (years); M (R) | 3.7 (0–35.6) | - |
| Prednisone equivalent (mg/d); M (R) | 8.8 (0–60) | - |
| Antimalarials; n (%) | 16 (25%) | - |
| Immunosuppressants; n (%) | 22 (34.4%) | - |
| Azathioprine; n (%) | 14 (21.9%) | - |
| Methotrexate; n (%) | 4 (6.3%) | - |
| Mycophenolate mofetil; n (%) | 3 (4.7%) | - |
| Oral cyclophosphamide; n (%) | 1 (1.6%) | - |
| ACE inhibitors and/or ARBs; n (%) | 50 (78.1%) | - |
| Time between biopsies (months); M (R) | 7.7 (5.0–15.6) | - |
SLE: systemic lupus erythematosus; LN: lupus nephritis; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker (angiotensin II receptor antagonist); M: median; R: range.
Patients received induction treatment with corticosteroids combined with cyclophosphamide (CYC, n=45), mycophenolate mofetil (MMF, n=11), rituximab (RTX, n=7), or azathioprine (AZA, n=1). For evaluation of treatment response, a post-treatment follow-up was conducted after a median time of 7.7 months (range: 5.0–15.6), including post-treatment renal biopsies (n=63).
Evaluation of sTNFR2 levels
Serum from patients was collected before starting (baseline) and after completion of induction treatment, and from controls at the time of recruitment, and was stored at −80°C until analysis. Serum levels of sTNFR2 were determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits from R&D Systems, Inc., Minneapolis, Minnesota, USA (Cat # DY726). Serum samples were diluted 1:100. All assays were undertaken according to the manufacturer’s protocol. Optical density at a wavelength of 450 nm was measured using an ELx808TM Absorbance Microplate Reader from BioTek Instruments, Inc., Winooski, Vermont, USA, and the concentrations of the samples were calculated using a standard curve. All samples were analysed in duplicate and all experiments were performed in a blinded manner. The mean coefficient of variation of the assays was 5.95% (range: 4.69–6.68%).
Determination of autoantibody and complement component levels
Serum levels of antibodies to double-stranded DNA (anti-dsDNA; reference values <5 IU/mL) were measured by multiplex immunoassay analysis on a BioPlex® 2200 System from Bio-Rad Laboratories, Inc., Hercules, California, USA. Levels of antibodies to complement component 1q (anti-C1q; reference values <14 U/mL) were determined using ELISA kits from Alegria, ORGENTEC Diagnostika GmbH, Germany.
Levels of complement component 3 (C3; reference range 0.67–1.29 g/L) and complement component 4 (C4; reference range 0.13–0.32 g/L) were determined by conventional nephelometry.
Monitoring methods and definitions
Global disease activity was assessed using the SLE Disease Activity Index 2000 (SLEDAI-2K) [33]. Urinary status was evaluated by urine test strips and urinary sediment. Proteinuria was estimated using the 24-hour urine albumin excretion (g/day). Renal function was assessed using plasma creatinine concentration (μmol/L) and the estimated glomerular filtration rate (eGFR), as determined by the Modification of Diet in Renal Disease (MDRD) Study equation [34].
Renal biopsies were evaluated using light, immunofluorescence and electron microscopy. The International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification system for LN [35] was used to classify the LN subsets. Renal activity and damage were assessed using Activity Index (AI) and Chronicity Index (CI) [36], respectively.
According to the American College of Rheumatology response criteria [37], reduced proteinuria by ≥50% to levels ≤2 g/day, normal or improved eGFR by ≥25%, and an inactive urinary sediment signified clinical responders (CR); all other cases were considered clinical non-responders (CNR). Patients showing improvement in their AI score by ≥50% were considered histopathological responders (HR); all other cases were considered histopathological non-responders (HNR) [38].
Patients were followed longitudinally for a median time of 11.3 years (range: 3.3–18.8) from baseline. Long-term renal outcome was assessed according to the last eGFR and the last CKD stage, as defined by the updated guidelines of the Kidney Disease Outcomes Quality Initiative by the National Kidney Foundation [39–41].
Statistics
For comparisons between baseline and post-treatment, we used the non-parametric Wilcoxon signed-rank test. Comparisons between independent samples were made using the Mann-Whitney U test. In order to assess the performance of baseline sTNFR2 levels as a predictor of treatment response and long-term renal outcome, receiver operating characteristic (ROC)-curves were constructed. Correlation analyses were performed using the Spearman’s rank correlation coefficient. Autoantibody levels were bounded by the detection limits of the assays. Prior to analysis, censored values were set to half the lower or twice the upper detection limit.
To investigate the role of serum sTNFR2 in long-term renal outcome, as well as in renal activity, renal damage, and global disease activity, linear mixed models for repeated measures were used. Separate models were built for each outcome of interest (AI, CI, eGFR, proteinuria, and SLEDAI-2K). Each one of these outcomes was separately included as the dependent variable in a linear mixed model, with patient visits as repeated and fixed effects, sTNFR2 levels as a covariate, and patients as a random effect. For the long-term renal outcome, the model was adjusted for the total observation time in years.
All tests were bilateral and p-values <0.05 were considered statistically significant. The statistical analyses were performed using the IBM SPSS Statistics 23 software (IBM Corp., Armonk, New York, USA).
Ethics and consent
Written informed consent was obtained prior to recruitment from all individuals participating in the study. The study protocol was reviewed and approved by the regional ethics review board at Karolinska Institutet, Stockholm, Sweden.
Results
In LN patients, the median sTNFR2 level was 9.8 ng/mL (range 3.4–41.5 ng/mL) at baseline and 6.0 ng/mL (range 2.0–18.8 ng/mL) post-treatment (Table 2). In controls, the median sTNFR2 was 3.6 ng/mL (range 1.6–66.0 ng/mL).
Table 2.
Comparisons between baseline and post-treatment outcomes
| Baseline | Post-treatment | p-value | |
|---|---|---|---|
| ISN/RPS class | |||
| I; II (+V); n | 0; 0 | 1; 15 (1) | - |
| III A (+V); III A/C (+V); III C (+V); n | 10 (3); 5 (2); 0 | 0; 9 (1); 8 (2) | - |
| IV S A (+V); IV S A/C (+V); IV S C (+V); n | 4; 3 (1); 0 | 0; 0; 0 | - |
| IV G A (+V); IV G A/C (+V); IV G C (+V); n | 9 (3); 11 (1); 0 | 2; 5 (1); 2 | - |
| V; n | 12 | 15 | - |
| Glomerular vasculitis; n | 0 | 1 | - |
| Activity Index; M (R) | 5 (0–13), n=64 | 2 (0–12), n=63 | <0.001 ↓ |
| Chronicity Index; M (R) | 1 (0–6), n=64 | 2 (0–8), n=63 | <0.001 ↑ |
| SLEDAI-2K; M (R); n=64 | 16 (6–28) | 4 (0–23) | <0.001 ↓ |
| PLN cases; M (R); n=52 | 16 (6–28) | 4 (0–20) | <0.001 ↓ |
| MLN cases; M (R); n=12 | 11.5 (10–23) | 7 (2–23) | 0.017 ↓ |
| sTNFR2 levels (ng/mL); M (R); n=64 | 9.8 (3.4–41.5) | 6.0 (2.0–18.8) | p<0.001 ↓ |
| PLN cases; M (R); n=52 | 9.8 (3.4–41.5) | 5.4 (2.0–12.2) | p<0.001 ↓ |
| MLN cases; M (R); n=12 | 9.0 (4.3–25.2) | 8.9 (2.0–18.8) | p=0.182 |
| anti-dsDNA (IU/mL); positive cases; M | 59 (94%), n=63; 110 | 48 (79%), n=61; 20 | <0.001 ↓ |
| Positive PLN cases; M | 49 (96%), n=51; 200 | 41 (84%), n=49; 26 | <0.001 ↓ |
| Positive MLN cases; M | 10 (83%), n=12; 21 | 7 (58%), n=12; 10.5 | 0.33 |
| anti-C1q (U/mL); positive cases; M | 46 (73%), n=63; 37.2 | 30 (47%), n=64; 12.8 | <0.001 ↓ |
| Positive PLN cases; M | 38 (75%), n=51; 45.4 | 26 (50%), n=52; 13.7 | <0.001 ↓ |
| Positive MLN cases; M | 8 (67%), n=12; 21.2 | 4 (33%), n=12; 9.9 | 0.060 |
| 24-h U-albumin (g/d); M (R) | 1.5 (0.04–8.4), n=63 | 0.3 (0–4.8), n=64 | <0.001 ↓ |
| P-creatinine (μmol/L); M (R) | 81 (46–284), n=64 | 76 (40–306), n=64 | 0.009 ↓ |
| eGFR (mL/min/1.73 m2); M (R) | 75 (17–138), n=64 | 81 (20–140), n=64 | 0.043 ↑ |
| C3 (g/L); M (R) | 0.54 (0.2–1.13), n=60 | 0.8 (0.36–1.51), n=61 | <0.001 ↑ |
| C4 (g/L); M (R) | 0.1 (0.02–0.51), n=60 | 0.13 (0.02–0.45), n=61 | <0.001 ↑ |
The lower and upper limits of the assay used for anti-dsDNA counts were 5 IU/mL and 300 IU/mL, respectively. The upper limit of the assay used for estimating anti-C1q levels was 100 U/mL. Cases with anti-dsDNA titer <5 IU/mL were regarded as negative. Cases with anti-C1q titer <14 U/mL were regarded as negative. Statistically significant p-values are in bold. Upward arrows (↑) signify significant increases following treatment. Downward arrows (↓) signify significant decreases following treatment.
ISN/RPS: International Society of Nephrology/Renal Pathology Society; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000 [33]; sTNFR2: soluble Tumor Necrosis Factor Receptor 2; anti-dsDNA: antibodies to double-stranded DNA; anti-C1q: antibodies to complement component 1q; PLN: proliferative lupus nephritis; MLN: membranous lupus nephritis; M: median; R: range.
Results from the evaluation of the renal biopsies, SLEDAI-2K scores, autoantibody levels, proteinuria, creatinine values and eGFR are presented in Table 2. According to baseline biopsies, 52 cases were classified as proliferative LN (PLN; ISN/RPS class III/IV±V), and 12 cases as membranous LN (MLN; ISN/RPS class V).
Serum sTNFR2 levels were elevated in LN patients compared with controls, both at baseline (p<0.001) and post-treatment (p<0.001). Baseline sTNFR2 levels did not differ between the PLN and MLN patient subgroups (p=0.49). Following induction treatment, significant reductions of sTNFR2 levels were observed within the entire patient cohort (p<0.001) and in the PLN subgroup (p<0.001), but not in MLN patients (p=0.18) (Table 2).
Serum sTNFR2 as a biomarker of renal damage
Baseline serum sTNFR2 levels correlated with CI scores in both baseline (r=0.34, p=0.006) and post-treatment (r=0.43, p<0.001) biopsies, and post-treatment sTNFR2 levels correlated with post-treatment CI scores (r=0.55, p<0.001). We also found that post-treatment, but not baseline, sTNFR2 levels correlated with post-treatment AI scores (r=0.28, p=0.03) and post-treatment proteinuria (r=0.42, p=0.001). No correlations were observed between baseline or post-treatment sTNFR2 and SLEDAI-2K, eGFR, C3 or C4 levels, prednisone equivalent dosages, anti-dsDNA, anti-C1q, or age (p=NS). Further, baseline serum sTNFR2 levels were associated with increasing CI scores in renal biopsies following treatment (p=0.003). No statistically significant association was found between baseline sTNFR2 levels and alterations in AI (p=0.26), eGFR (p=0.07), proteinuria (p=0.07), or SLEDAI-2K (p=0.90) following treatment.
Serum sTNFR2 as a biomarker of treatment response
Results from the assessment of clinical and histopathological outcomes following induction treatment are shown in Table 3. Serum levels of sTNFR2 decreased following treatment in both responders (clinical and histopathological) and non-responders (clinical and histopathological) in the combined patient cohort, and in the PLN subgroup. In the MLN subgroup, sTNFR2 levels decreased in clinical responders, but not in clinical non-responders (Table 3).
Table 3.
Comparisons between baseline and post-treatment serum sTNFR2 levels
| Baseline | Post-treatment | p-value | |
|---|---|---|---|
| All patients, n=64 | |||
| Clinical responders; M (R); n=48 | 9.9 (3.4–35.2) | 5.6 (2.0–12.2) | p<0.001 ↓ |
| Clinical non-responders; M (R); n=16 | 8.5 (4.3–41.5) | 7.0 (2.0–18.8) | p=0.049 ↓ |
| Histopathological responders; M (R); n=49 | 9.9 (3.4–41.5) | 5.7 (2.3–12.2) | p<0.001 ↓ |
| Histopathological non-responders; M (R); n=14 | 8.0 (4.3–20.0) | 6.0 (2.0–10.8) | p=0.008 ↓ |
| Proliferative lupus nephritis, n=52 | |||
| Clinical responders; M (R); n=41 | 9.8 (3.4–35.2) | 5.3 (2.3–12.2) | p<0.001 ↓ |
| Clinical non-responders; M (R); n=11 | 9.2 (4.6–41.5) | 6.6 (2.0–10.8) | p=0.008 ↓ |
| Histopathological responders; M (R); n=43 | 9.8 (3.4–41.5) | 5.2 (2.3–12.2) | p<0.001 ↓ |
| Histopathological non-responders; M (R); n=9 | 8.2 (4.7–20.0) | 7.3 (2.0–10.8) | p=0.021 ↓ |
| Membranous lupus nephritis, n=12 | |||
| Clinical responders; M (R); n=7 | 11.6 (4.7–25.2) | 9.1 (2.0–11.8) | p=0.028 ↓ |
| Clinical non-responders; M (R); n=5 | 6.0 (4.3–10.4) | 8.6 (4.0–18.8) | p=0.225 |
| Histopathological responders; M (R); n=6 | 14.6 (6.0–25.2) | 9.8 (6.8–11.8) | p=0.116 |
| Histopathological non-responders; M (R); n=5 | 4.7 (4.3–10.4) | 4.1 (2.0–9.9) | p=0.225 |
Comparisons between baseline and post-treatment serum sTNFR2 levels (ng/mL) in the entire patient cohort, in the proliferative lupus nephritis patient subgroup and in the membranous lupus nephritis patient subgroup, according to clinical and histopathological response to induction treatment. Statistically significant p-values are in bold. Downward arrows (↓) signify significant decreases following treatment. sTNFR2: soluble Tumor Necrosis Factor Receptor 2; M: median; R: range.
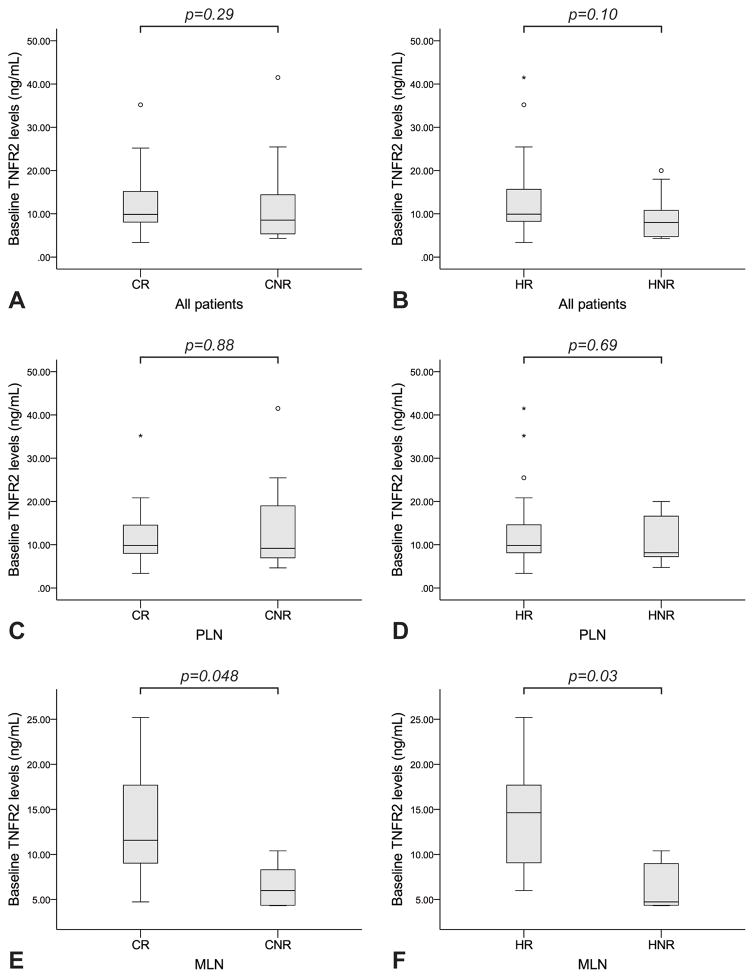
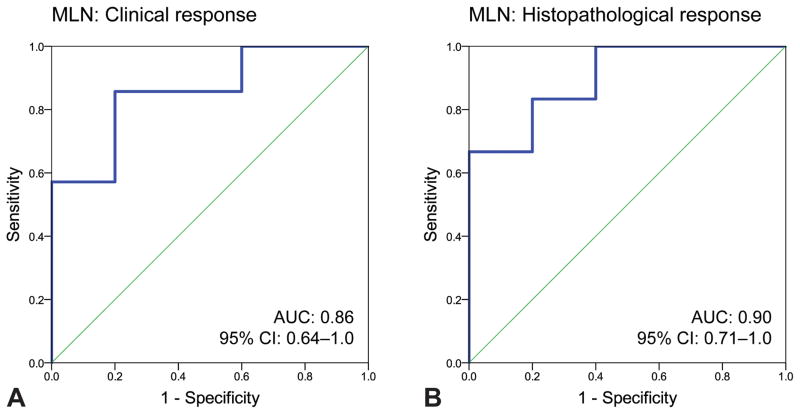
Baseline serum sTNFR2 levels did not differ between clinical responders (CR) and clinical non-responders (CNR) or between histopathological responders (HR) and histopathological non-responders (HNR) in the entire cohort (p=0.29 and p=0.10, respectively), or in the PLN subgroup (p=0.88 and p=0.69, respectively). On the contrary, in the MLN subgroup baseline sTNFR2 levels were higher in CR versus CNR (p=0.048), as well as in HR versus HNR (p=0.03) (Table 3, Figure 1). According to ROC-curve analysis, baseline sTNFR2 levels distinguished CR from CNR in the MLN subgroup (area under the curve, AUC: 0.86 (95% confidence interval, CI: 0.64–1.0), p=0.04), with a level of 8.6 ng/mL yielding a sensitivity of 85.7% and a specificity of 80.0%. Similarly, baseline sTNFR2 levels distinguished HR from HNR in MLN (AUC: 0.90 (95% CI: 0.71–1.0), p=0.03), with a level of 9.0 ng/mL yielding a sensitivity of 83.3% and a specificity of 80.0% (Figure 2).
Figure 1. Serum sTNFR2 as a predictor of response to induction treatment.
Baseline serum sTNFR2 levels did not differ between CR and CNR or HR and HNR in the entire cohort (A, B), or in the PLN subgroup (C, D). On the contrary, in the MLN subgroup baseline sTNFR2 levels were significantly higher both in CR versus CNR (E) and HR versus HNR (F).
Bounds of the boxes denote the 25th and 75th percentiles (IQR). Lines in the boxes denote the 50th percentile (median). Whiskers denote range. Circles (out values, 1.5–3 IQRs further from the closest box bound) and stars (far out or extreme values, ≥3 IQRs further from the closest box bound) denote outliers.
sTNFR2: soluble Tumor Necrosis Factor Receptor 2; CR: clinical responders; CNR: clinical non-responders; HR: histopathological responders; HNR: histopathological non-responders; PLN: proliferative lupus nephritis; MLN: membranous lupus nephritis; IQR: interquartile range.
Figure 2. Serum sTNFR2 as a predictor of response to induction treatment in MLN.
ROC-curves for baseline serum sTNFR2 levels by clinical (A) and histopathological (B) response following induction treatment in patients with MLN. Baseline sTNFR2 levels distinguished between CR and CNR (AUC: 0.86 (95% CI: 0.64–1.0), p=0.04), with a level of 8.6 ng/mL yielding a sensitivity of 85.7% and a specificity of 80.0%. Similarly, baseline sTNFR2 levels distinguished between HR and HNR (AUC: 0.90 (95% CI: 0.71–1.0), p=0.03), with a level of 9.0 ng/mL yielding a sensitivity of 83.3% and a specificity of 80.0%.
sTNFR2: soluble Tumor Necrosis Factor Receptor 2; ROC: receiver operating characteristics; AUC: area under the curve; CI: confidence interval; MLN: membranous lupus nephritis; CR: clinical responders; CNR: clinical non-responders; HR: histopathological responders; HNR: histopathological non-responders.
Serum sTNFR2 as a biomarker of long-term renal outcome in LN
At the last follow-up, the median eGFR was 80 mL/min/1.73 m2 (range 17–149), and patients were stratified into CKD stage 1 (n=22), stage 2 (n=26), stage 3 (n=12), and stage 4 (n=3). No patient had developed ESRD (CKD stage 5). One patient was lost to follow-up. Overall, there was no difference between eGFR at the last follow-up and either baseline (p=0.79) or post-treatment (p=0.21) eGFR.
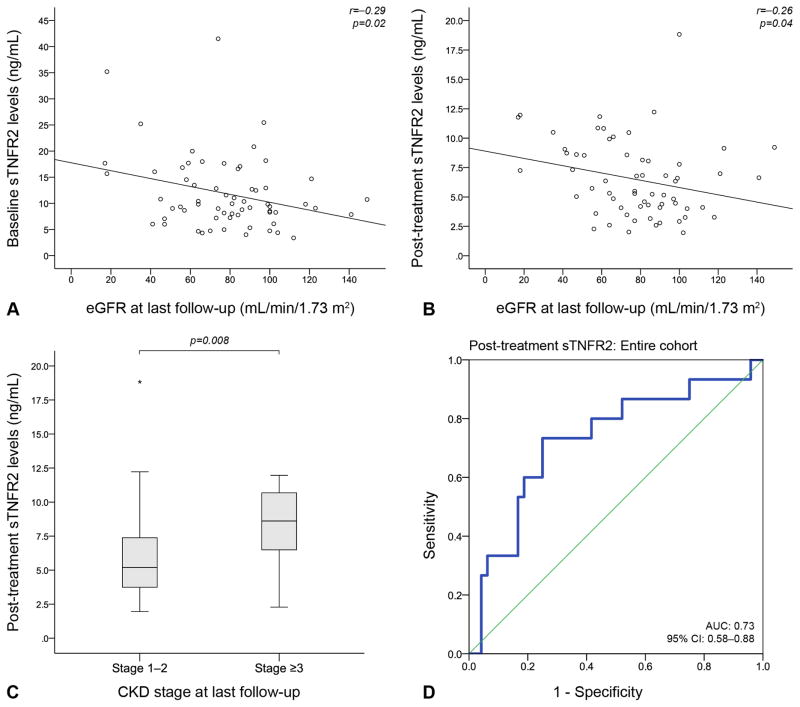
Long-term follow-up eGFR correlated inversely with both baseline (r=−0.29, p=0.02) and post-treatment (r=−0.26, p=0.04) sTNFR2 levels (Figure 3). Baseline sTNFR2 levels were associated with decreases in eGFR from baseline through the last follow-up (p=0.02); this association remained significant after adjustment for the follow-up duration estimated in years (p=0.046). Similarly, post-treatment sTNFR2 levels were associated with decreases in eGFR from post-treatment through the last follow-up, before (p=0.03) and after adjustment for follow-up duration (p=0.01).
Figure 3. Serum sTNFR2 as a predictor of long-term renal outcome.
Long-term follow-up eGFR correlated inversely with both baseline (A) and post-treatment (B) serum sTNFR2 levels. Post-treatment sTNFR2 levels were higher in LN patients with a CKD stage ≥3 at the last follow-up compared with patients with a CKD stage 1–2 (C). Post-treatment sTNFR2 levels could distinguish between patients with CKD stage 1–2 and ≥3 at the last follow-up (D), with a level of 7.1 ng/mL yielding a sensitivity of 73.3% and a specificity of 75.0%.
Bounds of the boxes denote the 25th and 75th percentiles (IQR). Lines in the boxes denote the 50th percentile (median). Whiskers denote range. Stars (far out or extreme values, ≥3 IQRs further from the closest box bound) denote outliers.
sTNFR2: soluble Tumor Necrosis Factor Receptor 2; eGFR: estimated glomerular filtration rate; CKD: chronic kidney disease; IQR: interquartile range.
Baseline sTNFR2 levels did not differ between LN patients with a CKD stage 1–2 and patients with a CKD stage ≥3 at the last follow-up (p=0.13). In contrast, post-treatment sTNFR2 levels were higher in LN patients with a long-term follow-up CKD stage ≥3 (median: 8.6 ng/mL; range: 2.28–11.96) compared with patients with a CKD stage 1–2 (median: 5.2 ng/mL; range: 1.95–18.83; p=0.008). Further, ROC-curve analysis showed that post-treatment sTNFR2 levels could distinguish between patients with a long-term follow-up CKD stage 1–2 versus ≥3 (AUC: 0.73 (95% CI: 0.58–0.88), p=0.008), with a level of 7.1 ng/mL yielding a sensitivity of 73.3% and a specificity of 75.0% (Figure 3).
Discussion
We investigated the performance of sTNFR2 as a biomarker of renal activity and damage, treatment response, and long-term outcome in LN. Serum levels of sTNFR2 decreased following induction therapy. In the PLN subset, sTNFR2 levels decreased regardless of the treatment outcome. In contrast, baseline sTNFR2 levels in MLN patients decreased only in CR, and high baseline levels were predictive of treatment response. Further, sTNFR2 levels correlated with CI scores in renal biopsies, and were also associated with long-term eGFR deterioration. Taken together, our results suggest sTNFR2 as a predictor of damage accrual and long-term prognosis in LN, and also as a potential marker of treatment response in MLN.
TNFRs have been demonstrated to be of importance in SLE [12, 13, 16, 20], but studies of their role in LN are limited. Circulating levels of sTNFR1 and sTNFR2 are usually strongly correlated both with each other and with TNF-α [19, 42]. Nevertheless, TNFR1 and TNFR2 have distinct roles in immune responses in general and in kidney diseases in particular [4, 23]. TNF-α and TNFR2 are usually absent in healthy renal tissue, whereas TNFR1 is found in glomerular endothelial cells, primarily localised within the Golgi apparatus [43]. During inflammation, however, TNF-α, TNFR1 and TNFR2 are expressed both in glomerular and in tubular cells [43, 44]. In mice subjected to immune complex-mediated glomerulonephritis, renal expression of TNFR2, but not TNFR1, was essential for glomerular complement deposition and the development of proteinuria and glomerulonephritis, whereas TNFR1 had an immunosuppressive role [45]. In a recent study of SLE and LN, both sTNFR1 and sTNFR2 levels were higher in SLE patients compared with healthy controls while sTNFR2 levels were also higher in patients with active LN compared with patients with quiescent SLE [30]. Taken together with the need for more reliable biomarkers for LN, these observations warrant further investigation of TNFR2 in LN, which was the scope of this study.
A previous study found elevated levels of sTNFR2 in patients with LN, both PLN and MLN, compared with non-renal SLE patients [15]. In a recent report of juvenile-onset SLE, sTNFR2 levels correlated negatively with eGFR and positively with the urinary albumin:creatitine ratio [42]. In accordance with another report of 13 patients with LN [11], we observed significant reductions of sTNFR2 levels following induction treatment for LN. However, we found no association between sTNFR2 and global SLE disease activity, suggesting a particular role of TNFR2 in LN. Indeed, the increased serum sTNFR2 levels during active LN may reflect increased expression of TNFR2 in the kidney. Of note, in a previous study of 113 patients with idiopathic membranous nephropathy [22] TNFR2 was predominantly expressed in tubules, and only rarely in glomeruli, and its expression was higher in patients with high versus low sTNFR2 levels.
In the combined patient cohort and in the PLN subgroup, sTNFR2 levels decreased regardless of the treatment outcome. In contrast, in the MLN subgroup sTNFR2 levels decreased only in CR, and higher baseline levels predicted both clinical and histopathological response with similar optimal threshold values according to ROC-curve analysis. Although validation is needed considering the low number of patients in the MLN subgroup, our results indicate a different role of TNFR2 in PLN and MLN, and endorse the prevailing hypothesis that these two LN subsets are driven by different pathogenic mechanisms.
A more striking finding was that sTNFR2 correlated with renal damage both at baseline and post-treatment, and was significantly associated with changes in Chronicity Index scores in renal biopsies. Consistently, sTNFR2 correlated with renal function at the last follow-up, and post-treatment sTNFR2 levels were higher in LN patients with a long-term follow-up CKD stage ≥3 versus stage 1–2. Taken together, these findings suggest that sTNFR2 levels may mirror chronic changes in the kidney tissue and portend renal damage accrual. Further, post-treatment proteinuria was recently demonstrated as a powerful predictor of the long-term renal outcome in LN [46, 47]. In the light of this, our finding that sTNFR2 correlated with proteinuria post-treatment also support the notion that sTNFR2 levels may be a useful predictor of the long-term renal outcome in LN. This is consistent with previous demonstrations that high sTNFR2 levels at the time of diagnosis of idiopathic membranous nephropathy were associated with renal function deterioration over time [22].
The associations of high sTNFR2 levels with renal damage and poor long-term renal outcome, together with the observation that higher baseline levels predicted good response to treatment in MLN, constitute a paradox, since responding patients may be expected to have a better long-term prognosis compared with non-responders. A possible explanation might be that patients with high baseline sTNFR2 levels represented a LN subset with a more severe disease phenotype, in which induction therapy was efficacious in reducing renal disease activity, but failed to prevent damage accrual in the long-term.
Whether the observed association between sTNFR2 levels and long-term follow-up CKD stage reflected an accumulation of sTNFR2 due to glomerular hypofiltration or renal TNFR2 overexpression and subsequent injury remains to be elucidated. Further, the degree of proteinuria might have influenced the estimated circulating sTNFR2 levels due to clearance in urinary losses. However, there was no inverse correlation between sTNFR2 and proteinuria, implying that this hypothesis is rather unlikely. Unfortunately, data on TNFR2 in urinary losses were not available in our cohort. Further investigation of TNFR2 in renal tissue and urinary losses might help clarify the mechanisms underlying these observations. Among previously suggested mechanisms, monocyte chemoattractant protein-1 (MCP-1) has been shown to contribute to the recruitment of inflammatory cells and tubulointerstitial damage in LN [48, 49]. Moreover, MCP-1 expression has been demonstrated to predict poor renal prognosis in paediatric LN [50]. This was further explored in a recent study, in which TNF-α effectively stimulated podocytes to produce MCP-1 [51]. Interestingly, TNFR2 was shown to be essential for mediating this effect of TNF-α on MCP-1 production while TNFR1 did not appear to be involved [51]. Being the link between TNF-α and MCP-1 production by podocytes, and also a mediator of glomerular complement deposition [45], TNFR2 emerges as a key player in renal injury and damage. However, the causes of its overexpression in renal tissue have yet to be clarified.
Considering the observation that post-treatment sTNFR2 levels were higher in patients with poorer long-term renal outcome, modulation of the TNF pathway might be a potential option for the treatment of LN. Previously, short-term TNF-α inhibition with infliximab combined with background immunosuppression was shown to reduce proteinuria levels [52] and induce long-term remission in patients with refractory LN, but prolonged administration led to severe adverse events [53–55]. In another study, long-term therapy with etanercept, a fusion protein containing sTNFR2, in addition to background immunosuppression had a more favourable safety profile and promising long-term efficacy in patients with refractory lupus arthritis [56]. Although TNF-α inhibition remains a controversial option for SLE, alternative ways to modulate this pathway, e.g. through specific inhibition of TNFR2, as previously suggested [23], might prove useful in the future. Supportive of more targeted inhibition was also a study of SLE-prone New Zealand Mixed 2328 mice, in which double deficiency of TNFR1 and TNFR2, but not deficiency of only one receptor, was highly deleterious to the host, resulting in accelerated nephritis features [14].
To our knowledge, our LN cohort is one of the largest with post-treatment renal biopsies, allowing a more reliable evaluation of response to treatment, including histopathological outcome [57]. Despite being limited in power by the size of the patient cohort, especially regarding the MLN patient subgroup, and the use of different therapeutic regimens, our study contributes to the understanding of the role of TNFR2 in LN and merits further investigation of TNF-α and its receptors in larger LN cohorts.
Conclusions
Our observations suggest that serum sTNFR2 is a non-invasive marker of kidney tissue damage, and a predictor of long-term prognosis in LN. Our data also suggest that sTNFR2 is a potential predictor of response to treatment in patients with MLN. Further evaluation of sTNFR2 in larger LN cohorts, especially MLN, might better clarify its role, and possibly reinvigorate the potential of TNF-α pathway modulation in future therapeutic approaches.
Acknowledgments
We express our gratitude to Birgitta Sundelin for evaluating the renal biopsies, Birgitta Tavaststjerna for performing the assays for autoantibody quantifications and Eva Jemseby for managing the serum samples. We thank all participating patients and controls, as well as all contributing medical staff from the Rheumatology Unit of Karolinska University Hospital.
This work was supported by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, Swedish Heart-Lung Foundation, Foundation in memory of Clas Groschinsky, Stockholm County Council, Karolinska Institutet Foundations, and also in part by R01 DK81872 from National Institutes of Health.
References
- 1.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 2.Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 3.Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors--structure and function. Immunol Res. 1992;11:81–90. doi: 10.1007/BF02918612. [DOI] [PubMed] [Google Scholar]
- 4.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36:261–70. doi: 10.1159/000342333. [DOI] [PubMed] [Google Scholar]
- 5.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–24. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–15. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson AC, Larsson TE, Helmersson-Karlqvist J, Larsson A, Lind L, Arnlov J. Soluble TNF receptors and kidney dysfunction in the elderly. J Am Soc Nephrol. 2014;25:1313–20. doi: 10.1681/ASN.2013080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37:226–34. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G, et al. Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care. 2013;36:2317–23. doi: 10.2337/dc12-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda Y, Gohda T, Suzuki Y, Omote K, Ishizaka M, Matsuoka J, et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS One. 2015;10:e0122212. doi: 10.1371/journal.pone.0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davas EM, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis PC. Serum IL-6, TNFalpha, p55 srTNFalpha, p75srTNFalpha, srIL-2alpha levels and disease activity in systemic lupus erythematosus. Clin Rheumatol. 1999;18:17–22. doi: 10.1007/s100670050045. [DOI] [PubMed] [Google Scholar]
- 12.Horiuchi T, Kiyohara C, Tsukamoto H, Sawabe T, Furugo I, Yoshizawa S, et al. A functional M196R polymorphism of tumour necrosis factor receptor type 2 is associated with systemic lupus erythematosus: a case-control study and a meta-analysis. Ann Rheum Dis. 2007;66:320–4. doi: 10.1136/ard.2006.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horiuchi T, Washio M, Kiyohara C, Tsukamoto H, Tada Y, Asami T, et al. Combination of TNF-RII, CYP1A1 and GSTM1 polymorphisms and the risk of Japanese SLE: findings from the KYSS study. Rheumatology (Oxford) 2009;48:1045–9. doi: 10.1093/rheumatology/kep166. [DOI] [PubMed] [Google Scholar]
- 14.Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol. 2009;182:2532–41. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud RA, El-Gendi HI, Ahmed HH. Serum neopterin, tumor necrosis factor-alpha and soluble tumor necrosis factor receptor II (p75) levels and disease activity in Egyptian female patients with systemic lupus erythematosus. Clin Biochem. 2005;38:134–41. doi: 10.1016/j.clinbiochem.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Morita C, Horiuchi T, Tsukamoto H, Hatta N, Kikuchi Y, Arinobu Y, et al. Association of tumor necrosis factor receptor type II polymorphism 196R with Systemic lupus erythematosus in the Japanese: molecular and functional analysis. Arthritis Rheum. 2001;44:2819–27. doi: 10.1002/1529-0131(200112)44:12<2819::aid-art469>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol. 2014;66:1888–99. doi: 10.1002/art.38573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12:454–61. doi: 10.1191/0961203303lu412oa. [DOI] [PubMed] [Google Scholar]
- 19.Svenungsson E, Gunnarsson I, Fei GZ, Lundberg IE, Klareskog L, Frostegard J. Elevated triglycerides and low levels of high-density lipoprotein as markers of disease activity in association with up-regulation of the tumor necrosis factor alpha/tumor necrosis factor receptor system in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2533–40. doi: 10.1002/art.11264. [DOI] [PubMed] [Google Scholar]
- 20.Tsao BP. Lupus susceptibility genes on human chromosome 1. Int Rev Immunol. 2000;19:319–34. doi: 10.3109/08830180009055502. [DOI] [PubMed] [Google Scholar]
- 21.Gabay C, Cakir N, Moral F, Roux-Lombard P, Meyer O, Dayer JM, et al. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol. 1997;24:303–8. [PubMed] [Google Scholar]
- 22.Lee SM, Yang S, Cha RH, Kim M, An JN, Paik JH, et al. Circulating TNF receptors are significant prognostic biomarkers for idiopathic membranous nephropathy. PLoS One. 2014;9:e104354. doi: 10.1371/journal.pone.0104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol. 2007;27:286–308. doi: 10.1016/j.semnephrol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–87. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware CF, Crowe PD, Vanarsdale TL, Andrews JL, Grayson MH, Jerzy R, et al. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J Immunol. 1991;147:4229–38. [PubMed] [Google Scholar]
- 26.Teh HS, Seebaran A, Teh SJ. TNF receptor 2-deficient CD8 T cells are resistant to Fas/Fas ligand-induced cell death. J Immunol. 2000;165:4814–21. doi: 10.4049/jimmunol.165.9.4814. [DOI] [PubMed] [Google Scholar]
- 27.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–63. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88:9292–6. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok CC, Ding HH, Kharboutli M, Mohan C. Axl, Ferritin, Insulin-Like Growth Factor Binding Protein 2, and Tumor Necrosis Factor Receptor Type II as Biomarkers in Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2016;68:1303–9. doi: 10.1002/acr.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T, Ding H, Han J, Arriens C, Wei C, Han W, et al. Antibody-Array-Based Proteomic Screening of Serum Markers in Systemic Lupus Erythematosus: A Discovery Study. J Proteome Res. 2016;15:2102–14. doi: 10.1021/acs.jproteome.5b00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 32.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 34.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 36.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75:382–91. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 37.Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response C. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421–32. doi: 10.1002/art.21625. [DOI] [PubMed] [Google Scholar]
- 38.Parodis I, Zickert A, Sundelin B, Axelsson M, Gerhardsson J, Svenungsson E, et al. Evaluation of B lymphocyte stimulator and a proliferation inducing ligand as candidate biomarkers in lupus nephritis based on clinical and histopathological outcome following induction therapy. Lupus Sci Med. 2015;2:e000061. doi: 10.1136/lupus-2014-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 40.Levey AS. A decade after the KDOQI CKD guidelines. Am J Kidney Dis. 2012;60:683–5. doi: 10.1053/j.ajkd.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 42.Patel M, Oni L, Midgley A, Smith E, Tullus K, Marks SD, et al. Increased concentration of plasma TNFR1 and TNFR2 in paediatric lupus nephritis. Lupus. 2016;25:1040–4. doi: 10.1177/0961203316631634. [DOI] [PubMed] [Google Scholar]
- 43.Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest. 2001;81:1503–15. doi: 10.1038/labinvest.3780364. [DOI] [PubMed] [Google Scholar]
- 44.Baud L, Ardaillou R. Tumor necrosis factor in renal injury. Miner Electrolyte Metab. 1995;21:336–41. [PubMed] [Google Scholar]
- 45.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115:1199–209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dall’Era M, Cisternas MG, Smilek DE, Straub L, Houssiau FA, Cervera R, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol. 2015;67:1305–13. doi: 10.1002/art.39026. [DOI] [PubMed] [Google Scholar]
- 47.Tamirou F, Lauwerys BR, Dall’Era M, Mackay M, Rovin B, Cervera R, et al. A proteinuria cut-off level of 0. 7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med. 2015;2:e000123. doi: 10.1136/lupus-2015-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoja C, Liu XH, Donadelli R, Abbate M, Testa D, Corna D, et al. Renal expression of monocyte chemoattractant protein-1 in lupus autoimmune mice. J Am Soc Nephrol. 1997;8:720–9. doi: 10.1681/ASN.V85720. [DOI] [PubMed] [Google Scholar]
- 49.Dai C, Liu Z, Zhou H, Li L. Monocyte chemoattractant protein-1 expression in renal tissue is associated with monocyte recruitment and tubulo-interstitial lesions in patients with lupus nephritis. Chin Med J (Engl) 2001;114:864–8. [PubMed] [Google Scholar]
- 50.Marks SD, Williams SJ, Tullus K, Sebire NJ. Glomerular expression of monocyte chemoattractant protein-1 is predictive of poor renal prognosis in pediatric lupus nephritis. Nephrol Dial Transplant. 2008;23:3521–6. doi: 10.1093/ndt/gfn270. [DOI] [PubMed] [Google Scholar]
- 51.Chung CH, Fan J, Lee EY, Kang JS, Lee SJ, Pyagay PE, et al. Effects of Tumor Necrosis Factor-alpha on Podocyte Expression of Monocyte Chemoattractant Protein-1 and in Diabetic Nephropathy. Nephron Extra. 2015;5:1–18. doi: 10.1159/000369576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumura R, Umemiya K, Sugiyama T, Sueishi M, Umibe T, Ichikawa K, et al. Anti-tumor necrosis factor therapy in patients with difficult-to-treat lupus nephritis: a prospective series of nine patients. Clin Exp Rheumatol. 2009;27:416–21. [PubMed] [Google Scholar]
- 53.Aringer M, Graninger WB, Steiner G, Smolen JS. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum. 2004;50:3161–9. doi: 10.1002/art.20576. [DOI] [PubMed] [Google Scholar]
- 54.Aringer M, Steiner G, Graninger WB, Hofler E, Steiner CW, Smolen JS. Effects of short-term infliximab therapy on autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 2007;56:274–9. doi: 10.1002/art.22327. [DOI] [PubMed] [Google Scholar]
- 55.Aringer M, Houssiau F, Gordon C, Graninger WB, Voll RE, Rath E, et al. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 2009;48:1451–4. doi: 10.1093/rheumatology/kep270. [DOI] [PubMed] [Google Scholar]
- 56.Cortes-Hernandez J, Egri N, Vilardell-Tarres M, Ordi-Ros J. Etanercept in refractory lupus arthritis: An observational study. Semin Arthritis Rheum. 2015;44:672–9. doi: 10.1016/j.semarthrit.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med. 2014;1:e000018. doi: 10.1136/lupus-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]