Key Points
Human, canine, ovine, and porcine platelets exhibit disparate biophysical signatures, whereas human and murine platelets are similar.
Multiple biophysical parameters integrate during clot formation, measured by bulk clot contraction, and attenuate biophysical differences.
Introduction
Clotting is an inherently mechanical process, as demonstrated by recent studies showing that biophysical parameters, such as platelet margination,1 thrombus porosity,2 shear forces,3 compression forces,4 and single-platelet forces,5 affect hemostasis and may be pathologically altered in disease states.6 However, little is known about the most basic biophysical differences between platelets of various animal species, especially dogs, mice, pigs, and sheep, that are commonly used as models for investigations into hemostasis and thrombosis because of their anatomical similarity to human blood vessels, organs, and cellular physiology. This lack of biophysical information hinders our interpretation of the animal models that have been used for various antiplatelet and anticoagulant therapeutic discoveries, including clopidogrel and bivalirudin,7 and models of various disease states, such as heart failure,8 trauma,9 hemophilia,10 and Glanzmann’s thrombasthenia.11,12 To address this knowledge gap, we leveraged characterized and novel biophysical assays, to map out and define a biophysical signature for the platelets of each species. These biophysical assays have shown the influence of cytoskeletal signaling pathways on the behavior of platelets, specifically the myosin light chain kinase13 and ρ-associated protein kinase5 pathways. Our study specifically focused on understanding the differences in platelet adhesion on collagen and fibrinogen, spreading area, single-platelet contraction forces, and volumetric bulk clot contraction, all of which may influence the initiation, propagation, and stability of blood clots. As the first study, to our knowledge, to comprehensively investigate interspecies biophysical differences, our work complements the existing literature that has demonstrated that platelet aggregation,14 coagulation,15 and dense granules16 differ among various animal species. Our new data help provide a rich picture of the interspecies biophysical differences that inform findings from various animal models of hemostasis.
Methods
We performed a comprehensive study of platelet biophysics in humans, C57bl/6 mice, Yucatan pigs, mixed-breed hound dogs, and Hampshire cross sheep. Blood collection for this study was approved by the University of Georgia’s Institutional Animal Care and Use Committee. Gel-filtered platelets were used for the adhesion assays, spreading area, and single-platelet contractile forces, as described previously.5 (Details are provided in the supplemental Methods.)
Cell adhesion and spreading assay
Platelets were diluted to 20 × 109/L to ensure the measurement of single platelets, not the aggregated ones; incubated on coverslips on 100 µg/mL human fibrinogen (Enzyme Research Laboratory) or type 1 rat tail collagen (VWR); and allowed to adhere for 2 hours. After imaging with bright-field microscopy, the cells were then counted and analyzed. (Details are provided in the supplemental Methods.)
Platelet contraction cytometry
Polyacrylamide hydrogels with pairs of fluorescently labeled fibrinogen (Fisher) microdots were fabricated at 75 kPa stiffness, and 2 × 109/L thrombin-activated platelets (1 U/mL thrombin; Haematologic Technologies) were then plated, causing the patterned microdot to displace. This concentration of platelets was used to eliminate platelet-platelet interactions, to ensure that the force of individual platelets could be measured. Individual platelet force is directly proportional to the microdot displacement, as we have demonstrated.5 (Details are provided in the supplemental Methods.)
Bulk clot contraction assay
Platelets are washed as described previously5 and then diluted to 200 × 109/L in a solution of fibrinogen (2 mg/mL), CaCl2 (5 mM), and thrombin (1 U/mL). Clots were left to form in cuvettes lined with gridded paper and measured after 2.5 hours. Samples that lacked sufficient platelets were not assessed.
Matrix protein
For the various biophysical assays, a common matrix protein was chosen for all animals, specifically human fibrinogen and type 1 rat tail collagen, as they are highly conserved. 17-20 It has also been shown that αIIbβ3 is a highly conserved integrin, especially among vertebrates.21-23 Moreover, mixing platelets and proteins from different species is commonly reported.24 Finally, this approach eliminates artifacts that may be introduced from isolated proteins of each animal.
Results and discussion
In this study, we found both significant biophysical differences and similarities among the platelets of the different species. When interspecies differences of platelet adhesion to fibrinogen and collagen were examined, we found that murine, canine, and ovine platelets all adhered in a manner similar to that of human platelets. However, pigs showed a pattern that was the absolute reverse of that of the other species, preferentially adhering to collagen over fibrinogen (Figure 1A-B; supplemental Figure 1). Collagen is involved early in the hemostatic process in which platelets adhere to exposed collagen after endothelial damage, and platelets from pigs may therefore exhibit enhanced binding to subendothelial collagen during these initial stages of clot formation,25 compared with platelets of other species. Conversely, during the later stages of hemostasis, when platelets adhere and interact with fibrin(ogen), the data suggest that platelets from pigs may have decreased or weaker interactions.
Figure 1.
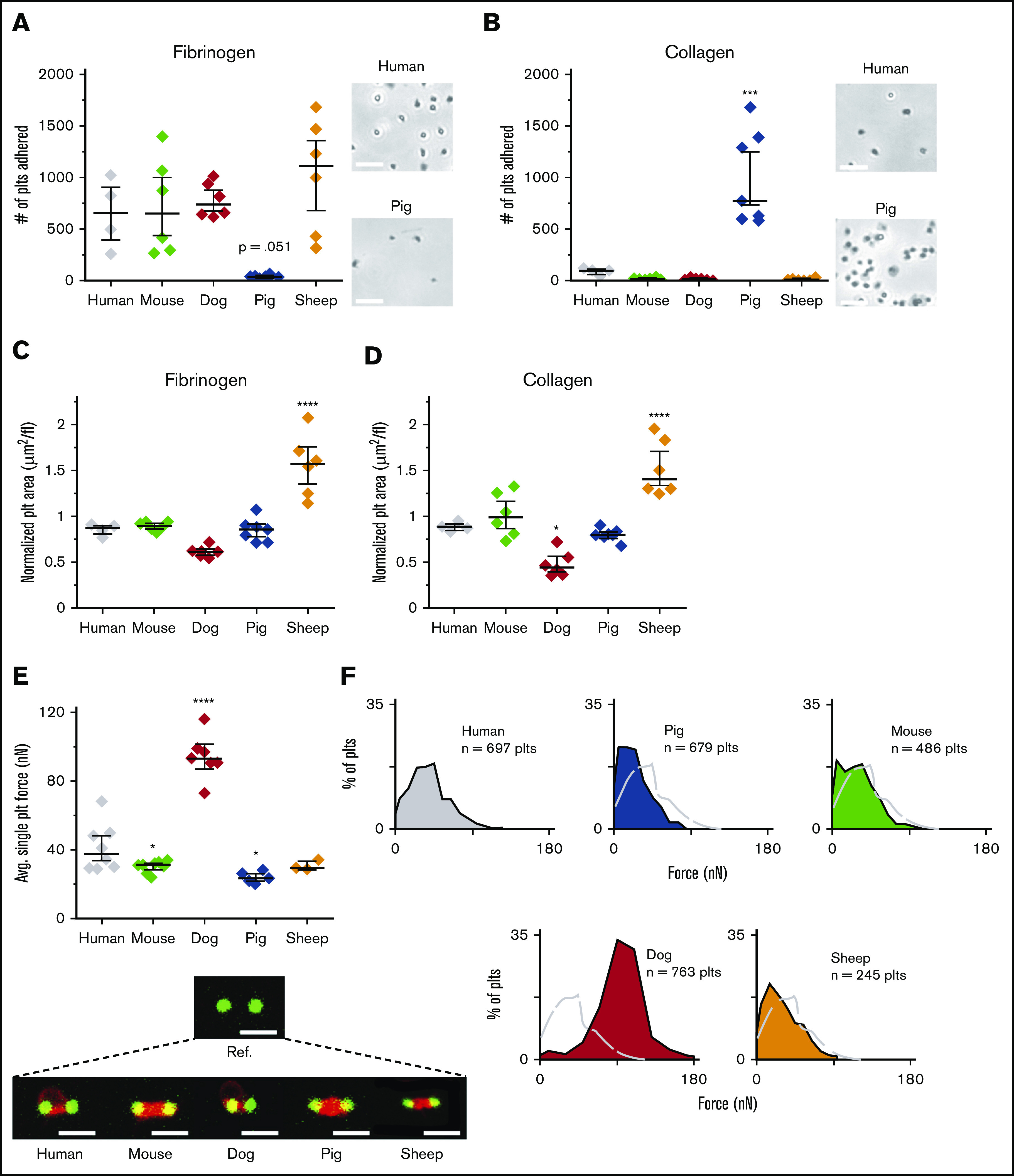
Single-platelet biophysics highlight differences among common models of hemostasis and thrombosis. Although murine, canine, ovine, and human platelets adhere similar to fibrinogen (A) and collagen (B), porcine platelets exhibit the opposite pattern, in which adhesion to collagen is significantly higher than adhesion to fibrinogen. Bright-field images at original magnification of ×30 were obtained demonstrating stark differences in adhesion between human and porcine platelets. Bars represent 10 μm. Using the platelets from the adhesion assays, we measured their spreading area on fibrinogen (C) and collagen (D), normalizing the spreading area by dividing by the mean platelet volume (MPV). The accounting of the platelet volume showed that murine platelets spread to similar surface areas, as compared with human platelets on both fibrinogen and collagen. Compared with other species, ovine platelets exhibited increased spreading on those surfaces. Adhesion and spreading data are shown as median ± standard error of the mean (SEM; n = 4, 6, 6, 7, and 6 for humans, mice, dogs, pigs, and sheep, respectively). All species were compared with humans, and statistical significance was determined by 1-way ANOVA followed by Tukey’s multiple-comparisons test. *P ≤ .05, ***P ≤ .001, ****P ≤ .0001. (E) Platelets from dogs generated single-platelet forces that were 230% higher than that of human platelets, whereas ovine and murine platelets contracted the most similarly at 75% and 73% the force of human platelets, respectively. Representative single-platelet contraction confocal images are shown for each species, with a reference image. All species were compared with humans, and statistical significance was determined by mixed model, to account for within-subject variation. Bars represent 4 μm. Contraction force data are shown as median ± SEM (n = 8, 8, 7, 5, and 3 for humans, mice, dogs, pigs, and sheep, respectively). *P ≤ .05, ****P ≤ .0001. (F) Because our system allows for the generation of single-platelet forces at high throughput, force profiles generated for each species further highlight the stark differences between humans and dogs.
Because studies have demonstrated that the platelet-spreading area is a measure of platelet activation and functionality,26,27 we examined the extent of spreading for each species when normalized to the platelet volume, which varies from 3.2 to 5.4 fL in sheep and mice and from 7.5 to 10 fL in humans, dogs, and pigs.28,29 On both fibrinogen and collagen, the normalized spreading area of murine and porcine platelets was not different from that of human platelets. Canine platelets had smaller spreading areas relative to their volume on fibrinogen and collagen. Conversely, ovine platelets spread ∼40% more than those of humans when normalized to their volume (Figure 1C-D; supplemental Figure 2).
Single-platelet contraction force, measured with a novel “biophysical” cytometer developed by our laboratory,5 identified the most significant differences between human platelets and those of the various nonhuman species (Figure 1E-F). Ovine and murine platelets were the most similar to human platelets with respect to contractile force, averaging 75% and 73% of the forces of human cells, respectively, whereas porcine platelets possessed significantly reduced forces, averaging 58% of that of human platelets. Surprisingly, canine platelets generated average forces that were 230% more than forces in human platelets. Although the biological significance of single-platelet contractile force is still being defined, mechanistically impaired single-platelet forces have been shown to correlate with phenotypic bleeding in humans,6 and our assay helped identify consistent functional differences between the various species.
To assess our data from a more global perspective and incorporate the 5 individual biophysical characteristics explored, we found that each species possessed a unique biophysical signature, which could help explain the difficulties in translating animal models to humans. Although murine platelets are smaller than those of humans, our data, as shown in radar plots, demonstrated that they are the most biophysically similar to human platelets, with a relative similarity of 95.4% based on the shape and area of the shaded regions (Figure 2A; supplemental Table 1). This finding is interesting, given that mice are the most used animal model for studying human disease. Minor differences between mice and humans could be attributable to surface receptor availability and density, which could explain differences between other species as well.30-32
Figure 2.
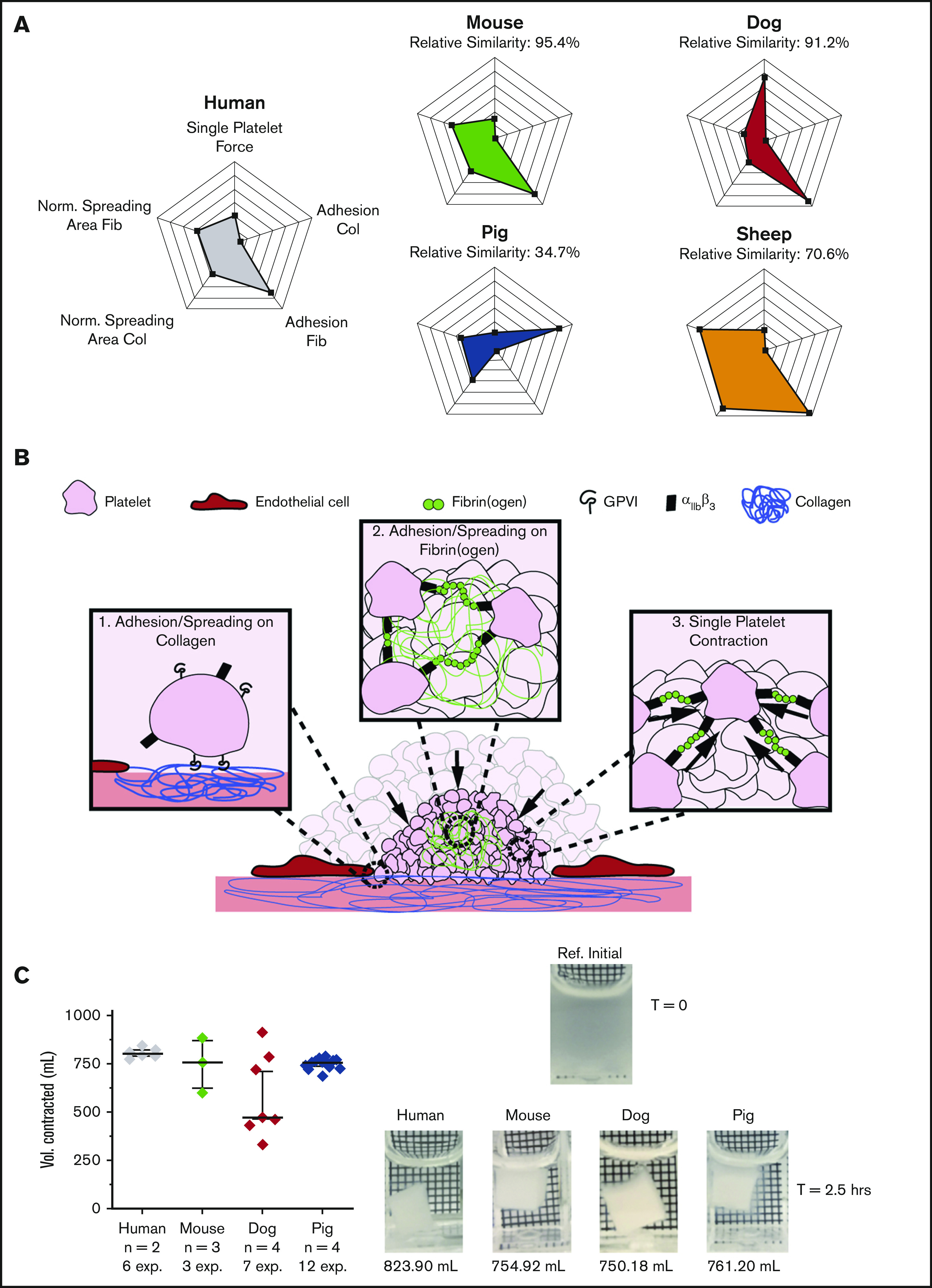
Bulk clot contraction integrates multiple single-cell biophysical parameters and demonstrates that the differences in single-platelet biophysics that exist across species attenuate during clot formation. (A) When observed collectively in 5 single-platelet assays (ie, including single-platelet contraction cytometry, adhesion on fibrinogen and collagen, and spreading area on fibrinogen and collagen), the murine platelet exhibited the most similarity to the human platelet, with a relative similarity of 95.4%. Mean and standard deviations were obtained for all single-platelet experiments for each species, and the percentage relative to humans was calculated. (B) The clotting environment requires a multitude of biophysical interactions. In the early stages, it requires platelet adhesion and spreading to collagen on the damaged vessel wall and platelet adhesion and spreading within a clot through fibrin(ogen) interactions linking platelets together. In the later stages of clot formation, platelets undergo canonical contraction to stabilize the clot. (C) An assay that combines many of these different aforementioned biophysical parameters and takes into account the entire clot formation process is volumetric bulk clot contraction. When focusing on this assay alone, we found that, despite all the single-platelet biophysical differences we noted in adhesion, spreading, and platelet contraction forces between species, there were no significant differences in bulk clot contraction between species. This result leads to the conclusion that biophysical differences attenuate during bulk clot formation, as deficiencies in some biophysical aspects could be overcome by surpluses in others. Bulk contraction data are shown as median ± standard error of the mean. All species were compared with humans, and statistical significance was determined by mixed model, to account for multiple experiments performed on the same subjects. A reference image and representative bulk clot images for each species are shown.
Although the platelets of each species exhibit unique biophysical signatures based on those 5 parameters, each in isolation, how those different biophysical variables ultimately interplay as the process of clot formation proceeds at the more global level has not been assessed. To that end, using platelets from different species, we compared the biophysics of clot contraction, which depends on and integrates those biophysical properties, specifically platelet adhesion, platelet spreading, and single-platelet contraction over time (Figure 2B), according to a well-established volumetric bulk clot contraction assay.33 Surprisingly, only minor differences were seen among the species (Figure 2C; supplemental Figure 3). As the clot contraction assay is a global assay of clot formation, this result suggests that the quantitative differences in the biophysical characteristics of platelets of different species “even out” during the hemostatic process and that certain biophysical behaviors may compensate for deficiencies in others.
In this study, we highlighted the importance of single-platelet biophysical assays and showed that use of the bulk clot contraction assay in isolation to measure platelet biophysics may lead to the false conclusion that the biophysical behavior of various species is largely similar. Our single-platelet data challenge this notion and provide key biophysical reference data that could be useful when translating experiments to humans. In contrast to currently used platelet function tests, such as aggregometry and platelet function analyzers, a benefit of our experimental assays is that these single-platelet results are independent of, and therefore are not confounded by, the platelet count, which thereby enables more direct comparison of platelet biophysics across different species. Several studies have shown stark differences between different species and humans regarding various agonists and inhibitors of platelet activity34-36; however, the lack of available biophysical information hinders our ability to develop a complete interpretation of these differences. As such, given the continual efforts to translate various animal models to humans and to develop new antiplatelet therapeutics that must be tested on various animal models, these single-platelet assays could help identify whether the hindrance to translation is related to the therapeutic agent or to intrinsic biophysical differences between humans and common animal models. Specifically, antiplatelet therapeutic agents are designed to target relevant biophysical aspects of platelet function, such as adhesion, aggregation, and contraction, and therefore our more nuanced examination of the biophysical properties of single platelets may inform and help address some of the challenges in translating common animal models to humans.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank David Myers for scientific guidance and advice and Andrew Shaw (Parker H. Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology) for technical assistance.
This work was supported by an American Society of Hematology Minority Medical Student Award Program Fellowship (O.O.) and National Institutes of Health, Institute of Heart, Lung, and Blood grants R01HL130918 and R35HL145000 (W.A.L.). The work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by National Science Foundation, division of Electrical, Communications, and Cyber Systems grant 1542174.
Footnotes
The data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request (Wilbur A. Lam; wilbur.lam@emory.edu).
Authorship
Contribution: B.B. and B.G.P. provided blood from various species; O.O., R.C., and Y.S. performed the experiments; O.O., R.C., and M.E.F. performed the data analysis; O.O. and T.L. conducted the statistical analysis; O.O. and W.A.L. wrote the paper; and all authors collaborated in designing the experiments and editing the paper.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Wilbur A. Lam, Emory University School of Medicine, 2015 Uppergate Dr NE, #448, Atlanta, GA 30322; e-mail wilbur.lam@emory.edu.
References
- 1.Walton BL, Lehmann M, Skorczewski T, et al. . Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh JD, Stalker TJ, Voronov R, et al. . A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124(11):1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. . A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665-673. [DOI] [PubMed] [Google Scholar]
- 4.Ju L, McFadyen JD, Al-Daher S, et al. . Compression force sensing regulates integrin αIIbβ3 adhesive function on diabetic platelets. Nat Commun. 2018;9(1):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers DR, Qiu Y, Fay ME, et al. . Single-platelet nanomechanics measured by high-throughput cytometry. Nat Mater. 2017;16(2):230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams EK, Oshinowo O, Ravindran A, Lam WA, Myers DR. Feeling the Force: Measurements of Platelet Contraction and Their Diagnostic Implications. Semin Thromb Hemost. 2019;45(3):285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagadeeswaran P, Cooley BC, Gross PL, Mackman N. Animal Models of Thrombosis From Zebrafish to Nonhuman Primates: Use in the Elucidation of New Pathologic Pathways and the Development of Antithrombotic Drugs. Circ Res. 2016;118(9):1363-1379. [DOI] [PubMed] [Google Scholar]
- 8.Houser SR, Margulies KB, Murphy AM, et al. ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology . Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111(1):131-150. [DOI] [PubMed] [Google Scholar]
- 9.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock. 2005;24(suppl 1):33-39. [DOI] [PubMed] [Google Scholar]
- 10.Yen C-T, Fan M-N, Yang Y-L, Chou S-C, Yu IS, Lin S-W. Current animal models of hemophilia: the state of the art. Thromb J. 2016;14(suppl 1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols TC, Hough C, Agersø H, Ezban M, Lillicrap D. Canine models of inherited bleeding disorders in the development of coagulation assays, novel protein replacement and gene therapies. J Thromb Haemost. 2016;14(5):894-905. [DOI] [PubMed] [Google Scholar]
- 12.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. . Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103(2):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Y, Brown AC, Myers DR, et al. . Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci USA. 2014;111(40):14430-14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelagalli A, Lombardi P, d’Angelo D, Della Morte R, Avallone L, Staiano N. Species variability in platelet aggregation response to different agonists. J Comp Pathol. 2002;127(2-3):126-132. [DOI] [PubMed] [Google Scholar]
- 15.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100(3):397-404. [PubMed] [Google Scholar]
- 16.Meyers KM, Holmsen H, Seachord CL. Comparative study of platelet dense granule constituents. Am J Physiol. 1982;243(3):R454-R461. [DOI] [PubMed] [Google Scholar]
- 17.Haq F, Ahmed N, Qasim M Comparative genomic analysis of collagen gene diversity. 3 Biotech 2019;9(3):83. [DOI] [PMC free article] [PubMed]
- 18.Doolittle RF. The structure and evolution of vertebrate fibrinogen. Ann N Y Acad Sci. 1983;408:13-27. [DOI] [PubMed] [Google Scholar]
- 19.Doolittle RF. The structure and evolution of vertebrate fibrinogen: a comparison of the lamprey and mammalian proteins. Adv Exp Med Biol. 1990;281:25-37. [DOI] [PubMed] [Google Scholar]
- 20.Runnegar B. Collagen gene construction and evolution. J Mol Evol. 1985;22(2):141-149. [DOI] [PubMed] [Google Scholar]
- 21.Hughes AL. Evolution of the integrin α and β protein families. J Mol Evol. 2001;52(1):63-72. [DOI] [PubMed] [Google Scholar]
- 22.Lipscomb DL, Bourne C, Boudreaux MK. DNA sequence of the canine platelet beta3 gene from cDNA: comparison of canine and mouse beta3 to segments that encode alloantigenic sites and functional domains of beta3 in human beings. J Lab Clin Med. 1999;134(3):313-321. [DOI] [PubMed] [Google Scholar]
- 23.Choi W, Karim ZA, Whiteheart SW. Protein expression in platelets from six species that differ in their open canalicular system. Platelets. 2010;21(3):167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herr AB, Farndale RW. Structural insights into the interactions between platelet receptors and fibrillar collagen. J Biol Chem. 2009;284(30):19781-19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2(4):561-573. [DOI] [PubMed] [Google Scholar]
- 26.Jirousková M, Jaiswal JK, Coller BS. Ligand density dramatically affects integrin alpha IIb beta 3-mediated platelet signaling and spreading. Blood. 2007;109(12):5260-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel D, Väänänen H, Jirousková M, Hoffmann T, Bodian C, Coller BS. Dynamics of GPIIb/IIIa-mediated platelet-platelet interactions in platelet adhesion/thrombus formation on collagen in vitro as revealed by videomicroscopy. Blood. 2003;101(3):929-936. [DOI] [PubMed] [Google Scholar]
- 28.Weiss DJ. Comparative Physiology of Platelets from Different Species In: Rao GHR, ed. Handbook of Platelet Physiology and Pharmacology, Boston, MA: Springer US; 1999:379-393. [Google Scholar]
- 29.Aurbach K, Spindler M, Haining EJ, Bender M, Pleines I. Blood collection, platelet isolation and measurement of platelet count and size in mice-a practical guide. Platelets. 2019;30(6):698-707. [DOI] [PubMed] [Google Scholar]
- 30.Burkhart JM, Vaudel M, Gambaryan S, et al. . The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73-e82. [DOI] [PubMed] [Google Scholar]
- 31.Zeiler M, Moser M, Mann M. Copy number analysis of the murine platelet proteome spanning the complete abundance range. Mol Cell Proteomics. 2014;13(12):3435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunster JL, Unsworth AJ, Bye AP, et al. . Interspecies differences in protein expression do not impact the spatiotemporal regulation of glycoprotein VI mediated activation. J Thromb Haemost. 2020;18(2):485-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weyrich AS, Denis MM, Schwertz H, et al. . mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109(5):1975-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigand A, Boos AM, Ringwald J, et al. . New aspects on efficient anticoagulation and antiplatelet strategies in sheep. BMC Vet Res. 2013;9(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomason J, Lunsford K, Mackin A. Anti-platelet therapy in small animal medicine. J Vet Pharmacol Ther. 2016;39(4):318-335. [DOI] [PubMed] [Google Scholar]
- 36.Burke SE, Lefer AM, Nicolaou KC, Smith GM, Smith JB. Responsiveness of platelets and coronary arteries from different species to synthetic thromboxane and prostaglandin endoperoxide analogues. Br J Pharmacol. 1983;78(2):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


