Abstract
Introduction and importance
SARS-CoV-2 is a novel infection that has affected millions of people around the world. Complications of the infection may affect multiple systems including cardiovascular, neurological, gastrointestinal, urinary, and pulmonary systems. Hypokalemia, which is a life-threatening condition that may lead to arrhythmia and possibly death, has been noticed in more than half of the COVID-19 patients. Further understanding of the disease process and its complications is necessary to guide in preventing the complications from happening in the first place and finding treatment for patients with an already established complications.
Case presentation
A 34-year old male from Philippines who lives in Saudi Arabia – Riyadh and works as health care provider with no previous history of any medical illness. Presented by himself to the emergency department (ED) with dry cough, shortness of breath, fever, malaise, and fatigability for five days. On examination (RR 25), (T 38.6 °C) and (O2 89% Room air), on auscultation there was a decrease on air entry bilaterally with scattered crepitations, no wheezing or stridor. Covid-19 swab was positive, (Day 1) potassium 2.91 (mmol/L) magnesium (mmol/L) with normal baseline before getting infected.
Clinical discussion
Patient while in the hospital was on daily potassium oral and IV replacement with IV magnesium replacement. Investigation showed 24Hr urine potassium 47.3 (mmol/L), 24Hr urine magnesium 5.52 (mmol/L), 24Hr urine Creatinine 9.25 (mmol/L), (TTKG) Transtubular Potassium Gradient 18 and (VBG) PH:7.38, Pco2:44 (mmHg) Po2:55 (mmHg) HCO3:25 (mEq/L). Patient has an increased renal potassium loss with normal VBG on separate days and normal Blood pressure that excludes diseases with associated acidemia or alkalemia. Our patient didn't want to go for any invasive diagnostic procedures and favored to wait for spontaneous recovery.
Conclusion
We followed up the potassium level of our patient for more than 5 months since he was diagnosed with COVID-19 to find out that he is still having hypokalemia, as well as, hypomagnesemia. Long term complications of COVID-19 infection such as hypokalemia and hypomagnesemia need to be observed and followed up closely to avoid life-threatening arrythmias and seizures. The attention of the scientific community to possible long term or permanent complications is needed to help find preventive measures and treatment for patients with complications.
Keywords: Case report, COVID-19, Hypokalemia, Hypomagnesemia, SARS-CoV-2
Highlights
-
•
COVID-19 is a new disease and its complications are not fully understood.
-
•
The alteration of the ACE2 receptors and RAS system dysregulation lasted for 5 months.
-
•
Presistent electrolytes disturbance after COVID-19 infection is a burden on both patients and their treating physicians.
1. Introduction
COVID-19 is a new pandemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. COVID-19 manifestations ranged from asymptomatic or mild symptoms to severe ones and death [3]. Fever, cough, and fatigue have been typical symptoms. Other less common symptoms of COVID-19 patients include nasal congestion, sore throat, aches and pains, headache, conjunctivitis, diarrhea, loss of taste and/or smell, discoloration of toes or fingers, or a skin rash. In severe cases, patients may suffer shortness of breath, chest pain and/or loss of movement or speech [2]. Although SARS-CoV-2 mainly affects the lungs and respiratory system, it can affect multiple organs and systems, including blood vessels, cardiovascular system, nervous system, liver, kidney and gastrointestinal tract [4]. However, many of the details of COVID-19 complications are not yet understood. In this report, we present a recovered COVID-19 case with persistent hypokalemia and hypomagnesemia for more than five months.
This case report has been reported in line with the SCARE 2020 Guideline [5].
2. Case description
A 34-year old male from Philippines who lives in Saudi Arabia – Riyadh and works as health care provider with no previous history of any medical illness. Presented to the emergency department (ED) with dry cough, shortness of breath, fever, malaise, and fatigability for five days. The patient shortness of breath has been increasing in severity over the last two days before coming to the ED. Upon arrival to ED, patient was awake, alert oriented to time, place and person, tachypneic, in distress, mobilizing without support, Glasgow coma scale was 15/15 and his temperature 38.6 °C, oxygen saturation 89% on room air, blood pressure 134/74 mmHg, respiratory rate 25 breaths per minute, and heart rate 97 beats per minute. Patient has no history of previous hospital admissions, surgical history, blood transfusion, smoking, alcohol drinking or drug abuse, allergies, or regular home medications. His father has diabetes, both parents are alive and well, no history of any sibling death or abortions, no family history of genetic diseases, malignancy, renal or gastrointestinal diseases. Patient unmarried, totally independent, lives with two other coworkers in an apartment.
On physical examination, patient had no chest wall deformity, finger clubbing, tar stains, needle marks or lymph nodes enlargement. Trachea was central and JVP is 8 cm. On auscultation there was a decrease on air entry bilaterally with scattered crepitations, no wheezing or stridor. Heart apex located over the left 5th intercostal space mid clavicular line, on auscultation patient had normal systolic and diastolic heart sounds with no added sounds or audible murmurs. Abdomen was soft non-tender with liver span is 8 cm and non-palpable spleen, no shifting dullness. On auscultation patient had normal bowel sounds and no renal bruits, and other physical examination unremarkable. Chest x-ray shown (Fig. 1).
Fig. 1.
Chest X-ray.
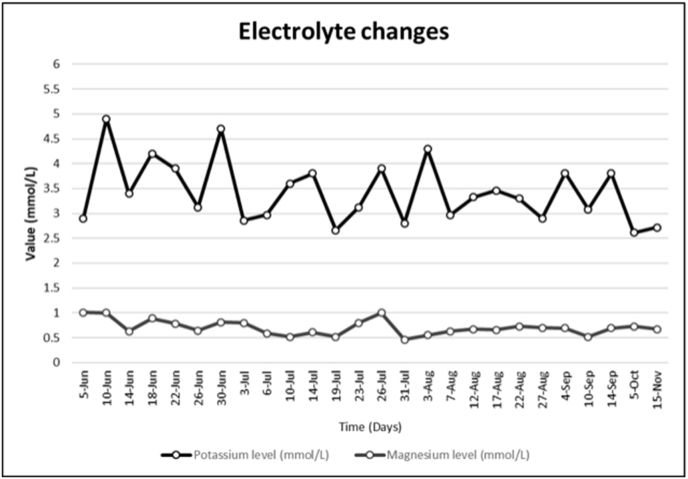
He was connected to supplemental 3 L/min oxygen via nasal cannula maintaining saturation 92% and was started on supportive management. Investigations were requested for him including ECG, chest x-ray, nasopharyngeal swab for COVID19, LFT, KFT, D-dimer, ferritin, electrolytes, and CBC with differential. During his first day in ED, his oxygen saturation started to drop, and oxygen requirement increased up to 12 L/min. He was then seen by an ICU doctor who has decided to admit the patient in the ICU-isolation after he was supported by mechanical ventilator. Initial lab results on day 1 showed HGB:14.9 (g/dl), WBC:9 (109/L), Lymphocyte:0.76 (109/L), Neutrophil:8.19 (109/L), PLT:190 (109/L), Na:134 (mmol/L), Cl:102 (mmol/L), K:2.91 (mmol/L), Mg: 1.01 (mmol/L), creatinine:75.3 (umol/L), ALT:112 (U/L), AST:95 (U/L), ALP:56 (U/L), Ferritin:4445 (ng/mL), D-dimer:2.5 (μg/ml), ESR:63 (mm/hr), CRP: 80.2 (mg/L). (VBG) PH:7.38, Pco2:44 (mmHg) Po2:55 (mmHg) HCO3:25 (mEq/L) Na:139 (mmol/L) Cl:109 (mmol/L), and normal anion gap. On day 2 and during his admission in ICU-isolation his COVID-19 result came positive. During his ICU-Isolation admission for around 24 days, the patient was treated with Vit-D, zinc sulphate, thiamine, interferon beta, ribavirin, lopinavir/ritonavir, and methylprednisolone in addition to other supportive management including prone positioning. Fortunately, the patient showed improvement and his COVID-19 became negative 2 times, first 6/24/2020 and the second one on 7/1/2020. After extubation, he was transferred to the general ward where he stayed for around 10 days due to ICU acquired weakness for Neurology evaluation and management along with Physiotherapy, he was still having persistent hypokalemia and hypomagnesemia. Since ICU admission patient has been receiving daily potassium replacement 30–40 mEq IV and magnesium 2g IV except on days when either potassium or magnesium reach normal levels as shown in (Fig. 2), the electrolytes replacement were based on frequent electrolytes lab results. Patient is always having normal blood pressure SBP ranges between (110–130). Patient wasn't having any diarrhea or loose bowel motions. Patient wasn't having polyuria, polydipsia or diaphoresis. Patient has normal Leukocyte count and morphology, TSH 3.84 (uIU/mL) FT4 15.4 (pmol/L) FT3 6.12 (pmol/L), 24Hr urine potassium 47.3 (mmol/L)A, (TTKG) Transtubular Potassium Gradient 18B. VBG is normal not showing acidosis, alkalosis or mixed picture, normal range Co2 and Hco3. 24Hr urine magnesium 5.52 (mmol/L)C, calculated Urine osmolality 285 (mOsm/kg), Serum osmolality 290 (mOsm/kg). 24Hr urine Creatinine 9.25 (mmol/L)D, 24Hr urine phosphorus 22.8 (mmol/L)E, 24Hr urine glucose 0.36 (mmol/L)F, PTH 7.6 (pmol/L)G Serum Phosphorus 1.14 (mmol/L), Corrected Serum Calcium 2.3 (mmol/L), 24Hr urine calcium 2.9 (mmol/L)H. During his 35 days admission, we noticed that the patient is having persistent hypokalemia and hypomagnesemia, therefore he needed continuous potassium and magnesium replacement even though he recovered the respiratory symptoms that was caused by COVOD-19 infection. Upon discharge time on 7/10/2020, most lab results were normal including ALT, AST, ESR, CRP, and lymphocyte count, except the potassium and magnesium levels that were persistently low. Thus, he was discharged on oral potassium (750 mg three times per day) and oral magnesium (400mg twice daily) replacements, as well as, nutritional advice. By going back in time before the patient got infected, we found Four normal results for his potassium level on (2/8/2018) K: 3.95 (mmol/L), on (8/14/2018) K: 4.15 (mmol/L), on (11/4/2019) K: 3.7 (mmol/L) and on (3/24/2020) K: 4 (mmol/L). We followed up with the patient periodically after he was discharged from the hospital through clinic visits for clinical examination and blood tests to replace and avoid complications of electrolyte disturbance. On August 7th his potassium level was 2.9 (mmol/L) and the magnesium level was 0.63 (mmol/L) on September 10th his potassium level was also low 3.08 (mmol/L) and magnesium level was 0.52 (mmol/L), on October 26th his potassium level was also low 2.77 (mmol/L). The patient was asked to stop oral supplements 3 days before taking blood to test his potassium and magnesium levels. On November 9th he continued on high potassium diet only, unfortunately his potassium dropped again to 2.6 (mmol/L) after 10 days without supplements. No complications developed after discharging the patient.
Fig. 2.
Diagram for the electrolytes changes.
A: Normal range (25–125) (mmol/day).
B: Normal range (8–9).
C: Normal range (1–10.5) (mmol/day).
D: Normal range (8.4–22) (mmol/day).
E: Normal range (13–42) (mmol/day).
F: Normal range (0–2.8) (mmol/L).
G: Normal range (1.1–8.4) (pmol/L).
H: Normal range (1–8.8) (mmol/day).
3. Discussion
SARS-CoV-2 belongs to a group of severe acute respiratory syndrome-related coronaviruses [6]. It started in December 2019 in China, and was declared a pandemic by World Health Organization (WHO) on 11th of March 2020 [7].
SARS-CoV-2 matches 79% of the genetic sequence homology with SARS-CoV that was identified on 2003, and they both target ACE2 receptor for their entrance to cells, but the novel virus has distinct mechanism which is direct kidney parenchyma infection and secondary endothelial injury, COVID-19 virus can directly infect the renal tubular epithelium and podocytes, which lead to proteinuria and acute kidney injury [8]. COVID-19 infection is also associated with high rates of extra-pulmonary complications that may continue to incur morbidity, disability, and delayed mortality in survivors. These include cardiac injury, acute ischemic or hemorrhagic stroke, neurological deficits, acute kidney injury, including the need for dialysis, and liver injury [[9], [10], [11], [12], [13]].
The adult body has total of 24g (2000 mEq or 1mol) of magnesium, approximately 50% is stored in the bones, while extracellular magnesium which represent 1% of total body magnesium, is found in the serum and red blood cells, around 55% of it is the free ionized bioactive form in the human body. Magnesium has an important role in limiting nuclear factor-κB (NF-κB) activation, cytokine IL-6 which lead to systemic inflammation in covid-19 patients [14]. Magnesium depends on intestines for absorption, bone for storage and kidneys for execration in order to maintain homeostasis. Bone provides a large exchangeable amount to regulate acute changes in serum magnesium concentration, kidneys are crucial in the maintenance normally 90% of filtered magnesium is being reabsorbed again, majority which accounts for up to 70% of reabsorption by thick ascending limb of the loop of Henle through passive paracellular transport of Mg2+ [15]. The pathophysiology behind increased urinary magnesium loss in Covid-19 patients is not yet understood, but it needs to be corrected otherwise it will promote hypokalemia by impairing the renal function of sodium-potassium ATPase pump [14]. hypokalemia has been noticed in the literature before as one of the complications among patients, in a cohort Chinese study that had a total of 175 patients 95 (55%) patients had hypokalemia, 31 (18%) had sever hypokalemia of less than 3 (mmol/L) and 64 (37%) patients had hypokalemia ranges between 3 and 3.5 (mmol/L).[ 16 ] Degree of hypokalemia found to be in direct correlation with disease severity, and an independent early marker of disease progression [17].Patients with severe or critical COVID-19 who survive may have a long road to recovery while experiencing the lasting effects of the infection and treatments [18]. Some of COVID-19 patients need long term follow up to observe and manage any new or established complication specifically if this complication has a potential life-threatening effect as in our case with hypokalemia and the sequela of its management. Both hypokalemia and hyperkalemia and their management may lead to arrhythmia and possibly death. Gastrointestinal loss and increased urinary loss are the two main proposed causes of hypokalemia in covid-19 patients, but the majority don't exhibit gastrointestinal symptoms and increased urinary potassium loss is primary cause of hypokalemia [16]. The same with our patient he didn't have any gastrointestinal symptoms of vomiting or diarrhea so we can exclude VIPoma, Zollinger-Ellison Syndrome and other gastrointestinal causes, while his urinary potassium-to-creatinine ratio was >1.5 with high TTKG both indicate an increase in urinary potassium excretion with normal acid-base balance so we can rule out renal tubular acidosis, Bartter's syndrome, Gitelman's syndrome and medications like loop diuretics, thiazide diuretics and anti-fungal use as Amphotericin B.
Arrhythmias in COVID-19 patients are common either by viral infection causing viral myocarditis or QT-prolonging medications used for treatment such as Hydroxychloroquine and/or Azithromycin, sinus bradycardia is the most common arrhythmia in infected patients [19]. Electrolyte imbalance especially magnesium, potassium and calcium could lead to arrhythmia and life‐threatening polymorphic ventricular tachycardia known as torsades de pointes. [20], which is why we had to monitor the patient closely even after discharge. There are many causes of hypokalemia but our patient had a normal blood pressure with normal acid-base balance so we can exclude causes like Hyperaldosteronism, Cushing, Liddle, Renal artery stenosis, renin secreting tumors, syndrome of apparent mineralocorticoid excess, remote diuretic use, profuse sweating, with normal thyroid panel and parathyroid hormone level we can rule out hyperthyroidism and Multiple endocrine neoplasia type 1 (MEN1), patient has a normal leukocyte count and morphology so we can eliminate Pseudohypokalemia secondary to leukemia from our differentials, patient also has normal glucose level and no history of hypoglycemia we can exclude insulinoma and factitious hypoglycemia, patient is not receiving Salbutamol so we can exclude salbutamol induced hypokalemia, there are no previously documented hypokalemia before getting infected with covid-19 or history of hospital admission due to generalized weakness nor family history of hypokalemia so we can exclude Hypokalemic periodic paralysis, patient also has more than 1.5 urinary potassium-to-creatinine ratio as in our patient, it will most likely caused by a renal-potassium loss given that he did not have gastrointestinal symptom nor he has received any medication that may explain his persistent hypokalemia [21].
In a healthy person, RAS activity is balanced by ACE1 (which increases RAS activity) and ACE2 (which decreases RAS activity) [22]. When SARS-CoV-2 binds and degrades ACE2, the ability of ACE2 to regulate RAS is reduced and it cannot antagonize ACEI-2. The final result is RAS activity is increased, which acts like secondary increased aldosterone [17]. Increased RAS activity enhances the distal delivery of sodium and water to collecting tubule of the kidney and the excretion of potassium, because previous literature also has shown that serum K+ is negatively associated with plasma renin activity [23].
In our case report we followed up with the patient for more than 5 months after he got infected by COVID-19. It was found that his hypokalemia and hypomagnesemia are persistent so far even though he does not have the symptoms of COVID-19 and two negative nasopharyngeal swabs, but it is fully managed by oral replacement. As of our best knowledge, persistent hypokalemia and hypomagnesemia have not been described in the literature before. Are the alterations caused by COVID-19 to ACE2 in the kidneys permanent or is there another mechanism causing this persistent hypokalemia and hypomagnesemia? Our patient didn't want to go for any invasive diagnostic procedures and favored to wait for spontaneous recovery.
4. Conclusion
It has been mentioned in a previous study that 55% of COVID-19 patients have hypokalemia [1]. Those patients need rigorous follow up to know if hypokalemia would spontaneously resolve after they recovered from COVID-19 pulmonary infection or it will last longer even though magnesium levels were corrected several times as shown in (Fig. 2). In our case report we showed that hypokalemia may last more than 5 months after they were diagnosed with COVID-19 pulmonary infection and started to have hypokalemia and even after the disappearance of the common COVID-19 symptoms. Is the effect on the ACE receptors permanent or temporary.
Sources of funding
Our study is case report no funding was needed, no sponsors, no data analysis was needed.
Ethical approval
Approval given.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Author contribution
Dr. Mohammed Alnafiey: Literature review, manuscript writing (discussion and case report), lab results collection and entry, editing journal requirement, following up closely with the patient for any new symptoms or complications, reviewing diagnostic guidelines for case approach, reviewing references. Dr. Abdullah Alangari: Literature review, manuscript writing (Abstract, discussion, case report), lab results collection and entry, graphs and images, editing journal requirement. Dr. Ahmed Abushara: Manuscript writing (discussion, introduction and conclusion parts), reviewing grammar and language errors. Dr. Abdullah Alarifi: Manuscript writing (discussion, introduction and conclusion parts).
Registration of research studies
-
1.
Name of the registry: N/A
-
2.
Unique Identifying number or registration ID: N/A
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): N/A
Guarantor
Dr. Mohammed Alnafiey.
Declaration of competing interest
None.
Contributor Information
Mohammed Obaid Alnafiey, Email: aamshmo@hotmail.com, malnafiey@ksmc.med.sa.
Abdullah Meshari Alangari, Email: a.alangari@ksmc.med.sa.
Abdullah Mohammed Alarifi, Email: dr.a.alarifi@hotmail.com, dr.abushara@gmail.com.
References
- 1.Phelan A., Katz R., Gostin L. The novel coronavirus originating in wuhan, China. J. Am. Med. Assoc. 2020;323(8):709. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.WHO Q&A on coronaviruses (COVID-19) 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses Who.int. Accessed.
- 3.Best Practice B.M.J. Coronavirus disease 2019 (COVID-19) - complications | BMJ best practice US. 2020. https://bestpractice.bmj.com/topics/en-us/3000168/complications Bestpractice.bmj.com. Accessed.
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Santos R., Ferreira A., Simões e Silva A. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008;93(5):519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 7.WHO WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Who.int. Accessed.
- 8.Su Hua, Yang Ming, Cheng Wan, Yi Li-Xia, Tang Fang, Zhu Hong-Yan, Fan Yi, Yang Hai-Chun, Fogo Agnes B., Nie Xiu, Zhang Chun. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(Issue 1):219–227. doi: 10.1016/j.kint.2020.04.003. 0085–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Leor O., Cid-Alvarez B. ST-segment elevation myocardial infarction care during COVID-19. JACC (J. Am. Coll. Cardiol.): Case Rep. 2020;2(10):1625–1627. doi: 10.1016/j.jaccas.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C., Shi L., Wang F. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/s2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace Taylor C. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020;39(8):685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 15.Jahnen-Dechent Wilhelm, Ketteler Markus. Magnesium basics. Clin. Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Dong, Li Xiaokuni, Song Qifa, Hu Chenchan, Su Feifei, Dai Jianyi. 2020. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-P Oscar. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 Mediterranean patients. Int. J. Infect. Dis. : IJID Off. Publ. Int. Soc. Infect. Dis. 17 Sep. 2020;100:449–454. doi: 10.1016/j.ijid.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babapoor-Farrokhran S., Rasekhi R.T., Gill D., Babapoor S., Amanullah A. Arrhythmia in COVID-19. SN Compr. Clin. Med. 2020 Aug:1–6. doi: 10.1007/s42399-020-00454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Digby G.C., Pérez Riera A.R., Barbosa Barros R. Acquired long QT interval: a case series of multifactorial QT prolongation. Clin. Cardiol. 2011 Sep;34(9):577–582. doi: 10.1002/clc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jameson, J. Larry et al. Harrison's Principles of Internal Medicine, 20E.
- 22.Chen D., Li X., Song Q. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in wenzhou, China. JAMA Netw. Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielecka-Dabrowa A., Mikhailidis D., Jones L., Rysz J., Aronow W., Banach M. The meaning of hypokalemia in heart failure. Int. J. Cardiol. 2012;158(1):12–17. doi: 10.1016/j.ijcard.2011.06.121. [DOI] [PubMed] [Google Scholar]