Abstract
Background
Healthcare workers (HCWs) are highly exposed to SARS-CoV-2 infection given their specific tasks. The IgG-IgM serological assay has demonstrated good accuracy in early detection in symptomatic patients, but its role in the diagnosis of asymptomatic patients is uncertain.
The aim of our study was to assess IgM and IgG prevalence in sera in a large cohort of HCWs previously subjected to Nasopharyngeal swab test (NST) after accurate risk assessment due to positive COVID-19 patient exposure during an observation period of 90 days.
Methods
2407 asymptomatic HCWs that had close contact with COVID-19 patients in the period between April 8th and June 7th were screened with NST based on the RT-PCR method. In parallel, they underwent large-scale chemiluminescence immunoassays involving IgM-IgG serological screening to determine actual viral spread in the same cohort.
Results
During the 90-day observation period, 18 workers (0.75%) resulted positive for SARS-CoV-2 infection at the NST, whereas the positivity rates for IgM and IgG were 11.51% and 2.37%, respectively (277 workers). Despite high specificity, serological tests were inadequate for detecting SARS-CoV-2 infection in patients with previous positive NST results (IgM and IgG sensitivities of 27.78% and 50.00%, respectively).
Conclusions
These findings indicate a widespread low viral load of SARS-CoV-2 among hospital workers. However, serological screening showed very low sensitivity with respect to NST in identifying infected workers, and negative IgG and IgM results should not exclude the diagnosis of COVID-19. IgG-IgM chemiluminescence immunoassays could increase the diagnosis of COVID-19 only in association with NST, and this association is considered helpful for decision-making regarding returning to work.
Keywords: Serological screening, COVID-19, Healthcare workers
1. Background
SARS-CoV-2 is a huge challenge for healthcare workers worldwide. The specific tasks of healthcare workers include daily contact with infected people, and the Hospital Health Administration is forced to rapidly adapt work conditions to avoid nosocomial cluster (Karuppiah et al., 2020). However, after the first large European wave of infection between March and May, the most recent literature focuses attention on asymptomatic patients as an effective and efficient source of contagion (Bhattacharya et al., 2020); the ability to intercept these patients is crucial to avoid new clusters and lockdown measures.
To date, among all available diagnostic methods for detecting SARS-CoV-2, real-time reverse transcription polymerase chain reaction (RT-PCR) using respiratory samples is the gold standard for COVID-19 diagnosis, but the combination of IgM and IgG antibodies offer increased sensitivity (Böger et al., 2020). Moreover, Deeks et al. affirmed that IgM antibody detection is a sensitive and specific tool to diagnose recent SARS-CoV-2 infection at least 15 days after close contact with an infected individual if NST was negative (Deeks et al., 2020).
To date, automated chemiluminescent immunoassay (CLIA) is the most validated serological test and seems to increase RT-PCR sensitivity (Soleimani et al., 2020). Recently, high sensitivity rates were described in IgM and IgG CLIA determination (88% and 100% after 12 days of symptom onset) (Padoan et al., 2020; Nicol et al., 2020). On the other hand, rapid detection SARS-CoV-2 antibody tests, e.g., lateral flow immunoassays (LFIAs), seem to have lower accuracy (Guedez-López et al., 2020; Zhang et al., 2020); in particular, the immunochromatographic antibody test is burdened by the high incidence of false positive results of IgG (Shibata et al., 2020).
The longitudinal profile of IgM and IgG kinetics revealed seroconversion for both within 6 days with pike times of 18 and 23 days, respectively (Shu et al., 2020). A positive IgG and/or IgM result in a single sample collected 2 weeks after symptoms in patients who were negative based on NST suggests SARS-CoV-2 infection; however, today, minimal evidence is available for the asymptomatic population (Long et al., 2020).
The aim of this study was to assess IgM and IgG prevalence in sera in a large cohort of HCWs previously subjected to NST after accurate risk assessment due to positive COVID-19 patient exposure during an observation period of 90 days.
2. Methods
Study group. All HCWs of the University Hospital of Bari, Italy underwent a preventive protocol that required them to undergo a NST in case of close contact with COVID-19 patients or evidence of SARS-CoV-2 symptoms onset (anosmia, ageusia, fever, asthenia, sore throat, rhinorrhea, cough, diarrhea, and dyspnea). All HCWs subject to NST, after 14–21 days, underwent sera collection for SARS-CoV-2 IgM and IgG determination. Occupational risk assessment was performed according to Italian Guidelines for Biological Occupational Risk and CDC guidance (Centre for Disease Contro, 2019; Alessio et al., 2005). The three risk categories were high, medium, and low.
Specimen collection and analysis. All the selected workers were submitted to a collection of nasopharyngeal SWAB specimens by trained staff following adequate standard operating procedures (SOPs), and during the collection, all specimens were handled carefully according to World Health Organization (WHO) criteria (World Health Organization, 2018; ecommended Surveilla; Guideline for the collect, 2000). The specimens underwent Nucleic Acid Amplification Tests (NAAT) for COVID-19. This method is based on the detection of unique sequences of virus RNA by real-time Reverse-Transcription Polymerase Chain Reaction (rRT-PCR). HCWs who had non-PPE guarded contact with confirmed COVID patients were placed in home isolation and therefore NST was performed at their home. On the other hand HCWs who had PPE-guarded contact have continued to work and therefore NST was performed at the Hospital Virologic Laboratory. Biological safety requirements were adhered to in both scenarios.
Serological test. IgG and IgM serological tests were performed according the Abbott tm method by the Abbott Company, which won the contract proclaimed by the Italian Government as an emergency provision for serological COVID tests.
Serum samples were inserted into an Abbott Architect Instrument i2000sr using the Abbott-specific assay based on chemiluminescent microparticle immunoassay with a positivity cutoff of 1.40 AU/ml for IgG and 1.0 AU/ml for IgM.
Statistical analysis. Data were collected using an Excel file (Microsoft Corporation tm). Absolute prevalence and percentage were calculated for positive results for NTS and serological tests. Descriptive statistics were performed to define the baseline characteristics of the study sample. The results are reported as the mean ± sd and frequency with percentages for continuous and categorical variables, respectively. To assess the role of different individual (sex, age) and occupational (biological risk, operative units, job titles) variables on the results of SWAB nasopharyngeal tests and serological tests, separate univariate logistic regressions were performed. Multiple logistic regression analyses were used to investigate the risk of observing serological positive tests after positive nasopharyngeal SWAB results (as an indicator of infection of COVID virus) by adjusted for sex, age, biological risk, selected job titles and operative units. The risk, both in univariate and multiple regressions, was expressed as an odds ratio (OR) with the relative 95% confidence interval (CI). All the results with a p-value<0.05 were considered statistically significant. The diagnostic accuracy of serological tests compared with the SWAB test was also evaluated. The measures considered included sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). All analyses were conducted with SAS software (version 9.4).
3. Results
During the 90-day observation period, 2407 of 5750 HCWs (41.86%) referred to at least one close contact with COVID-19 patients and underwent NST and serological determination in a period between the 14th and 21st days after NST. Table 1 shows all sociodemographic, professional and clinical characteristics of the HCWs enrolled. Participants were middle-aged (45.67 ± 11.96) and predominantly women (56.5%). A large part of the sample (approximately 70%) was composed of physicians and nurses (60.85%) mostly exposed, during their daily practice, to a medium biological risk, i.e. HCWs that provide direct assistance to patients, in the absence of procedures that generate aerosols, in the patient room/ward.The absolute prevalence of HCWs with a nasopharyngeal SWAB test positive for SARS-COV-2 infection was 18 out of 2470 (0.75%). The prevalence of a positive IgM serological test was 277 HCWs out of 2470 (11.51%), and the prevalence for IgG was 57 out of 2470 (2.37%). Table 2 shows the association between the HCWs and the three main outcomes of the survey. The only determinant associated with the positive SWAB test was job title (p-value = 0.027); subjects with SARS-COV-2 infection were most frequently physicians (72.2%).
Table 1.
Sociodemographic, professional and clinical characteristics of the HCWs enrolled.
| N | 2407 |
| AGE | 45.67 ± 11.96 |
| GENDER | |
| M | 1047 (43.5%) |
| F | 1360 (56.5%) |
| JOB TITLE | |
| Physician | 831 (34.52%) |
| Nurse | 809 (33.61%) |
| Support operator | 347 (14.42%) |
| Healthcare professions | 53 (2.2%) |
| Other | 367 (15.25%) |
| OPERATIVE UNIT | |
| Other clinical units | 1373 (57.16%) |
| Infectious diseases | 70 (2.91%) |
| Emergency room | 107 (4.45%) |
| Intensive care unit, anesthesiology | 357 (14.86%) |
| COVID units | 118 (4.91%) |
| Nonclinical units (no contact with patients) | 377 (15.7%) |
| BIOLOGICAL RISK | |
| Low risk | 281 (11.78%) |
| Medium risk | 1452 (60.85%) |
| High risk | 653 (27.37%) |
| SWAB | |
| Negative | 2389 (99.25%) |
| Positive | 18 (0.75%) |
| TEST IGM | |
| Negative | 2130 (88.49%) |
| Positive | 277 (11.51%) |
| TEST IGG | |
| Negative | 2350 (97.63%) |
| Positive | 57 (2.37%) |
Table 2.
Association between HCWs and survey outcome.
| SWAB |
IGM |
IGG |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NEGATIVE (%) | POSITIVE (%) | PVALUE | NEGATIVE (%) | POSITIVE (%) | PVALUE | NEGATIVE (%) | POSITIVE (%) | PVALUE | ||
| N | 2389 (99.3) | 18 (0.7) | 0.4113 | 2130 (88.5) | 277 (11.5) | 2350 (97.6) | 57 (2.4) | 0.0604 | ||
| Age | 45.7 ± 12.0 | 43.2 ± 11.8 | 46.0 ± 11.9 | 43.3 ± 12.2 | 0.0004 | 45.6 ± 12.0 | 48.9 ± 11.5 | |||
| Gender | M | 1041 (43.6) | 6 (33.3) | 0.8676 | 932 (43.8) | 115 (41.5) | 0.4794 | 1019 (43.4) | 28 (49.1) | 0.386 |
| F | 1348 (56.4) | 12 (66.7) | 1198 (56.2) | 162 (58.5) | 1331 (56.6) | 29 (50.9) | ||||
| Biological risk | Low risk | 281 (11.9) | 0 (0.0) | 0.3918 | 269 (12.7) | 12 (4.5) | 0.0004 | 278 (11.9) | 3 (5.5) | 0.2088 |
| Medium risk | 1440 (60.8) | 12 (70.6) | 1273 (60.1) | 179 (67.0) | 1413 (60.6) | 39 (70.9) | ||||
| High risk | 648 (27.4) | 5 (29.4) | 577 (27.2) | 76 (28.5) | 640 (27.5) | 13 (23.6) | ||||
| Job title | Physician | 818 (34.2) | 13 (72.2) | 0.027 | 718 (33.7) | 113 (40.8) | 0.0187 | 813 (34.6) | 18 (31.6) | 0.2921 |
| Nurse | 805 (33.7) | 4 (22.2) | 711 (33.4) | 98 (35.4) | 792 (33.7) | 17 (29.8) | ||||
| Support operator | 346 (14.5) | 1 (5.6) | 312 (14.6) | 35 (12.6) | 333 (14.2) | 14 (24.6) | ||||
| Health care professions | 53 (2.2) | 0 (0.0) | 48 (2.3) | 5 (1.8) | 52 (2.2) | 1 (1.8) | ||||
| Other | 367 (15.4) | 0 (0.0) | 341 (16.0) | 26 (9.4) | 360 (15.3) | 7 (12.3) | ||||
| Operative unit | Other clinical units | 1360 (57.0) | 13 (72.2) | 0.3089 | 1190 (55.9) | 183 (66.5) | <0.0001 | 1334 (56.8) | 39 (70.9) | 0.2516 |
| Infectious diseases | 70 (2.9) | 0 (0.0) | 68 (3.2) | 2 (0.7) | 69 (2.9) | 1 (1.8) | ||||
| Emergency room | 106 (4.4) | 1 (5.6) | 103 (4.8) | 4 (1.5) | 104 (4.4) | 3 (5.5) | ||||
| Intensive care unit, anesthesiology | 353 (14.8) | 4 (22.2) | 305 (14.3) | 52 (18.9) | 351 (15.0) | 6 (10.9) | ||||
| COVID units | 118 (4.9) | 0 (0.0) | 100 (4.7) | 18 (6.5) | 115 (4.9) | 3 (5.5) | ||||
| Nonclinical units (no contact with patients) | 377 (15.8) | 0 (0.0) | 361 (17.0) | 16 (5.8) | 374 (15.9) | 3 (5.5) | ||||
The IgM test results were associated with age (p-value = 0.0004), biological risk (p-value = 0.0004), job title (p-value = 0.0187) and operative units (p-value≤0.0001). In particular, seropositive workers were younger (43.3 ± 12.2), were more exposed to medium (67.0%) biological risk and were more frequently physicians (40.8%) working in clinical operative units (66.5%) compared with workers testing negative. The IgG test results were not associated with any of the determinants considered. Four HCWs remained asymptomatic until healing. The contact tracing protocol revealed that 17 of 18 HCWs had been in close contact with COVID-19 cases in non-workplace settings (households, schools, group-living and other social environments); only one, a 56-year-old physician, was in close contact with a COVID-19 patient at work.Univariate analyses were performed to express the previous associations in terms of odds ratios with 95% confidence intervals. The results are shown in Table 3 . The probability of obtaining positive results on the nasopharyngeal SWAB test was higher for physicians compared with all other job titles [OR = 4.99 (1.77–14.06)].
Table 3.
Univariate analyses.
| SWAB |
IGM |
IGG |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.98 (0.95–1.02) | 0.386 | 0.98 (0.97–0.99) | 0.0004 | 1.03 (1–1.05) | 0.0386 |
| Gender | ||||||
| M vs F | 0.65 (0.24–1.73) | 0.386 | 0.91 (0.71–1.18) | 0.4795 | 1.26 (0.75–2.13) | 0.3869 |
| Biological risk | ||||||
| Low risk YES vs NO | not estimable | 0.31 (0.17–0.57) | 0.0001 | 0.41 (0.13–1.33) | 0.1395 | |
| Medium risk YES vs NO | 1.32 (0.49–3.52) | 0.582 | 1.23 (0.95–1.6) | 0.1207 | 1.44 (0.82–2.53) | 0.2086 |
| High risk YES SI vs NO | 1.03 (0.37–2.91) | 0.95 | 1.02 (0.77–1.35) | 0.9023 | 0.79 (0.42–1.48) | 0.4588 |
| Job title | ||||||
| Physician YES vs NO | 4.99 (1.77–14.06) | 0.002 | 1.36 (1.05–1.75) | 0.0199 | 0.87 (0.5–1.54) | 0.6362 |
| Nurse YES vs NO | 0.56 (0.18–1.71) | 0.311 | 1.09 (0.84–1.42) | 0.5078 | 0.84 (0.47–1.48) | 0.5409 |
| Support operator YES vs NO | 0.35 (0.05–2.62) | 0.305 | 0.84 (0.58–1.23) | 0.3703 | 1.97 (1.07–3.65) | 0.0302 |
| Health care professions YES vs NO | not estimable | 0.8 (0.32–2.02) | 0.6332 | 0.79 (0.11–5.81) | 0.8164 | |
| Other YES vs NO | not estimable | 0.54 (0.36–0.83) | 0.0044 | 0.77 (0.35–1.72) | 0.5298 | |
| Operative unit | ||||||
| Other clinical units YES vs NO | 1.97 (0.7–5.54) | 0.2 | 1.54 (1.18–2) | 0.0013 | 1.65 (0.94–2.9) | 0.082 |
| Infectious diseases YES vs NO | not estimable | 0.22 (0.05–0.91) | 0.0358 | 0.59 (0.08–4.33) | 0.604 | |
| Emergency room YES vs NO | 1.27 (0.17–9.61) | 0.819 | 0.29 (0.11–0.79) | 0.0155 | 1.2 (0.37–3.9) | 0.7619 |
| Intensive care unit, anesthesiology YES vs NO | 1.65 (0.54–5.04) | 0.381 | 1.38 (1–1.92) | 0.0504 | 0.67 (0.29–1.57) | 0.3578 |
| COVID units YES vs NO | not estimable | 1.41 (0.84–2.37) | 0.1921 | 1.08 (0.33–3.51) | 0.8984 | |
| Nonclinical units YES vs NO | not estimable | 1.41 (0.84–2.37) | 0.1921 | 1.08 (0.33–3.51) | 0.8984 | |
Older HCWs were at a lower probability of being positive on the IgM test [OR = 0.98 (0.97–0.99)]. The workers exposed to a low biological risk exhibited a lower probability of being positive on the IgM test [OR = 0.31 (0.17–0.57)]. The physicians and the workers of the clinical operative units exhibited an increased probability of obtaining positive IgM test results [OR = 1.36 (1.05–1.75) and OR = 1.54 (1.18–2), respectively]. Unexpectedly, working at infectious disease units is a protective factor [OR = 0.22 (0.05–0.91)]. The probability of a positive IgG results was directly associated with the age expressed as years [OR = 1.03 (1–1.05)] and with the job of the support operators [OR = 1.97 (1.07–3.65)].
Table 4 presents the multivariate analysis results. The probability of positive IgM test results after positive nasopharyngeal SWAB test results as an indicator of infection adjusted for sex, age, biological risk, selected job titles and operative units was significantly high [OR = 2.91 (1.01–8.39)]. The probability of observing positive IgG tests after a positive nasopharyngeal SWAB test adjusted for the same variables was also relevant [OR = 77.32 (26.07–229.34)]. The multivariate analyses confirmed that age, biological risk (medium and high) and operative units (infectious diseases) are factors associated with IgM test positivity. Age was confirmed as a risk factor for IgG test positivity.
Table 4.
Multivariate analyses.
| IGM |
IGG |
|||
|---|---|---|---|---|
| AdjOR (95% CI) | p-value | AdjOR (95% CI) | p-value | |
| SWAB | ||||
| Positive vs Negative | 2.91 (1.01–8.39) | 0.0485 | 77.32 (26.07–229.34) | <.0001 |
| AGE | 0.98 (0.97–1) | 0.0075 | 1.03 (1.01–1.06) | 0.018 |
| Gender | ||||
| F vs M | 0.97 (0.75–1.27) | 0.8404 | 0.73 (0.41–1.29) | 0.2751 |
| Risk | ||||
| Medium vs Low | 2.91 (1.57–5.39) | 0.0007 | 3.03 (0.9–10.21) | 0.0744 |
| High vs Low | 2.84 (1.5–5.39) | 0.0014 | 2.32 (0.62–8.62) | 0.2091 |
| Job title | ||||
| Physician Yes vs No | 0.97 (0.72–1.3) | 0.8454 | 0.68 (0.35–1.35) | 0.2739 |
| Operative unit | ||||
| Infectious diseases Yes vs No | 0.2 (0.05–0.85) | 0.0289 | 0.85 (0.11–6.7) | 0.8733 |
Table 5 presents the accuracy of serological tests (both IgM and IgG) using the SWAB test as the gold standard.
Table 5.
Accuracy of serological tests (IgM and IgG) using the SWAB test as the gold standard.
| IGM | IGG | |
|---|---|---|
| Sensitivity | 27.78% | 50.00% |
| Specificity | 88.61% | 97.99% |
| Positive Likelihood Ratio | 2.44 | 24.89 |
| Negative Likelihood Ratio | 0.82 | 0.51 |
| Disease prevalence | 0.75% | 0.75% |
| Positive Predictive Value | 1.81% | 15.79% |
| Negative Predictive Value | 99.39% | 99.62% |
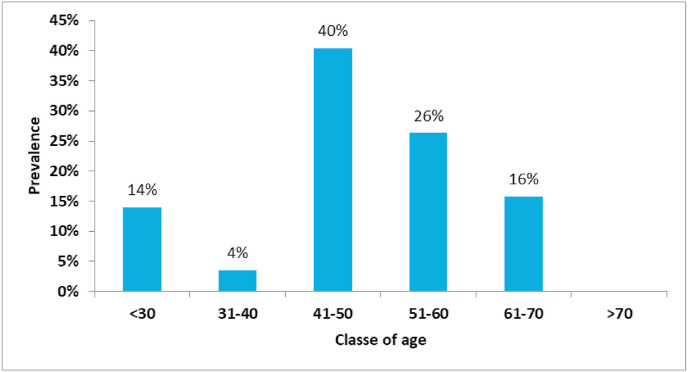
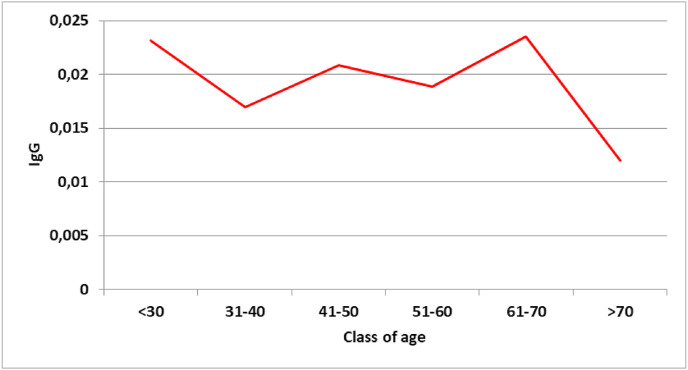
Sensitivity in identifying virus carriers was low for both IgM (27.78%) and IgG test (50.00%). The serological tests fail to indicate the presence of SARS-CoV-2 positivity in patients with a previous positive NST result. Specificity was very high for both IgM and IgG tests; true negatives are classified as such in a high percentage of cases. The age class with the highest prevalence of positive IgG was middle-aged workers (41–50) (Fig. 1 ). The classes with the highest antibody titers were very young workers (<30) and senior workers (61–70) (Fig. 2 ).
Fig. 1.
Prevalence of positive IgG results.
Fig. 2.
Geometric mean values of IgG.
4. Discussion
In the 90-day observation period, all HCWs subjected to combined screening were enrolled after accurate risk assessment postexposure to confirmed COVID patients. According to European Center of Disease Control (EDCD) guidelines (European Center for Disea), all HCWs with high biological risk were equipped with a full Personal Protective Equipment (PPE) kit (gloves, safety googles, FFP2 protection mask or better, disposable gown), and the gold standard test (RT-PCR) was mandatory after each close contact. Only a negative test result would allow HCWs to keep working, and serological tests were reserved for epidemiological purposes (Center for Disease Contro, 2019).
After double screening, we found an unexpectedly high serological prevalence of SARS-CoV-2 infection despite a low rate of RT-PCR positivity. IgM positivity was higher in each HCW category (nurse, physician, SHOs) with respect to IgG prevalence.
Considering the high specificity of the chemiluminescence immunoassay found in previous studies (Padoan et al., 2020) and calculated in our work using NST as the gold standard, these results could indicate the existence of numerous undiagnosed COVID-19 cases among HCWs in the assistant setting; infected people likely remained asymptomatic, which could explain the trouble in identifying these workers through NST (Liu et al., 2020). Moreover, the large difference in positive results between serological and nasopharyngeal tests could indicate many sources of unknown exposure to SARS-CoV-2 despite the implementation of preventive measures. An Italian cross-sectional study highlighted that approximately half of infected HCWs had no significant personal history of SARS-CoV-2 exposure, suggesting that many sources of contagion were unapparent. Thus, the exclusive use of RT-PCR screening is complex and not very useful (Lahner et al., 2020). Moreover, an appropriate window period seems to be essential to enhance the sensitivity of serological tests, increasing the gap in the positive result rate with respect to RT-PCR. In a multicenter retrospective study in Wuhan, China, 47% of SARS-CoV-2-infected people, who were mostly asymptomatic, were diagnosed with serological tests after negative RT-PCR (Tang et al., 2020), as demonstrated by Clarke and colleagues. A high seroprevalence of SARS-CoV-2 antibodies was noted in asymptomatic or PCR-negative patients receiving in-center hemodialysis, suggesting that current diagnostic screening strategies may be limited in their ability to detect acute infection (Clarke et al., 2020). In our study, we found a high seroprevalence of SARS-CoV-2 with the Abbott test, but low sensitivity in identifying virus carriers and low positive predictive values in 2407 serum samples. Thus, serological tests did not adequately indicate SARS-CoV-2 infection in patients with a previous positive NST result (IgM and IgG sensitivity of 27.78% and 50.00%, respectively). These results are in contrast to those from Batra and colleagues (Batra et al., 2020), who found that the test had a specificity of 100% and sensitivity of 99.1% for specimens collected >14 days post symptom onset or >5 days post-RNA testing. On the other hand, Meschi et al. (2020) showed that the Abbott system could have lower sensitivity, which is more similar to our results (61.9% on the fourteenth day).
These results suggest that NST based on the RT-PCR method should be the gold standard for guiding decisions on quarantine and readmission to work in occupational health surveillance.
At present, serological tests cannot replace the molecular diagnostic test as they are not characterized by sufficient validity due to their low sensitivity in identifying virus carriers. Therefore, there are no recommendations for their use for both diagnostic (early identification of infected subjects) and prognostic purposes in occupational settings, nor to determine the work suitability for each worker.
Serological tests, on the other hand, are very important in the epidemiological evaluation of viral circulation in workplaces and in risk assessment. Fundamentally, they allow the estimation of the number of subjects infected, as well as tracking the spread of the diseases through a specific population, setting or environment over time. Accordingly, serological screening programs in the different hospital departments can contribute, for example, to the identification of the departments with the higher risk of virus circulation. Further investigations are required to better understand the utility of these tests in decisions regarding the return to work of HCWs with positive serological tests.
5. Conclusion
We found a high unexpected prevalence of SARS-CoV-2 among 2407 HCWs of University Hospital of Bari, Italy despite a low positivity rate for RT-PCR tests, which is considered the gold standard for diagnosis. These results could suggest that serological screening of SARS-CoV-2 antibodies should be implemented to better track positive HCWs but only in association with RT-PCR. Due to the low sensitivity in identifying virus carriers of the Abbott Serological Test highlighted in our study, further investigations are needed to utilize serological test results in decisions regarding the return of HCWs to work.
Author contributions
Luigi Vimercati had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization and Project administration: LV, PLL, LG, GM and ST. Data curation: DC, MQ, LDM, AC, PS, SS and AMVL. Writing (original draft - review & editing): LDM, AC and MQ. Formal analysis: GG. Methodology and Supervision: LV and ST.
Funding source
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Patient consent
Informed and written consent was obtained from the participants. The patients were informed that data from the research protocol would be treated in an anonymous manner based on scientific methods and for scientific purposes in accordance with the principles of the Helsinki Declaration.
Ethical approval is not necessary because all medical and instrumental examinations were performed according to Italian laws concerning the protection of workers exposed to occupational risks (D. Lgs. 81/2008).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alessio L., Porru S., Aparo U.L., Bassetti D., Beltrame A., Buzzi F., Cipolloni L., Germano T., Lombardi R., Longo F., et al. Linee Guida per la Sorveglianza Sanitaria dei Lavoratori della Sanità esposti a rischio biologico. Soc. Ital. Med. Lavoro ed Igiene Ind.: PIME: Pavia, Italy. 2005;ume 17 [Google Scholar]
- Batra R., Olivieri L.G., Rubin D., Vallari A., Pearce S., Olivo A., Prostko J., Nebbia G., Douthwaite S., Rodgers M., Cloherty G. A comparative evaluation between the Abbott Panbio™ COVID-19 IgG/IgM rapid test device and Abbott Architect™ SARS CoV-2 IgG assay. J. Clin. Virol. 2020 Sep 16;132:104645. doi: 10.1016/j.jcv.2020.104645. Epub ahead of print. PMID: 32961429; PMCID: PMC7493757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Basu P., Poddar S. Changing epidemiology of SARS-CoV in the context of COVID-19 pandemic. J Prev Med Hyg. 2020;61(2):E130–E136. doi: 10.15167/2421-4248/jpmh2020.61.2.1541. Published 2020 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19 [published online ahead of print, 2020 Jul 10] Am. J. Infect. Contr. 2020;S0196–6553(20):30693. doi: 10.1016/j.ajic.2020.07.011. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
- Centre for Disease Control and Prevention Centre for disease control and prevention: interim operational considerations for public health management of healthcare workers exposed to or with suspected or confirmed covid-19: non-u.s. healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/public-health-management-hcw-exposed.html Available online:
- Clarke C., Prendecki M., Dhutia A., et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening [published online ahead of print, 2020 jul 30] J. Am. Soc. Nephrol. 2020 doi: 10.1681/ASN.2020060827. ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Dinnes J., Takwoingi Y., et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6(6):CD013652. doi: 10.1002/14651858.CD013652. Published 2020 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Recommended Surveillance Standards WHO/CDS/CSR/ISR/99.2 https://www.who.int/csr/resources/publications/sur veillance/whocdscsrisr992.pdf
- European Center for Disease Control (ECDC) https://www.ecdc.europa.eu/sites/default/files/documents/Infection-prevention-and-control-in-healthcare-settings-COVID-19_4th_update.pdf
- Guedez-López G.V., Alguacil-Guillén M., González-Donapetry P., et al. Evaluation of three immunochromatographic tests for rapid detection of antibodies against SARS-CoV-2 [published online ahead of print, 2020 Aug 17] Eur. J. Clin. Microbiol. Infect. Dis. 2020:1–9. doi: 10.1007/s10096-020-04010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideline for the Collection of Clinical Specimens during Field Investigation of Outbreaks https://www.who.int/ihr/publications/WHO_CDS_ CSR_EDC_2000_4/en/ WHO/CDS/CSR/EDC/200.4.
- Karuppiah A., Bharadwaj S., Crimmins S., Elsamadicy E., Rabin J., Kodali B.S. Adaptation of labor and delivery to COVID-19. Am J Disaster Med. 2020;15(2):93–97. doi: 10.5055/ajdm.2020.0359. [DOI] [PubMed] [Google Scholar]
- Lahner E., Dilaghi E., Prestigiacomo C., et al. Prevalence of SARS-CoV-2 infection in health workers (HWs) and diagnostic test performance: the experience of a teaching hospital in Central Italy. Int. J. Environ. Res. Publ. Health. 2020;17(12):4417. doi: 10.3390/ijerph17124417. Published 2020 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lian R., Zhang G., et al. Changes in serum virus-specific IgM/IgG antibody in asymptomatic and discharged patients with reoccurring positive COVID-19 nucleic acid test (RPNAT) [published online ahead of print, 2020 Aug 18] Ann. Med. 2020:1–14. doi: 10.1080/07853890.2020.1811887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q., Liu B., Deng H., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Meschi S., Colavita F., Bordi L., Matusali G., Lapa D., Amendola A., Vairo F., Ippolito G., Capobianchi M.R., Castilletti C. INMICovid-19 laboratory team. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J. Clin. Virol. 2020 Aug;129:104539. doi: 10.1016/j.jcv.2020.104539. Epub 2020 Jul 6. PMID: 32679298; PMCID: PMC7336910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol T., Lefeuvre C., Serri O., et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J. Clin. Virol. 2020;129:104511. doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoan A., Cosma C., Sciacovelli L., Faggian D., Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020;58(7):1081–1088. doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- Shibata S., Ishiguro T., Kobayashi Y., et al. High incidence of false-positive results of IgG antibody against SARS-CoV-2 with rapid immunochromatographic antibody test due to human common cold coronavirus infection. Respir Med Case Rep. 2020;31:101180. doi: 10.1016/j.rmcr.2020.101180. . Published 2020 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H., Wang S., Ruan S., et al. Dynamic changes of antibodies to SARS-CoV-2 in COVID-19 patients at early stage of outbreak [published online ahead of print, 2020 jul 27] Virol. Sin. 2020:1–8. doi: 10.1007/s12250-020-00268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani R., Khourssaji M., Gruson D., et al. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID-19 patients [published online ahead of print, 2020 Aug 14] J. Med. Virol. 2020 doi: 10.1002/jmv.26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Tian J.B., Dong J.W., et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in wuhan, China [published online ahead of print, 2020 jul 3] Am. J. Kidney Dis. 2020;S0272–6386(20):30786. doi: 10.1053/j.ajkd.2020.06.008. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2018. Protocol to Investigate Non-seasonal Influenza and Other Emerging Acute Respiratory Diseases.https://www.who.int/influenza/resources/publicati ons/outbreak_investigation_protocol/en/ 2018. [Google Scholar]
- Zhang C., Zhou L., Liu H., et al. Establishing A high sensitivity detection method for SARS-CoV-2 IgM/IgG and developing a clinical application of this method [published online ahead of print, 2020 aug 17] Emerg. Microb. Infect. 2020:1–27. doi: 10.1080/22221751.2020.1811161. [DOI] [PMC free article] [PubMed] [Google Scholar]