Graphical abstract
The worldwide outbreak of COVID-19 since December 2019 has caused great challenges to health organizations, and brought tremendous impact on the global economy. There have been over 62.3 million confirmed infection cases and 1.4 million deaths reported until now (December 1 st, 2020), and the numbers are still growing [1]. Although not as influential as COVID-19, the other two large pandemics, severe acute respiratory syndrome (SARS, outbroke in 2002) and Middle East respiratory syndrome (MERS, outbroke in 2012), were also caused by coronaviruses and resulted in severe public health and economic crises in several countries [2,3]. Accompanied by the globalization, the highly connected human society through air travel and conventions offers convenience for the rapid spreading of viruses. Moreover, the occurrence of undetected viruses and their high spontaneous mutation rate challenge the cognitive process and the development of antiviral agents. In this view, the development of broad-spectrum antiviral strategies for prevention and control of viral transmission is of great importance for protecting our human society.
Viruses are obligate parasites that can only replicate inside living cells. Viruses in form of independent particles are called virions, which are composed of at least two parts: (1) genetic material (DNA or RNA) and (2) a protein coat surrounding and protecting the genetic material. Some viruses may also be enveloped in a lipid capsule, when they are released from the host cells. The spreading of viruses varies greatly depending on the specific species, infectivity, and the subsequent relationship with the host. Viruses causing epidemic diseases, e.g., COV-2019, always spread through many routes such as air, water, respiratory droplets, food, and surface contact etc. Much research has focused on the development of antiviral agents that can disrupt the viral replication cycle or destroy them completely [4]. However, the utilization of antiviral materials to block the viral transmission, before they contact human cells, can be meaningful in many applications, such as the healthcare, pharmaceutical manufacturing, food processing, public transport and facilities and scientific research etc.
The utilization of metallic copper (Cu) for antimicrobial applications has attracted for a long-time enormous attention in infection control, including medical implants. The proper addition of Cu in alloys has been confirmed to be biologically safe and efficient in inhibition of many microorganisms non-selectively [5]. Several mechanisms have been proposed to illustrate the intrinsic mechanisms of the copper-mediated antimicrobial function including disruption of cellular membranes resulting from direct contact, reactive hydroxyl radicals generated through Fenton reactions, and ligand interactions mediated by copper ions destroying the structure and integrity of DNA and RNA etc. [6,7]. Since metallic copper can react with multiple active sites without selectivity to the cells, its broad-spectrum antimicrobial efficacy is well known besides the low possibility for pathogens to develop a resistance. Besides the antibacterial and antifungal properties, metallic copper has also been reported for antiviral applications as a constituent in alloys or as a functional additive [8,9].
Cu bearing metals, such as Cu-bearing stainless steels and Cu-bearing titanium alloys, have been widely investigated for their antibacterial properties [10,11]. The increase of copper content, within the limit ranges, has been proven to enhance the antimicrobial activity [12,13]. However, the addition of copper in selective metallic materials might hamper the corrosion resistance and mechanical properties, leading to the non-desirable performance for practical applications [14,15]. Liu et al. illustrated that at least 5% (w/w) of Cu in Ti-Cu alloys was required to obtain a stable antibacterial efficiency [12], but only a 75% reduction of bacterial cell numbers was achieved for Ti-10 Cu alloy (containing 10% Cu) against anaerobic Porphyromonas gingivalis over 24 h of incubation [16]. This reduction is insufficient to warrant an industrial application. Recently, the high-entropy alloys (HEAs), accomplished by the combination of multiple principle elements in relatively high concentrations (often in equi-atomic concentrations), have attracted much attention because of their exceptional properties [17]. One interesting approach for manufacturing structural materials with antiviral abilities can be achieved by the fabrication of Cu-bearing HEAs (Cu-HEAs). Owing to the huge design space of HEAs, their antiviral function can lead to a promising future for preparing antiviral materials.
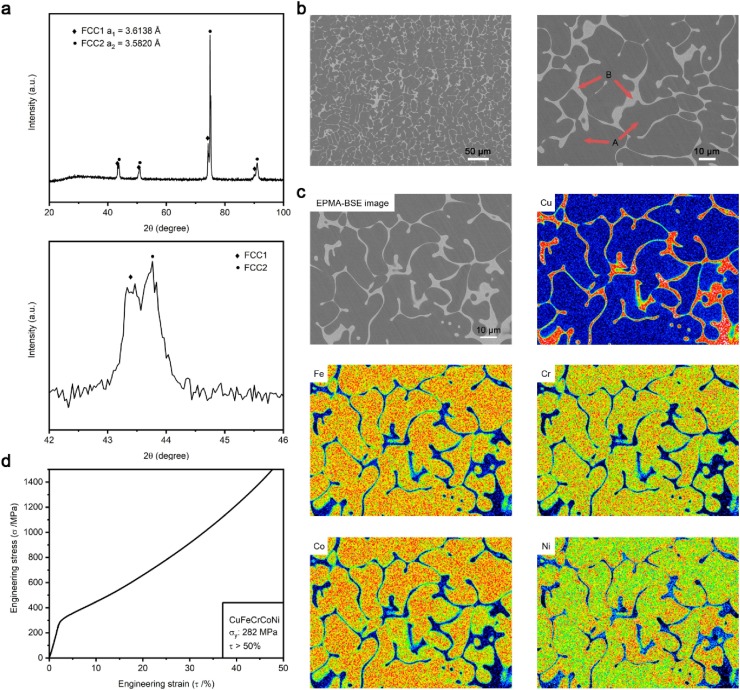
To investigate the feasibility of Cu-HEAs for antiviral applications, the HEA ingots were prepared by vacuum arc melting in a Ti-gettered argon atmosphere. Equal molar ratios of commercially pure Co, Cr, Cu, Ni (99.9%, w/w), and Fe (99.5%–99.6%, w/w) were put into a water-cooled copper crucible. During the arc melting, the ingots were flipped over and remelted for at least five times to ensure the chemical homogeneity. The phase constitution of this CuFeCrCoNi HEA was measured using an X-ray diffractometer (XRD, Empyrean) through scanning 2θ degree from 20° to 100° at a rate of 4°/min. As shown in Fig. 1 (a), two disordered face-centered-cubic solid solid-solution phases (FCC1 and FCC2) were observed on the CuFeCrCoNi surfaces. According to the Bragg equation, the lattice constants of the two FCC phases were 3.6138 Å and 3.5820 Å, respectively. The microstructures and micro-area compositions were also analyzed using an electron probe microanalyzer (EPMA, JXA-8530 F PLUS) in back scattered electron (BSE) mode equipped with a wavelength dispersion spectrometer (WDS). The BSE-images are shown in Fig. 1(b) and the chemical compositions of different regions are listed in Table 1 . It is clear that the alloy exhibited typical dendrite and interdendrite structures. The WDS results indicated that the gray dendrite regions (illustrated as “A” in Fig. 1(b)) enriched with Co, Cr, Fe and Ni elements, and the four elements uniformly distributed in the alloy. However, the white interdendrite regions (illustrated as “B” in Fig. 1(b)) were mainly composed of the Cu element, accounting for about 80% of the atomic ratio. These results were further corroborated by the elemental mapping distribution, as demonstrated in Fig. 1(c). The compressive property of the CuFeCrCoNi HEA was conducted at room temperature according to the standard of ASTM E9−09 using cylindrical samples of ϕ5 × 10 mm at a compression of 10−3 s−1. Fig. 1(d) displays the engineering compression curve showing that the CuFeCrCoNi HEA exhibited excellent plasticity with a compression ratio of over 50% and its yield strength was 282 MPa.
Fig. 1.
Characterization of the material properties of the CuFeCrCoNi HEA: (a) XRD analysis, (b) EPMA-BSE images, (c) elemental mapping distribution, and (d) engineering compressive curve. In Fig. 1(b), “A” indicates the dendrite regions and “B” indicates the interdendrite regions. In Fig. 1(c), those images are from the same field of the EPMA-BSE image.
Table 1.
Chemical composition (at%) of different regions of the CuFeCrCoNi HEA measured by EPMA-WDS.
| Region | Cu | Fe | Cr | Co | Ni |
|---|---|---|---|---|---|
| Dendrite region (A) | 9.88 | 23.28 | 23.00 | 23.67 | 20.18 |
| Interdendrite region (B) | 84.00 | 3.21 | 2.65 | 3.38 | 6.76 |
The antiviral properties of the CuFeCrCoNi HEA against influenza virus H1N1 and enterovirus 71 were analyzed by the Guangdong Detection Center of Microbiology (Guangzhou, China) according to the ISO 21702:2019 standard. Coupons with a surface size of 10 mm × 10 mm were cleaned using 70% (v/v) ethanol. Then 0.1 mL virus suspension at approximately 106 plaque forming units (PFU) per mL was dipped on the surface. A polyethylene film with a thickness of 0.1 mm was covered on the surface. After 24 h of incubation at 25 °C with a relative humidity no less than 90%, 2 mL maintenance medium was added to recover the viruses. A serial of 10-fold dilutions were prepared and 0.1 mL of each solution was added into the wells containing the host cell monolayers. After incubation at 34 °C in the CO2 incubator for 1 h, 3 mL of the agar medium was poured into the wells for plaque assay. After 3 d incubation in the CO2 incubator, formalin solution was used to fix the cells and methylene blue solution was used to dye the cells. If half of the wells were infected by the corresponding viruses, the dilution numbers (D) were recorded. The tissue culture infective doses (TCID50) were calculated as 10D. The antiviral efficacy was calculated as [(U 0−U t)−(U 0−U c)]/U c, wherein U 0 means the TCID50 of the control samples (304 stainless steel) at 0 h, U t the TCID50 of the treated samples at 24 h, and U c the TCID50 of control samples at 24 h.
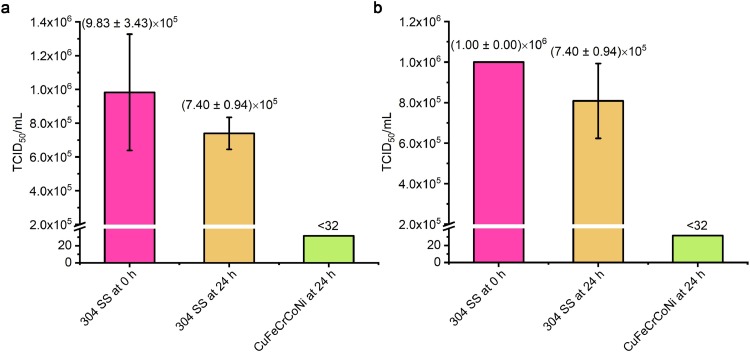
As shown in Fig. 2 , after 24 h incubation on 304 SS coupons, over 70% of H1N1 viruses were still alive, and were still able to infect Madin-Darby canine kidney (MDCK) cells. However, more than 99.99% of the H1N1 viruses on the CuFeCrCoNi HEA had been inactivated after 24 h of incubation (Fig. 2(a)). Also, over 99.99% of the enterovirus 71 viruses had been inactivated, whereas over 93.3% of the viruses on 304 SS surfaces were still able to infect Vero cells after 24 h (Fig. 2(b)). These results indicate that CuFeCrCoNi HEA is capable of efficient inactivation of these virus types.
Fig. 2.
Antiviral properties of the CuFeCrCoNi HEA against two viruses: (a) influenza virus H1N1 and (b) enterovirus 71. Standard deviations are from at least 3 parallel tests.
The addition of element aluminum into HEAs has been verified to induce the formation of new phases and then to precipitate out in the HEAs, which increases the mechanical strengths of HEAs [18,19]. Various research has focused on the effect of the aluminum content on the microstructure and properties of different HEAs, including AlxCoCrCuFeNi [20,21], AlxCoCrFeNi [22], and TiC reinforced AlxFeCoNiCu [23] and so on. A gradual change from single FCC phase to duplex FCC-BCC phase, then to single BCC phase, was observed with an increase of aluminum content. The formation of BCC phase in the FCC phase increases the strength. However, it hampers the ductility, which leads to brittleness at ambient temperature [24]. Consequently, the addition of aluminum at an appropriate content is necessary to achieve excellent properties. Moreover, because of its low mass density, the addition of aluminum is an effective way to reduce the HEA density. Herein, we tested further the corrosion and antiviral properties of Al0.4CoCrCuFeNi HEA [25], which also possesses both FCC1 and FCC2 phases, to illustrate the influence of aluminum addition on the properties of Cu-HEAs and to pave the way for further pursuing antiviral HEAs with remarkable performance.
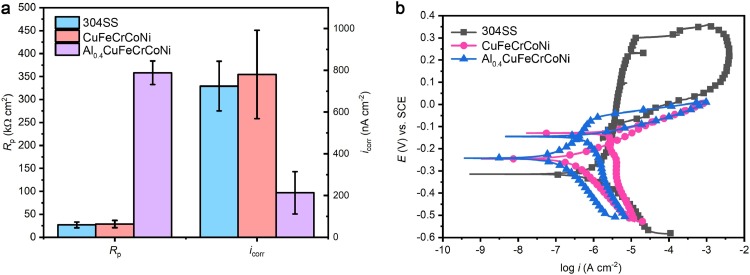
In Fig. 3 , data from electrochemical analyses are presented to analyze the influence of aluminum addition on the corrosion properties of Cu-HEAs. The polarization resistance (R p) of the Cu-HEAs increased from 28.8 ± 7.9 kΩ cm2 for the CuFeCrCoNi HEA to 358.3 ± 25.7 kΩ cm2 for the Al0.4CuFeCrCoNi HEA (Fig. 3(a)) in 3.5% NaCl. This suggests significant increase in corrosion resistance. An R p value of 27.0 ± 6.3 kΩ cm2 was measured for 304 stainless steel (304SS), which is similar to the CuFeCrCoNi HEA and far below that of the Al0.4CuFeCrCoNi HEA (Fig. 3(a)). The corrosion parameters, including corrosion potential (E corr) and corrosion current density (i corr), were calculated through the Tafel curve fitting of the cyclic polarization curves in Fig. 3(b). The open current potential (E OCP) and E corr were almost the same for the two Cu-HEAs (Table 2 ). However, the i corr decreased from 780 ± 212 nA cm−2 for the CuFeCrCoNi HEA, which was similar with the value for 304SS, to 213 ± 101 nA cm−2 for the Al0.4CuFeCrCoNi HEA (Fig. 3(a)). This confirms further the decrease of corrosion tendency of CuFeCrCoNi HEA due to an aluminum addition. Moreover, with the addition of Al, the passivation behavior was observed on the polarization curve of Al0.4CuFeCrCoNi HEA, which did not occur with the CuFeCrCoNi HEA. The breakdown potential of the Al0.4CuFeCrCoNi HEA was lower than that of 304SS, indicating a better pitting corrosion resistance of 304 SS.
Fig. 3.
Electrochemical analysis of the corrosion resistance of two Cu-HEAs: (a) Rp and icorr and (b) polarization curves. Standard deviations are from at least 3 parallel tests.
Table 2.
Electrochemical parameters of the two Cu-HEAs.
| EOCP (mV) vs. SCE | Ecorr (mV) vs. SCE | |
|---|---|---|
| 304SS | −258.3 ± 35.1 | −285.7 ± 42.7 |
| CuFeCrCoNi | −195.4 ± 47.7 | −228.6 ± 23.1 |
| Al0.4CuFeCrCoNi | −196.5 ± 28.3 | −232.0 ± 18.5 |
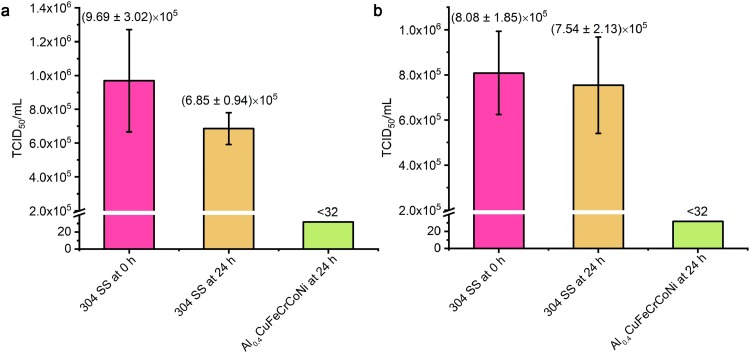
The antiviral properties of the Al0.4CuFeCrCoNi HEA against the influenza virus H1N1 and the enterovirus 71 were also tested. As shown in Fig. 4 (a), after 24 h of treatment, over 99.99% of H1N1 viruses have been inactivated on Al0.4CuFeCrCoNi HEA surfaces and were non-infectious anymore. Also, an antiviral effect of over 99.99% was obtained for the Al0.4CuFeCrCoNi HEA against enterovirus 71 (Fig. 4(b)). The Al0.4CuFeCrCoNi HEA displayed a similar antiviral activity as compared with the CuFeCrCoNi HEA. Obviously, the addition of Al with proper content did not influence antiviral efficiency of the Cu-HEAs.
Fig. 4.
Antiviral properties of the Al0.4CuFeCrCoNi HEA against two viruses: (a) influenza virus H1N1 and (b) enterovirus 71. Standard deviations are from at least 3 parallel tests.
HEAs are currently in the focus of interesting metallurgy because of many possible innovative applications in various industries. One main reason of the use of HEAs distinctive from conventional materials are the outstanding mechanical properties. The most noticeable examples are the FCC CrCoNi-based alloys [26,27]. Here, the CuFeCrCoNi HEA was made through adding the two metals Fe and Cu into the CrCoNi-based alloy. In the product only FCC phases were observed by XRD analysis. The excellent yield stress values of these Cu-HEAs (282 MPa for the CuFeCrCoNi HEA and 318 MPa for the Al0.4CuFeCrCoNi HEA [25]), which by far exceed those of 304 Cu-SS (185 MPa [25]) and 316 L/317 L N Cu-SS (∼230 MPa [28,29]), are a result of the specific interactions among the various metal atoms in these HEAs. Consequently, such materials can meet the performance requirements for various special applications. Besides excellent mechanical properties, the next generation of HEAs should focus on novel materials with unprecedented properties [30,31]. In this study, excellent antiviral properties were observed through the incorporation of copper into HEA. Cu accounts for approximately 20 at.% in the Cu-HEAs, which far exceed that of classical antimicrobial metals such as antibacterial stainless steels and antibacterial titanium alloys. Previously, the Al0.4CuFeCrCoNi HEA had demonstrated its broad-spectrum antibacterial performance [25]. In view of this, the addition of other antimicrobial metals, such as argentum (Ag) and zinc (Zn), might be used also for the development of antiviral HEAs.
Depending on various antiviral mechanisms, numerous antiviral materials have been developed, such as metallic nanoparticles, carbon-based nanomaterials, organic nanomaterials, and antiviral biopolymers which were synthesized either artificially or extracted from natural resources [32,33]. Such functional nanomaterials are restricted always by high cost due to the complicated manufacturing process. In addition, though much attention has been paid to their unique physicochemical characteristics, nanomaterials are still questionable considering their potential toxicity toward humans and their environmental risks [33,34]. The large specific surface area of nanomaterials enables them to interact closely with intracellular substances, e.g. nucleic acids, proteins and enzymes, which might cause yet unknown detrimental effects on cells and tissues [35]. Biopolymers, mostly isolated from natural resources, are promising candidates for antiviral applications. Some polysaccharides [36], poly- and oligonucleotides [37], peptides [38], and proteins [39] have been confirmed to possess broad-spectrum antiviral activities and low adverse effects. However, because of their low mechanical strength and low product yields, those antiviral biopolymers can only be utilized in highly specialized areas, such as biomedical applications and fabrication of protective appliances. Copper alloys have long been recognized for their antiviral properties against various viruses including herpes simplex virus [40], noroviruses [41], and influenza virus [42]. Several mechanisms have been proposed to elucidate the inactivation of viruses by metallic copper. The released copper ions (i.e., Cu+ and Cu2+) during aqueous corrosion might chelate with the nucleic acids and the proteins, resulting in the denaturation of virus [40,43]. The oxidative agents produced during the electrochemical corrosion processes can also participate and accelerate the inactivation processes [40,44]. On the other hand, the direct contact of solid metallic oxides, especially Cu2O, formed on the alloy surfaces can also lead to the denaturation of structural proteins, which results in the inactivation of viruses [42]. Engineered Cu-HEAs offer a viable strategy for usable antiviral structural materials, which can be applied in food manufacturing, public transportation, medical instruments, therapeutic appliance, and public sanitary places etc.
In conclusion, we propose a novel concept for the design of antiviral metallic materials. The addition of copper into HEAs endows the materials with excellent antiviral properties. Further optimization of alloying compositions, e.g. an aluminum addition, can neutralize the drawbacks caused by copper addition. Furthermore, we illustrate two Cu-HEAs with efficient antiviral properties. Over 99.99% of influenza virus H1N1 and enterovirus 71 were inactivated on the surfaces of such Cu-HEAs and, thus, non-infectious anymore after 24 h of treatment. The design concepts proposed here can guide the design of various types of antiviral Cu-HEAs with excellent mechanical properties and corrosion resistant performance.
Acknowledgements
This research was financially supported by the Fundamental Research Funds for the Central Universities (Nos. N2002020 and N2002019), the National Natural Science Foundation of China (Nos. 51871050, 5184022, and 51901039), the National Key Research and Development Program of China (Nos. 2019YFA0209901 and 2018YFA0702901), the fund of the State Key Laboratory of Solidification Processing in NWPU (No. SKLSP201902), and the Fund of Science and Technology on Reactor Fuel and Materials Laboratory (STRFML-2020-04).
References
- 1.WHO . 2020. Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Anderson R.M., Fraser C., Ghani A.C., Donnelly C.A., Riley S., Ferguson N.M., Leung G.M., Lam T.H., Hedley A.J. Philos. Trans. R. Soc. Lond. B-Biol. Sci. 2004;359:1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhamlan F.S., Majumder M.S., Brownstein J.S., Hawkins J., Al-Abdely H.M., Alzahrani A., Obaid D.A., Al-Ahdal M.N., BinSaeed A. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-011865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paintsil E., Cheng Y.C. In: Encyclopedia of Microbiology. 4th edn. Schmidt T.M., editor. Academic Press; 2019. pp.176-225.65. [Google Scholar]
- 5.Zhang E.L., Fu S., Wang R.X., Li H.X., Liu Y., Ma Z.Q., Liu G.K., Zhu C.S., Qin G.W., Chen D.F. Rare Met. 2019;38:476–494. [Google Scholar]
- 6.Grass G., Rensing C., Solioz M. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent M., Duval R.E., Hartemann P., Engels-Deutsch M. J. Appl. Microbiol. 2018;124:1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- 8.Gross T.M., Lahiri J., Golas A., Luo J., Verrier F., Kurzejewski J.L., Baker D.E., Wang J., Novak P.F., Snyder M.J. Nat. Commun. 2019;10:1979. doi: 10.1038/s41467-019-09946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warnes S.L., Summersgill E.N., Keevil C.W. Appl. Environ. Microbiol. 2015;81:1085–1091. doi: 10.1128/AEM.03280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Yang C., Yang K. ACS Appl. Mater. Interfaces. 2020;12:361–372. doi: 10.1021/acsami.9b19596. [DOI] [PubMed] [Google Scholar]
- 11.Li M., Ma Z., Zhu Y., Xia H., Yao M., Chu X., Wang X., Yang K., Yang M., Zhang Y., Mao C. Adv. Healthc. Mater. 2016;5:557–566. doi: 10.1002/adhm.201500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Li F., Liu C., Wang H., Ren B., Yang K., Zhang E. Mater. Sci. Eng. C. 2014;35:392–400. doi: 10.1016/j.msec.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Zhang E., Ren J., Li S., Yang L., Qin G. Biomed. Mater. 2016;11 doi: 10.1088/1748-6041/11/6/065001. [DOI] [PubMed] [Google Scholar]
- 14.Chen S., Dong X., Ma R., Zhang L., Wang H., Fan Z. Mater. Sci. Eng. A. 2012;551:87–94. [Google Scholar]
- 15.Chen S.Q., Dong X.P., Xiong X.Q., Ma R., Fan Z.T. Adv. Mater. Res. 2012;463−464:52–57. [Google Scholar]
- 16.Bai B., Zhang E., Liu J., Zhu J. Dent. Mater. J. 2016;35:659–667. doi: 10.4012/dmj.2016-001. [DOI] [PubMed] [Google Scholar]
- 17.George E.P., Raabe D., Ritchie R.O. Nat. Rev. Mater. 2019;4:515–534. [Google Scholar]
- 18.Chuang M.H., Tsai M.H., Wang W.R., Lin S.J., Yeh J.W. Acta Mater. 2011;59:6308–6317. [Google Scholar]
- 19.Chen S.T., Tang W.Y., Kuo Y.F., Chen S.Y., Tsau C.H., Shun T.T., Yeh J.W. Mater. Sci. Eng. A. 2010;527:5818–5825. [Google Scholar]
- 20.Wu J.M., Lin S.J., Yeh J.W., Chen S.K., Huang Y.S., Chen H.C. Wear. 2006;261:513–519. [Google Scholar]
- 21.Tong C.J., Chen Y.L., Chen S.K., Yeh J.W., Shun T.T., Tsau C.H., Lin S.J., Chang S.Y. Metall. Mater. Trans. A. 2005;36:881–893. [Google Scholar]
- 22.Wang W.R., Wang W.L., Wang S.C., Tsai Y.C., Lai C.H., Yeh J.W. Intermetallics. 2012;26:44–51. [Google Scholar]
- 23.Sun X., Zhu H., Li J., Huang J., Xie Z. Mater. Sci. Eng. A. 2019;743:540–545. [Google Scholar]
- 24.Kim W.J., Jeong H.T., Park H.K., Park K., Na T.W., Choi E. J. Alloy. Compd. 2019;802:152–165. [Google Scholar]
- 25.Zhou E., Qiao D., Yang Y., Xu D., Lu Y., Wang J., Smith J.A., Li H., Zhao H., Liaw P.K., Wang F. J. Mater. Sci. Technol. 2020;46:201–210. [Google Scholar]
- 26.Slone C.E., Miao J., George E.P., Mills M.J. Acta Mater. 2019;165:496–507. [Google Scholar]
- 27.Yang M., Yan D., Yuan F., Jiang P., Ma E., Wu X. Proc. Natl. Acad. Sci. U.S.A. 2018;115:7224–7229. doi: 10.1073/pnas.1807817115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xi T., Shahzad M.B., Xu D., Sun Z., Zhao J., Yang C., Qi M., Yang K. Mater. Sci. Eng. C. 2017;71:1079–1085. doi: 10.1016/j.msec.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Xi T., Yang C., Babar Shahzad M., Yang K. Mater. Des. 2015;87:303–312. [Google Scholar]
- 30.Ma E., Wu X. Nat. Commun. 2019;10:5623. doi: 10.1038/s41467-019-13311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Sarkar A., Wang D., Velasco L., Azmi R., Bhattacharya S.S., Bergfeldt T., Düvel A., Heitjans P., Brezesinski T., Hahn H., Breitung B. Energ. Environ. Sci. 2019;12:2433–2442. [Google Scholar]
- 32.Zhou J., Hu Z., Zabihi F., Chen Z., Zhu M. Adv. Fiber Mater. 2020;2:123–139. doi: 10.1007/s42765-020-00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang L., Ahamed A., Ge L., Fu X., Lisak G. ChemPlusChem. 2020;85:2105–2128. doi: 10.1002/cplu.202000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nho R. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102242. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Castranova V. J. Toxicol. Environ. Health B. 2011;14:593–632. doi: 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]
- 36.Wang W., Wang S.X., Guan H.S. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaillant A. Antiviral Res. 2016;133:32–40. doi: 10.1016/j.antiviral.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Brice D.C., Diamond G. Curr. Med. Chem. 2020;27:1420–1443. doi: 10.2174/0929867326666190805151654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun E., Hotter D., Koepke L., Zech F., Gross R., Sparrer K.M.J., Muller J.A., Pfaller C.K., Heusinger E., Wombacher R., Sutter K., Dittmer U., Winkler M., Simmons G., Jakobsen M.R., Conzelmann K.K., Pohlmann S., Munch J., Fackler O.T., Kirchhoff F., Sauter D. Cell Rep. 2019;27:2092–2104. doi: 10.1016/j.celrep.2019.04.063. e2010. [DOI] [PubMed] [Google Scholar]
- 40.Sagripanti J.-L., Routson L.B., Bonifacino A.C., Lytle C.D. Antimicrob. Agents Ch. 1997;41:812–817. doi: 10.1128/aac.41.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warnes S.L., Keevil C.W. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minoshima M., Lu Y., Kimura T., Nakano R., Ishiguro H., Kubota Y., Hashimoto K., Sunada K. J. Hazard. Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michels H., Wilks S., Noyce J., Keevil C. Stainless Steel. 2005;77000:27. [Google Scholar]
- 44.Scully J.R. NACE International; 2020. The COVID-19 Pandemic, Part 1: Can Antimicrobial Copper-based Alloys Help Suppress Infectious Transmission of Viruses Originating From Human Contact With High-touch Surfaces? [Google Scholar]