Abstract
Abstract: Literature has been published stating that thrombosis is occurring at higher rates in patients who are positive for COVID-19. This experience is more with limb ischemia. Reports of mesenteric ischemia are coming in from different parts of the globe. We share our early experience of managing two patients with acute mesenteric ischemia.
Keywords: AMI, Acute, Mesenteric, Ischemia, COVID -19, SARS CoV-2
INTRODUCTION
Since the rapid spread of the novel corona virus (COVID-19; SARS CoV-2) evidence has been published about its association with thrombosis.1 Early publications attributed computerized tomography (CT) chest findings to endothelial injury, thrombosis and decreased fibrinolysis which was later on detected in other areas of the vascular tree.2 The exact mechanism behind this pathology is not very well known, however, proposed hypothesis suggests a severe inflammatory response that leads to thrombo-inflammation.1 Disease manifestations vary between mild symptoms to critical illness. Studies to understand the pathophysiology of this pathology are ongoing as the data is gathered and experience shared. We present 2 cases of acute mesenteric ischemia (AMI), which were COVID positive on nasopharyngeal polymerase chain reaction test; an arterial and nonocclusive each, and their outcome.
Case 1
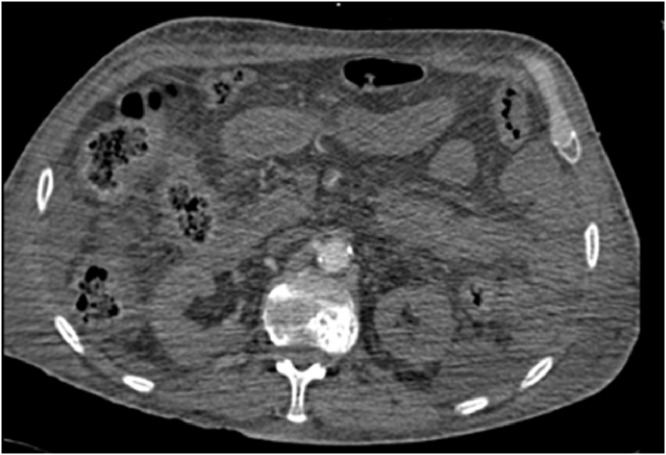
A 51-year-old male with no co-morbidities presented to our emergency department with a 2-day history of shortness of breath and fever. His inflammatory markers, ferritin, fibrinogen, and D dimer levels were elevated (Table 1 ). The chest X-ray revealed bilateral patchy consolidation. He was intubated as he was desaturating; shifted to the intensive care unit (ICU) and was proven to be COVID positive. A CT chest confirmed acute respiratory distress syndrome changes. He was managed with Azithromycin, Oseltamivir, Methylprednisolone, prophylactic dose of enoxaparin 40 mg once a day and esomeprazole 40 mg once a day, intermittent prone ventilation for 16 hours, nasogastric feeds, and minimal inotropic support. On the 11th day of admission he was extubated. Three days later his conscious level deteriorated and was re-intubated. Imaging of the brain and cerebral spinal fluid studies were normal. Day 13 postreintubation he was febrile with raised inflammatory markers but there was no growth on blood culture. Chest X-ray revealed worsening consolidation, despite upgrading antibiotics, and he required inotropes. His arterial blood gas showed an element of compensated metabolic acidosis with a lactate of 7. CT angiography (CTA) of the abdomen revealed non occlusive AMI (Fig. 1 ). The small bowel was hypo-perfused, aorta, celiac trunk, superior mesenteric artery (SMA), and inferior mesenteric artery were well opacified with no filling defects, furthermore, left lower limb deep vein thrombosis was noticed at the left common iliac vein which was extending to the inferior vena cava confluence. Therapeutic dose of Enoxaparine - 40 mg (1 mg × 40 kg twice a day) was administered. A laparotomy to resect nonviable bowel was planned, however, his hemodynamic stability worsened and the family refused surgical intervention. He succumbed about 12 hours later.
Table 1.
Lab values of case 1 and case 2.
| Normal lab values | Case 1 | Case 2 |
|---|---|---|
| Hb (11.5–15.5 g/dl) | 5.9 g/dl | 10 mg/l |
| WBC (4.5 to 11.0 × 109/L) | 30 × 109/L | 16 × 109/L |
| Fibrinogen (1.7–3.6 g/l) | 10 g/l | 5 g/l |
| Ferritin (30–400 ug/L) | 687 ug/L | 619 ug/l |
| D dimer (0.2–.0.7 mg/L) | 2.5 mg/L | 10 mg/l |
Fig. 1.
Abdominal CT Angiography demonstrating hypo perfused small bowel consistent with non occlusive AMI.
Case 2
A 51-year-old patient was tested positive for COVID 19 virus 4 days prior to presentation to our emergency department with a 3 day history of generalized abdominal pain and poor oral intake, not associated with nausea or vomiting. Examination of his abdomen revealed signs of peritonitis. His inflammatory markers, fibrinogen, ferritin and D dimer levels were elevated (Table 1). AMI was suspected and a CTA of the abdomen revealed SMA thrombosis and nonenhancing proximal ileal loops consistent with small bowel ischemia (Fig. 2 ). At laparotomy about 30 cm segment of gangrenous jejunum, 20 cm from the duodenojejunal flexure, was resected. SMA thrombectomy was performed successfully. The distal 20 cm of ileum was dusky in color, however; it was not ischemic; the abdomen was closed temporarily, unfractionated heparin (UFH) infusion started for anticoagulation, and a re-look laparotomy performed after 24 hours. At the re-look a further 10 cm gangrenous jejunal segment was resected; cecum and terminal ileum were dusky in color and a second re-look laparotomy was planned after 24 hours. At the second re-look the ischemic cecum and terminal ileum were resected and side to side jejuno-colic anastomosis performed. He was continued on UFH infusion for anticoagulation. He did not require ionotropic support between the re-look surgeries and was extubated on day 1 post-op. TPN was started post-op and oral feeds as tolerated were resumed a week later. His symptoms of short gut syndrome were managed medically.
Fig. 2.
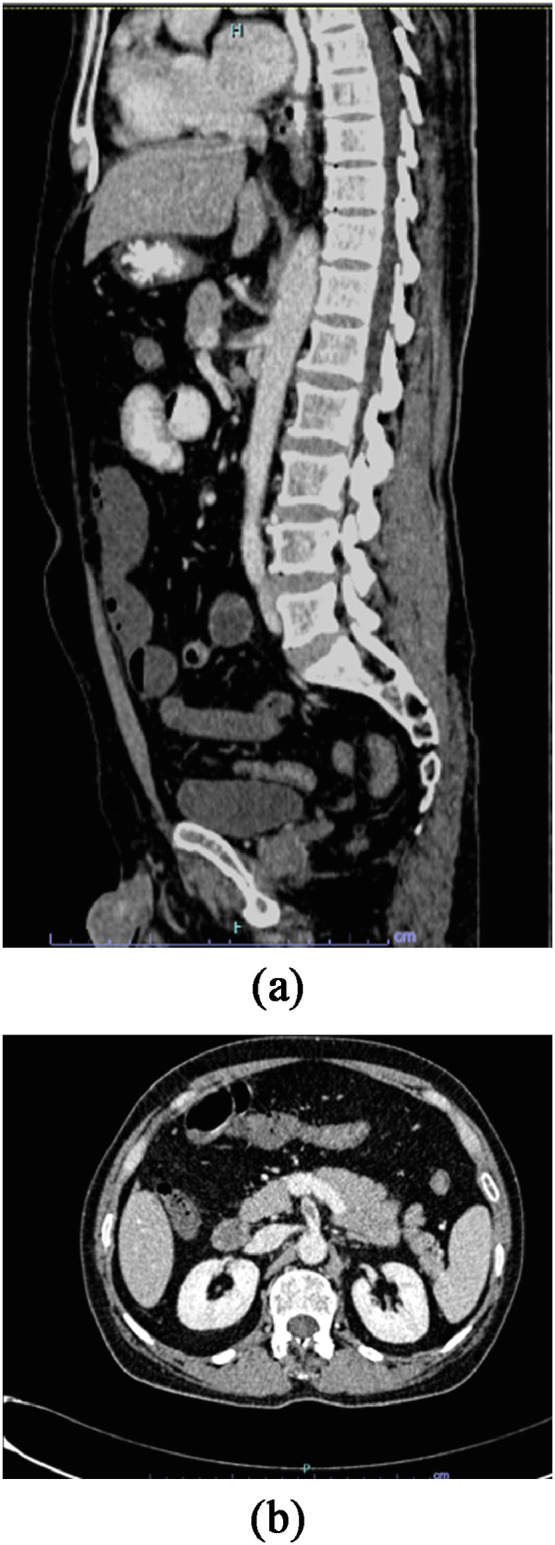
A, B: Abdominal CT Angiography demonstrating superior mesenteric artery thrombosis and nonenhancing proximal ileal loops consistent with arterial AMI.
Discussion
AMI is defined as a sudden disruption in the blood supply within the mesenteric circulation and patients often present with “more symptoms, than signs” of the acute ischemia. It accounts for 1 in every 1000 hospital admissions and the mortality rate ranges between 60% and 80%. Hence, it is required of clinicians to have a high index of suspicion and perform a CTA as soon as possible.3., 4., 5. The causes of AMI are arterial embolism/thrombosis (60%–70%), nonocclusive (20%–30%), and venous occlusion (5%–10%). Emboli preferentially affect SMA because of its small take-off angle, compared with those of the coeliac and inferior mesenteric artery.3 The thrombus in the SMA is seen shortly after the origin, unlike the findings in atheromatous lesions of the SMA.6
Gastrointestinal (GI) symptoms have been described in 10% of patients with COVID-19 infection.7 In a recent meta-analysis of 29 studies the COVID-10 induced GI symptoms symptoms were anorexia (21%), nausea/vomiting (7%), diarrhea (9%), and abdominal pain (3%).8
Critical illness may cause a hypercoagulable state due to immobilization, mechanical ventilation, central venous access devices, and nutritional deficiencies. In patients with COVID-19, studies have shown that the virus has the ability to spark a cycle of inflammation and thrombosis through angiotensin converting enzyme 2 receptors located on the alveoli surface.1 Angiotensin converting enzyme 2 is expressed in lung alveolar epithelium, enterocytes of small intestine, and vascular endothelium, therefore small bowel vasculature maybe susceptible to SARS-CoV-2 infection.9 Moreover, there are direct effects on the coagulation pathway as shown by the presence of microthrombi and fibrinogen deposits in the histopathology of COVID-19 patients. Autopsies of ten African-American patients with severe COVID-19 revealed an element of microthrombi and microangiopathic pathology supporting the fact that this virus may induce in situ thrombosis in small blood vessels rather than an embolic event.10
Patients with severe COVID-19 complicated by AMI may present classically with abdominal pain out of proportion to examination, vomiting, diarrhoea, abdominal distention, or worsening systemic status/sepsis.4 , 11 , 12 Lab investigations should include D-dimer, fibrinogen, and ferritin levels as these are often elevated.8 In a retrospective study describing seven patients with COVID-19 induced GI symptoms; 4 out of 7 were found to have an element of AMI out of which 6 had elevated levels of D dimer.8 It was found that a longitudinal increase in the level of D Dimer during hospitalization is associated with high mortality rates in COVID-19 patients.1 , 10 Both our patients had elevated levels of D dimer.
The imaging study of choice used to diagnose AMI is CTA; its specificity is >95%; findings of intestinal pneumatosis, portal venous gas, lack of bowel wall enhancement, and ischemia of other organs should rise the suspicious of AMI.13 Patients with severe COVID-19 who are on mechanical ventilation may display an element of pneumatosis on CT; therefore, this finding should be interpreted cautiously.11
The initial care of patients with AMI and COVID-19 positive tests should follow the established guidelines for resuscitation and intervention.12 Recent studies recommend the administration of prophylactic dose of low molecular weight heparin to all patients admitted with COVID-19 because of its thromboembolic complications. Further to this, in order to reduce morbidity and mortality patients require fluid resuscitation, restoration of flow in the mesenteric arterial/venous tree with or without bowel resection, and inotropic support.14 , 15
Conclusion
AMI in COVID patients should be suspected when they present with sudden abdominal pain out of proportion to the abdominal signs and a CTA performed as soon as possible to confirm the diagnosis. The cause is quite likely to be micro-thrombosis and microangiopathy secondary to the virus which directly affect the coagulation pathway. An increased use of re-look laparotomy, temporary closure of abdomen/lapaostomy should be considered along with anticoagulation. Till such time that a better understanding of the cause of hypercoagulability and a balance between therapeutic and intense anticoagulation has clarity; morbidity and mortality in this subset of patients is likely to be high.
Footnotes
Conflict of interest: The author has no conflict of interest.
References
- 1.Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thrombosis Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhayana R, Som A, Li MD, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;11 doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florim S, Almeida A, Rocha D, et al. Acute mesenteric ischaemia: a pictorial review. Insights Imaging. 2018;17:673–682. doi: 10.1007/s13244-018-0641-2. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clair DG, Beach JM, Mesenteric Ischemia, Campion EW, editors. N Engl J Med. 2016;10:959–968. doi: 10.1056/NEJMra1503884. 374. [DOI] [PubMed] [Google Scholar]
- 5.Kassahun WT, Schulz T, Richter O, et al. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg. 2008;393:163–171. doi: 10.1007/s00423-007-0263-5. [DOI] [PubMed] [Google Scholar]
- 6.Mastoraki A, Mastoraki S, Tziava E, et al. Mesenteric ischemia: pathogenesis and challenging diagnostic and therapeutic modalities. World J Gastrointest Pathophysiol. 2016;15:125–130. doi: 10.4291/wjgp.v7.i1.125. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norsa L, Pietro B, Indriolo A, et al. Poor outcome of intestinal ischemic manifestations of COVID 19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;1:185–192. doi: 10.1007/s11684-020-0754-0. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 11.Parry AH, Wani AH, Yaseen M. Acute mesenteric ischemia in severe coronavirus-19 (COVID-19): possible mechanisms and diagnostic pathway. Acad Radiol. 2020;27:1190. doi: 10.1016/j.acra.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emile SH, Khan SM, Barsoum SH. Predictors of bowel necrosis in patients with acute mesenteric ischemia: systematic review and meta-analysis. Updates Surg. 2020 doi: 10.1007/s13304-020-00857-9. [DOI] [PubMed] [Google Scholar]
- 13.Stephen E, Sarfaraz ZK, Abdelhedy I, et al. Acute mesenteric ischemia: the what, why, and when? Indian J Vasc Endovasc Surg. 2016;3:24–28. [Google Scholar]
- 14.Ferner Robin E, Levi Marcel, Sofat Reecha, et al. On behalf of the Oxford COVID-19 Evidence Service Team; 2020. Thrombosis in COVID-19: clinical outcomes, biochemical and pathological changes, and treatments. [Google Scholar]
- 15.Singh B, Kaur P. COVID-19 and acute mesenteric ischemia: a review of literature. Hematol Transfus Cell Ther. 2020 doi: 10.1016/j.htct.2020.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]