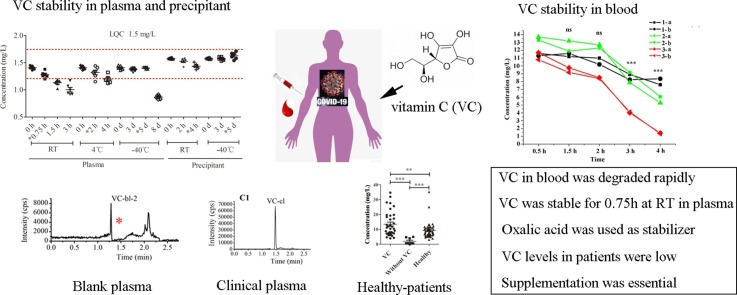
Graphical abstract
Keywords: Vitamin C, COVID-19, UPLC-MS/MS, Oxalic acid, Stability
Abstract
To administer vitamin C (VC) with precision to patients with the coronavirus disease (COVID-19), we developed an ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method to assess plasma VC concentrations. 31 patients with COVID-19 and 51 healthy volunteers were enrolled. VC stability was evaluated in blood, plasma, and precipitant-containing stabilizers. A proportion of 7.7 % of VC was degraded in blood at room temperature (RT) (approximately 20–25 °C) at 1.5 h post administration with respect to the proportion degraded at 0.5 h, but without statistical difference. VC was stable in plasma for 0.75 h at RT, 2 h at 4 °C, 5 days at −40 °C, and 4 h in precipitant-containing stabilizer (2 % oxalic acid) at RT. The mean plasma concentration of VC in patients with COVID-19 was 2.00 mg/L (0.5–4.90) (n = 8), which was almost 5-fold lower than that in healthy volunteers (9.23 mg/L (3.09. 35.30)) (n = 51). After high-dose VC treatment, the mean VC concentration increased to 13.46 mg/L (3.93. 34.70) (n = 36), higher than that in healthy volunteers, and was within the normal range (6–20 mg/L). In summary, we developed a simple UPLC-MS/MS method to quantify VC in plasma, and determined the duration for which the sample remained stable. VC levels in patients with COVID-19 were considerably low, and supplementation at 100 mg/kg/day is considered highly essential.
1. Introduction
Vitamin C (VC), also known as ascorbic acid, is well known for its high antioxidant activity [1], which helps protect the body from oxidative damage and dysfunction. However, in critically ill patients, VC requirements are considerably increased [2]. VC levels are inversely proportional to the severity of the patient’s condition [3]. Notably, VC has been shown to have potential therapeutic effects on acute pancreatitis [1], lymphocytes [4], and infectious diseases [5,6]. In particular, treatment with high doses of VC helps combat infections [5,6], e.g., by reducing susceptibility to lower respiratory tract infections [5] and the duration of colds [6]. A recently published small sample size study with 17 patients, suggested that the administration of intravenous (IV) VC might decrease inflammatory marker levels and be useful in the treatment of patients with moderate to severe COVID-19 disease [7]. In Wuhan, China, a high-dose of VC (24 g/day for 7 days) was reported to be effective in the treatment of 308 critically ill patients with COVID-19 [8] (NCT04264533; clincialtrials.gov). Similarly, in Shanghai, China, high-dose IV VC was used in the treatment of patients with moderate-to-severe COVID-19, and was shown to be beneficial in terms of inflammatory response, and immune and organ function enhancement, in patients with severe COVID-19 patients [9]. Although VC has been used in the treatment of patients for several decades, and generally has low toxicity [10], in take of 2 g of VC per day increases human oxaluria and the risk of developing kidney stones [11], and doses > 2 g may cause adverse gastrointestinal events, such as, abdominal pain, diarrhea, and/or nausea [12]. Rare cases even reported that long-term, high-dose VC in take could lead to oxalate nephropathy [13]. Unfortunately, acute VC deficiency generally goes unnoticed because its symptoms are similar to those of critical illnesses. In addition, plasma VC concentrations in patients with COVID-19 are usually unknown. Therefore, it is necessary to administer a suitable dose of VC in such patients based on plasma concentration measures.
The first step in measuring drug concentrations is to develop quantification methods. As at now, several methods, such as high-performance liquid chromatography with electrochemical detection [14,15] and LC-mass spectrometry (MS) [16], have been developed. However, it is difficult to use these methods to analyze samples of patients with highly infectious diseases because of the complex sample preparation processes involved; these include requirements such as, placing on dry ice [14], and drying under nitrogen [15].
In this study, we developed a simple ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for the quantification of VC, taking into consideration its stability, and controlling its circulation time in blood and plasma. Oxalic acid, being one of the degradation products of ascorbic acid, was used to stabilize it [17], as it inhibits the oxidation of VC by reacting competitively with oxygen [18], and decreasing the pH, thereby inhibiting VC degradation [19]. The developed UPLC-MS/MS method was then used to quantify plasma VC concentrations in 31 patients with COVID-19 and 51 healthy volunteers.
2. Materials and methods
2.1. Study design and participants
This observational study included 31 COVID-19 patients (25 treated with VC and 6 not treated with VC), and 60 healthy volunteers (51 for VC concentration measurement, 6 for blank plasma obtention, and 3 for whole blood stability analyses). As this study involved human participants, the study protocol was established in accordance with the World Medical Association’s Declaration of Helsinki, and was approved by the Ethics Committee of the Shanghai Public Health Clinical Center, Fudan University, China (Approval No. YJ-2020-S053−02). This study was also registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn, NO: ChiCTR2000035629, and ChiCTR2000032716).
All volunteers were aged 18 years or older, and signed written consent to participate in the study. Patients with COVID-19 were administered IV VC at a dose of 100 mg/kg/day. Plasma VC concentrations were measured before dosing, and 5–15 days after dosing. One to three fasting blood samples (2 mL in ethylene diamine tetraacetic acid-containing tubes) were collected from each volunteer. Liver function parameters (including alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), and renal function parameters (such as creatinine and estimated glomerular filtration rate [eGFR]) were determined using general biochemical methods.
2.2. Plasma VC concentration measurement
2.2.1. Chemicals and reagents
VC was purchased from Laboratory of the Government Chemist (Augsburg, Germany), and VC-d6 (internal standard [IS]) was purchased from Shandong Yingsheng Biology Co., Ltd. (Jinan, Shandong, China). Anhydrous oxalic acid was purchased from Aladdin Biochemical Technology (Shanghai, China). Methanol (MeOH) and acetonitrile (ACN) were purchased from ANPEL Laboratory Technologies (Shanghai, China). Ultra-pure water was prepared using a Milli-Q system (Millipore, Bedford, MA, USA). All chemicals were of analytical or LC–MS grade.
2.2.2. Blank plasma
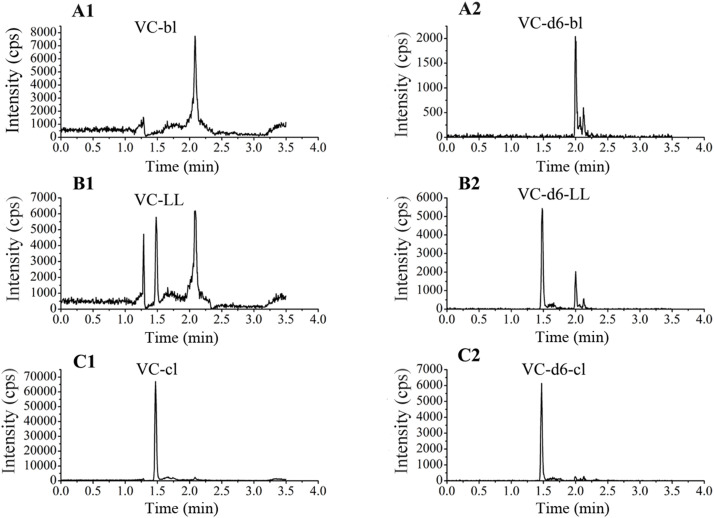
Plasma samples from six healthy volunteers (20 mL of whole blood for each) were kept at room temperature (RT) (approximately 20–25 °C) for approximately 12 h; then they were stored at -40 °C for at least one month and freeze-thawed at least three times. Then, 50 μL of the plasma was pipetted and treated according to the "sample preparation" method to detect the presence of VC. Plasma samples with undetectable VC were used as blank matrices in the subsequent analyses.
2.2.3. Standard solution
The working solutions of VC were diluted with 2% oxalic acid (weight (W)/ volume (V)) to obtain standard solutions of 500, 250, 100, 40, 20, and 10 mg/L, and quality control (QC) solutions of 400, 200, 80, 15, and 10 mg/L. Then, the working solutions were spiked into blank plasma samples with a dilution factor of 10. All working solutions were stored at 4 °C for ≤ 10 days. The spiked plasma samples were stored at −40 °C for ≤ 5 days.
2.2.4. The UPLC-MS/MS system
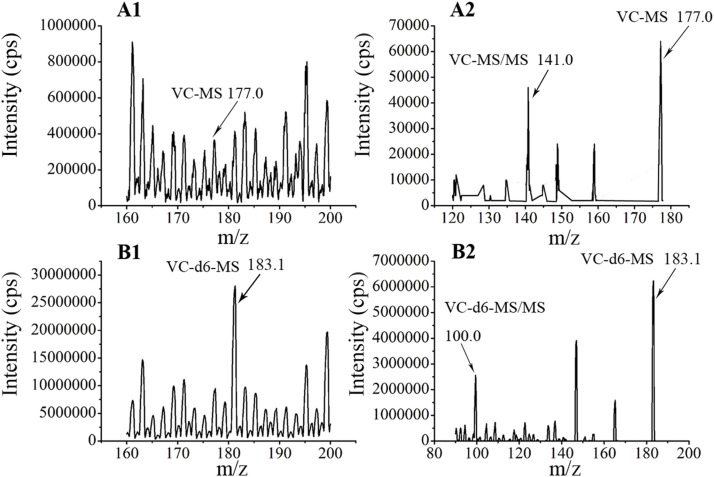
VC concentrations were analyzed using an ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS system), made up of a Waters Acquity UPLC (Waters Corporation, Milford, USA) and an AB Sciex Triple Quad 5500 MS (AB SCIEX company, Boston, USA). The chromatographic column was a Kinetex® 2.6 μm Phenyl-Hexyl column 100 Å (50 mm × 3.0 mm) (Phenomenex Company, USA). Chromatographic separation was carried out using a mobile phase composed of 0.08 % (W/V) oxalic acid (A), and a methanol solution containing 0.008 % (W/V) oxalic acid (B). Gradient elution was employed with the following variations: 0–1 m in. 2 % phase B, at a flow rate of 0.1 mL/min; 1.2–1.8 m in. From 10 %–80 % phase B, 0.5 mL/min; 2.0–2.3 m in. From 80 %–95 % phase B, 0.5 mL/min; 2.5–2.51 m in. From 95 % to 2 % phase B, 0.4 mL/min; and 2.51–3.50 m in. 2% phase B, 0.4 mL/min. The analytes were quantified using multiple reaction monitoring transitions under positive ion mode with ion pairs of m/z 177.0/141.0 and 183.1/100.0 for VC and IS, respectively. A sample volume of 10 μL was injected into the UPLC-MS/MS system. The samples were stored at 4 °C prior to injection.
2.2.5. Method validation
This method was validated according to the guidelines of the Food and Drug Administration [20], by assessing linearity, selectivity, lower limit of quantitation (LLOQ), precision and accuracy, matrix effect, extraction recovery, and stability.
In this study, we focused on the stability of VC in different matrices, including whole blood, plasma, and precipitant-containing stabilizers. To assess stability in whole blood, three healthy volunteers were enrolled. For each individual, 3 mL of blood (3 mL/tube) were collected into 2 K2EDTA tubes and placed separately at RT for 30 min, 1.5 h, 2 h, 3 h, and 4 h. At each time point, six blood aliquots (n = 3 individuals × 2 samples/individual) (approximately 200 μL) were separated and plasma was obtained (50 μL). To determine the stability of VC in plasma, blank plasma samples (without VC) spiked with low and high concentrations of QC solutions (high QC (HQC) and low QC (LQC)) were used. The 2 types of plasma samples spiked with QC solutions were kept at RT for 0.75, 1.5, or 3 h; at 4 °C for 2 or 4 h; at −40 °C for 3, 5, or 8 days; or freeze-thawed thrice at −40 °C within 12 h. Furthermore, we evaluated the stability of VC by adding the precipitant containing 2 % oxalic acid (stabilizer) to the HQC and LQC samples, and stored them at RT for 2 or 4 h, or at −40 °C for 3 or 5 days. In addition, we determined the 24 h stability under autosampler settings (4 °C).
2.2.6. Clinical sample preparation and detection
Blood samples were processed for VC detection within 30 min after collection, at RT. First, the samples were centrifuged. After the blood was centrifuged for 10 min, plasma was collected. Within 45 min, the plasma (50 μL) was precipitated with 150 μL of organic solution (ACN-MeOH-2 % oxalic acid (containing 250 μg/L of IS) in the ratio 45: 45: 10, V: V: V). If plasma could not be processed immediately, it was stored at −40 °C for ≤ 5 days. After centrifugation at 4 °C for 10 min, the supernatant was stored at −40 °C for ≤ 5 days or directly diluted within 4 h with 0.01 % oxalic acid, with a dilution factor of 5. Finally, 10 μL was injected into the LC–MS/MS system.
2.3. Statistical analysis
Data are represented as means and standard deviations, or scatter plots showing median values. Plasma VC concentrations below the quantifiable limit were recorded as 1/2 LLOQ [21]. Concerning the VC stability data, the 15th and 85th percentiles were used as accuracy boundaries, based on the theoretical value. GraphPad Prism version 5 was used to compare plasma VC concentrations. A two-tailed nonparametric t-test was used to calculate P values. The correlation between VC concentrations and the covariates (age, weight, ALT, AST, and CR levels, and eGFR) was also evaluated using GraphPad Prism version 5. Values of P < 0.05 were considered as statistically significant.
3. Results
3.1. Patient characteristics and clinical samples
For this study, 31 COVID-19 patients (25 treated with VC and 6 not treated with VC) and 51 healthy volunteers were recruited from the Shanghai Public Health Clinical Center. The demographic and clinical characteristics of all patients are shown in Table 1 .
Table 1.
Participant characteristics.
| Items | Sex, male% | Age, y | Weight, kg | Dose, g | #Concentration, mg/L | *Days, d | AST, U/L | ALT, U/L | Cr, μmol/L | eGFR, mL/min |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients treated with VC (n = 25) | ||||||||||
| Average | 68 | 39.00 | 68.42 | 5.99 | 13.46 (n = 36) | 7.03 | 32.83 | 43.91 | 63.68 | 127.72 |
| SD | 14.56 | 14.89 | 1.35 | 8.32 | 2.66 | 33.33 | 35.10 | 16.32 | 30.12 | |
| Min | 19.00 | 43.00 | 2.00 | 3.93 | 5.00 | 7.00 | 6.00 | 44.20 | 58.54 | |
| Max | 75.00 | 100.00 | 10.00 | 34.70 | 15.00 | 106.00 | 118.00 | 112.38 | 215.49 | |
| Median | 38.00 | 69.00 | 5.00 | 10.80 | 6.00 | 22.50 | 32.00 | 64.53 | 128.32 | |
| Patients treated without VC (n = 6) | ||||||||||
| Average | 75 | 35.63 | 75.06 | 2.00 (n = 8) | 23.71 | 44.71 | 75.43 | 105.47 | ||
| SD | 8.52 | 13.82 | 2.08 | 4.79 | 19.91 | 10.64 | 8.79 | |||
| Min | 22.00 | 50.00 | 0.5 (BQL) | 7.00 | 13.00 | 45.96 | 89.98 | |||
| Max | 44.00 | 98.50 | 4.90 | 30.00 | 69.00 | 85.59 | 140.31 | |||
| Median | 38.00 | 98.50 | 0.5 (BQL) | 24.00 | 46.00 | 78.06 | 106.37 | |||
| Healthy (n = 51) | ||||||||||
| Average | 0 | 31.42 | 56.88 | 9.23 | 17.82 | 13.54 | 53.43 | 126.10 | ||
| SD | 6.61 | 5.61 | 5.11 | 3.86 | 7.22 | 7.01 | 19.77 | |||
| Min | 21.00 | 46.80 | 3.09 | 10.00 | 6.00 | 37.98 | 78.54 | |||
| Max | 44.00 | 68.40 | 35.30 | 28.00 | 43.00 | 75.31 | 191.95 | |||
| Median | 31.00 | 55.50 | 8.01 | 12.00 | 17.50 | 52.21 | 128.45 | |||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; Cr, creatinine; VC, vitamin C.
the days from first dosing to blood withdrawal.
BQL represents below quantifiable limit, and was recorded as 1/2 LLOQ.
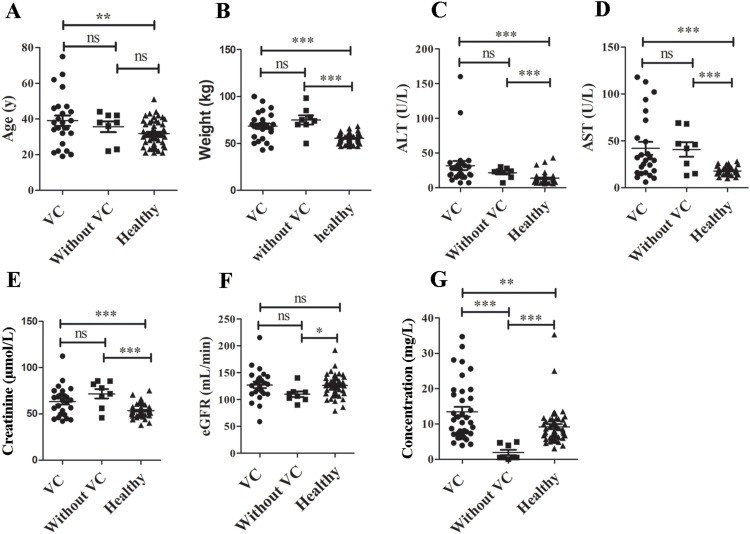
Statistical differences were observed between patients with COVID-19 (treated with or without VC) and healthy volunteers in terms of age (Fig. 1 A), weight (Fig. 1B), ALT (Fig. 1C), AST (Fig. 1D), and creatinine (Fig. 1E) levels, eGFR (Fig. 1F), and VC concentrations (Fig. 1G). Except for age and weight, all parameters differed between the healthy individuals and patients, as all healthy volunteers were female and younger. However, the other three parameters (ALT, AST, and creatinine levels) might have differed between these groups due to the disease. No significant difference in eGFR was observed.
Fig. 1.
Statistical difference between patients with coronavirus disease-2019 (COVID-19) (with or without VC treatment) and healthy volunteers. A, for age; B, weight; C, ALT; D, AST; E, creatinine level; F, eGFR, and G, VC concentration. VC, Vitamin C; ns, no significant difference; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Concerning the 25 patients treated with VC, 2 samples were collected before dosing, and 36 samples were collected at trough time points within 5–15 days of dosing. For patients who were not or not yet on VC therapy, and healthy volunteers, only one sample was collected; whereas, after dosing, 1–3 samples were collected. In total, 36, 8, and 51 samples were collected from the group receiving VC, group not receiving VC or before dosing, and the healthy group, respectively.
3.2. Quantification of VC by UPLC-MS/MS
3.2.1. UPLC-MS/MS method development
A UPLC-MS/MS method was established for the determination of the concentration of VC in plasma. The ion pairs of VC and IS (VC-d6) were detected at m/z 177.0/141.0 and 183.1/100.0, respectively (Fig. S1). For VC, a standard curve with linearity was established in the range of 1–50 mg/L, and the LLOQ was determined to be 1 mg/L.
3.2.2. UPLC-MS/MS method validation
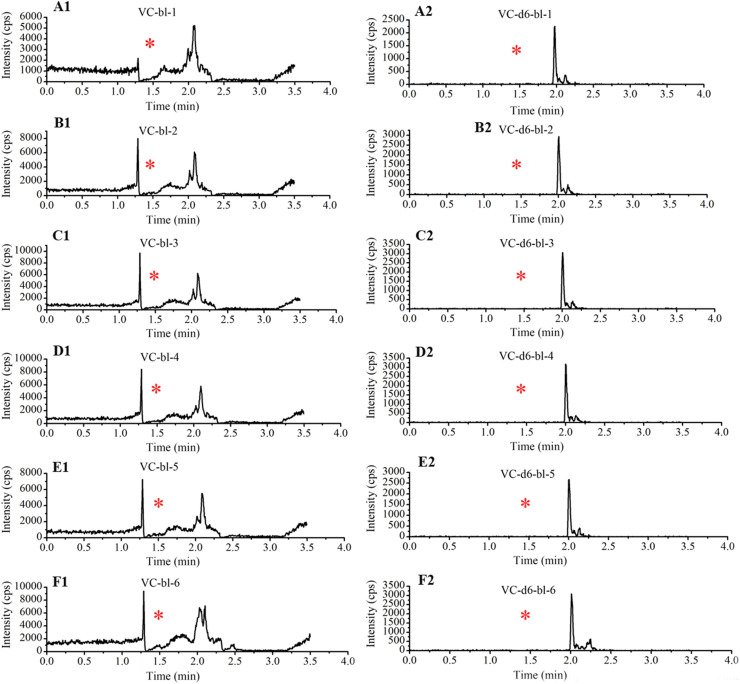
Enough blank plasma samples were prepared according to the instability character of VC through placing at RT, long term freeze, and freeze-thaw cycles. No VC peak was detected for any of the six plasma samples (Fig. S2). Typical chromatograms of blank plasma, blank plasma spiked with VC concentrations of LLOQ and its IS, and clinical samples are shown in Fig. S3. The compounds (VC and VC-d6) were eluted at 1.5 min.
The intra-day and inter-day precisions of VC (at the spiked concentrations of 1, 1.5, 8, 20, and 40 mg/L) were less than 4.47 % and 8.18 %, respectively, and the deviations of accuracy were within ± 15 % (Table S1). The matrix effects (MEs) and extraction recoveries (ERs) at low (1.5 mg/L) and high (40 mg/L) concentrations (LQC and HQC solutions) were 112.76 % and 84.64 %, and 86.26 % and 86.36 %, respectively.
3.2.3. Stability of VC
VC has high reducibility and is easily oxidized; therefore, in this study, we paid particular attention to its stability during the processes of sample treatment and analysis. To this end, oxalic acid (2%) was added to the analyte and IS working solutions during the sample preparation process. Our results showed that the working solutions were stable at 4 °C for 10 days (data not shown). The IS in precipitant could only be stable for 3 days under the experimental conditions (4 °C or RT).
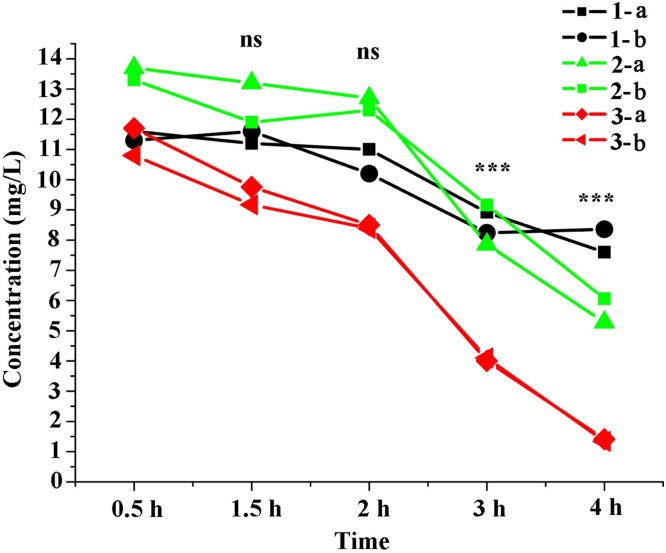
To evaluate the stability of VC in blood, we collected 6 blood samples (two tubes / individual) from three healthy volunteers and stored them at RT for 0.5, 1.5, 2, 3, or 4 h. At each time point, the blood was centrifuged at 4 °C for 10 min to obtain plasma. As shown in Table 2 and Fig. 2 , VC plasma concentrations decreased by 7.7 %, 12.8 %, 41.6 % (P < 0.001), and 58.5 % (P < 0.001) at 1.5, 2, 3, and 4 h, respectively, with respect to the concentrations at 0.5 h. Although no significant differences in concentration were observed at 1.5 and 2 h, a loss of 16 % and 25 % was noted in the samples collected from volunteer number 3 at these time points with respect to the concentration at 0.5 h (Fig. 2). Therefore, in this study, blood samples were centrifuged within 30 min of collection.
Table 2.
Stability of VC in whole blood under room temperature (about 20–25 °C), n = 6.
| Time | Concentration (mg/L) | Standard deviation | Fold-degradation (compared with 0.5 h) | P value (compared with 0.5 h) |
|---|---|---|---|---|
| 0.5 h | 12.07 | 1.16 | NA | NA |
| 1.5 h | 11.14 | 1.47 | 7.7 | NS |
| 2 h | 10.52 | 1.84 | 12.8 | NS |
| 3 h | 7.05 | 2.37 | 41.6 | 0.0009 |
| 4 h | 5.01 | 3.02 | 58.5 | 0.0003 |
NS: no significant difference. NA: not applicable.
Fig. 2.
Stability of vitamin C in blood at room temperature.
Human venous blood was collected from three healthy volunteers; samples were duplicated and placed at room temperature (approximately 20–25 °C) for 0.5, 1.5, 2, 3, and 4 h. “1-,” “2-,” and “3-” indicate different blood donors, and “a” and “b” represent the duplicate samples from each volunteer. Ns: no significant difference; ***: P < 0.001.
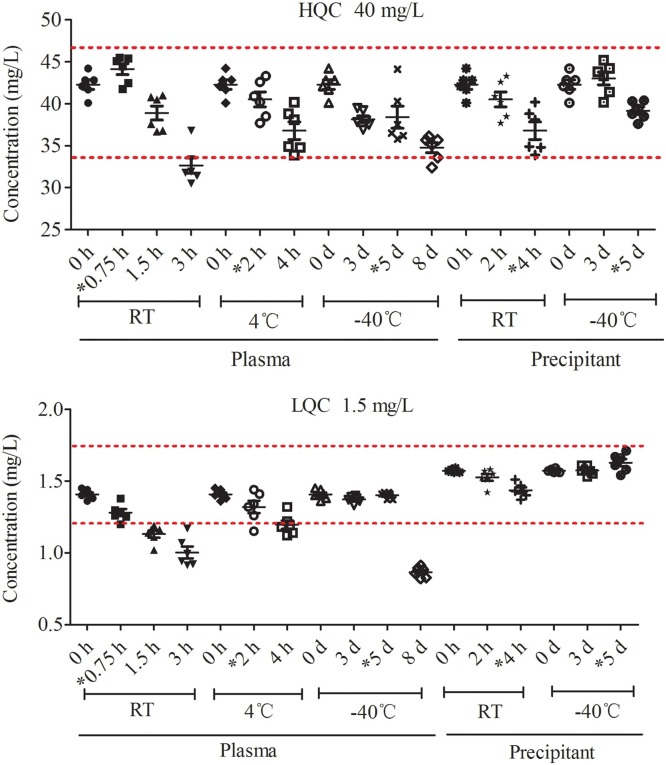
For the determination of the stability of VC in plasma and precipitant under different conditions, spiked LQC (1.5 mg/L) and HQC (40 mg/L) samples were used. As shown in Table 3 and Fig. 3 , VC was stable in plasma for 0.75 h at RT, 2 h at 4 °C, and for 5 days at −40 °C. After precipitation with an organic solvent containing the stabilizer, VC was stable for 4 h at RT, 5 days at −40 °C, and 24 h in the sampler. It was also stable after three freeze-thaw cycles within 12 h.
Table 3.
Stability of VC in plasma and precipitant under various storage conditions (n = 6).
| Matrix | Storage conditions | Time | Concentration (mg/L) |
Precision | Accuracy | |
|---|---|---|---|---|---|---|
| Spiked | Measured | (%) | (%) | |||
| Plasma | Room temperature (RT) | 0.75 h | 1.5 | 1.32 | 3.67 | 88.17 |
| 40 | 44.13 | 3.59 | 110.3 | |||
| 1.5 h | 1.5 | 1.13 | 6.15 | 75.17 | ||
| 40 | 38.9 | 5.34 | 97.31 | |||
| 3 h | 1.5 | 1.02 | 10.04 | 67.82 | ||
| 40 | 32.63 | 6.95 | 81.6 | |||
| 4°C | 2 h | 1.5 | 1.32 | 8.09 | 88.1 | |
| 40 | 40.53 | 5.41 | 101.31 | |||
| 4 h | 1.5 | 1.2 | 5.85 | 79.67 | ||
| 40 | 36.78 | 6.99 | 91.92 | |||
| −40°C | 3 d | 1.5 | 1.37 | 1.92 | 91.33 | |
| 40 | 38.15 | 2.57 | 95.36 | |||
| 5 d | 1.5 | 1.49 | 6.88 | 99.69 | ||
| 40 | 41.82 | 7.62 | 104.62 | |||
| 8 d | 1.5 | 0.87 | 4.12 | 58.23 | ||
| 40 | 34.77 | 4.17 | 86.94 | |||
| Precipitant | RT | 2 h | 1.5 | 1.53 | 4.23 | 101.93 |
| 40 | 39.12 | 2.12 | 97.76 | |||
| 4 h | 1.5 | 1.44 | 3.46 | 95.74 | ||
| 40 | 38.08 | 2.16 | 95.23 | |||
| Long term (-40°C) | 3 d | 1.5 | 1.57 | 2.07 | 104.95 | |
| 40 | 42.85 | 7.49 | 107.18 | |||
| 5 d | 1.5 | 1.63 | 3.81 | 108.48 | ||
| 40 | 39.17 | 2.86 | 97.9 | |||
| Autosampler | Autosampler (4°C) | 24 h | 1.5 | 1.63 | 4.32 | 108.48 |
| 40 | 44.47 | 3.32 | 111.17 | |||
| Plasma | Three-freeze-thaw cycles | 1.5 | 1.35 | 3.23 | 89.91 | |
| 40 | 43.15 | 1.34 | 107.82 | |||
Fig. 3.
Stability of VC in plasma and precipitant under various storage conditions. HQC (n = 6) and LQC (n = 6) were used. The accuracy of 85 %-115 % was highlighted with red dotted lines. VC, vitamin C; HQC, high-concentration quality control; LQC, low-concentration quality control; RT, room temperature; *, time for which VC was stable.
3.3. Quantification of clinical samples
The verified UPLC-MS/MS method was used to quantify VC concentrations in healthy volunteers (n = 51) and patients with COVID-19 (n = 31), among whom 6 and 25 were treated without and with VC, respectively. The mean plasma VC concentrations of 8 samples (i.e., 6 obtained from patients not treated with VC and 2 obtained before dosing) were 2.00 mg/L (0.5–4.90). Concentrations of VC in patients were almost 5-fold lower than in healthy volunteers, with a mean VC concentration of 9.23 mg/L (3.09−35.30.) (n = 51). After the administration of VC, the mean plasma concentration increased to 13.46 mg/L (3.93–34.70) (n = 36), which was similar to that in the healthy volunteer group (Table 1 and Fig. 1G).
4. Discussion
VC is an essential vitamin involved in many biochemical functions [22]. To maintain the optimal physiological levels of VC, the recommended daily intake is 200 mg/day, which can be obtained from normal diet, resulting in a steady-state concentration of 70–85 μmol/L (12.33–14.98 mg/L) [22,23]. A population study demonstrated that the optimal plasma VC concentration is 40 μmol/L [24]. In epidemiological studies, plasma VC concentrations less than 11.4 μM (2.00 mg/L) indicated VC deficiency [25,26]. Based on this value, more than 7 % of the population (> 20 million people) in the USA [25], 25–46 % of the low-income population in the UK [27], and smokers [28,29] are deficient in VC. Approximately one-third of patients with shock have a VC concentration of less than 11 μmol/L [3]. Similarly, the plasma VC concentrations in 80 % of patients with leukemia (n = 123) was reported to be below 2 mg/L (11 μmol/L), with only 12 patients having VC concentrations above 4 mg/L [30]. VC deficiency can influence human disease progression. Similarly, a disease can also cause VC deficiency. Therefore, it is necessary to supplement patients with VC. There have been reports of high-dose administration of VC in patients with COVID-19 [8,9]. However, there are no data on plasma VC levels of patients with COVID-19. For this reason, we developed a rapid, sensitive, and specific UPLC-MS/MS method. To the best of our knowledge, our study is the first to report on VC concentrations in COVID-19 patients.
In this study, we focused on the stability of VC in whole blood, plasma, and precipitant. First, we assessed the stability of VC in the blood. As reported in a study by Karlsen [31], the highest VC levels were observed in heparin and EDTA plasma, with no significant difference after blood was placed at RT for 30 min. In this study, we used K2EDTA as an anticoagulant, and blood was placed at RT for 0.5, 1.5, 2, 3, and 4 h. A significant increase in the degradation of VC was observed between 30 min to 1.5 h. Blood sample collection and centrifugation take less than 30 min in clinical practice. Our stability analysis results were similar to those reported in the study by Karlsen [31], where VC degradation was rapid between 30 min–90 min. Therefore, plasma needs to be separated from whole blood as soon as possible. In this study, plasma was separated within 30 min of blood sample collection. Furthermore, because of the instability of VC, it is very important to select a suitable stabilizer in plasma and precipitants. However, There are some methods used to stabilize VC, for example, placing blood in ice and treating it with a solution of methanol/EDTA [14,32]. However, they are difficult to employ for infectious samples because ice is usually not available in BSL-3 or isolation wards. Furthermore, there are several studies in which metaphosphoric acid [31,33] was used to stabilize VC; however, metaphosphoric acid must be added to the plasma sample separately from an organic solution because it is insoluble in organic solutions. Therefore, metaphosphoric acid was not suitable for this study. In addition, trichloroacetic acid [34] or perchloric acid [35] was also used to stabilize VC, but sample processing was complicated (placing on ice) and time-consuming (even up to 2 h) [34]. In this study, we chose 2% oxalic acid for the stabilization of VC because it is a degradation product of VC [17], and can prevent the oxidation of VC by reacting competitively with oxygen [18]. Furthermore, oxalic acid can inhibit the degradation of VC by decreasing the pH [19]. Moreover, it is soluble in organic solvents. To ensure the stability of VC, we comprehensively evaluated its stability in plasma at different temperature conditions, and found it to be stable in plasma for 0.75 h at RT, 2 h at 4 °C, and 5 days at −40 °C, as well as after three freeze-thaw cycles within 12 h. We also analyzed VC stability in a precipitant (organic solvent containing 2 % oxalic acid) and found that it could be stable for 4 h at RT, 5 days at −40 °C, and 24 h in an autosampler. In summary, in this study, we determined the stability of VC in plasma from blood sample collection to MS detection, and successfully measured VC concentrations in patients with COVID-19.
After ensuring the stability of VC, we successfully developed a simple UPLC-MS method for the measurement of plasma VC concentrations. This method showed a good linear relationship within the range of 1–50 mg/L, with intra-day and inter-day precision, accuracy, ME, and ER meeting FDA guideline recommendations. Compared to previous methods [14,15,32], the method used in this study was simple and more suitable for clinical applications. For example, in previous studies, blood was placed on dry ice [14,32], and the solution was dried under nitrogen after extraction [15]. However, in this study, blood samples were collected, and plasma obtained within 30 min of blood collection at RT, followed by protein precipitation with an organic solution containing the IS. The supernatant was then diluted and loaded in to the mass spectrometer.
The validated method was used to quantify plasma VC, and its concentrations in COVID-19 patients were significantly lower than those in healthy volunteers. After receiving a high dose of VC, VC concentrations in patients significantly increased. Furthermore, in this study, we observed that plasma VC concentrations in patients with COVID-19 were similar to in other patients, with concentrations lower than 11 μmol/L (2.00 mg/L) [3,30]. These results suggest the benefits of a therapeutic protocol with high-dose IV VC in patients with COVID-19 [7,9].
This study had two main limitations. First, only healthy female volunteers were enrolled, to eliminate the effect of smoking on VC concentrations (with a 50 % decrease in smokers compared to non-smokers) [28,29]. In China, smoking is more common in males (more than 50 %) than in females (less than 2.1 %). Furthermore, no significant differences in plasma VC concentrations have been reported between the male and female populations. Moreover, the main aims of this study were to develop an LC–MS method to measure VC concentrations in plasma, and to determine the necessity of high-dose VC therapy in patients with COVID-19 (non-smokers in our ward). Despite these reasons, it would still have been more appropriate to include some non-smoking males. Second, this was an observational study involving only 31 COVID-19 patients, rather than a multicenter randomized controlled trial. Therefore, these data, along with their clinical significance, need to be further validated in larger cohorts.
5. Conclusions
In this study, we developed an UPLC-MS/MS method to quantify plasma VC in patients with COVID-19. Our results showed that patients with COVID-19 had significant VC deficiency. Hence, it is essential to supplement such patients with VC. This study provided data to further support the use of high-dose VC therapy in patients with COVID-19.
Author contributions
L.J. Zhang, Y.R. Xing, and B. Zhao wrote the manuscript. L.J. Zhang, E.Q Mao, and H.Z. Lu designed the research. L. Yin, M.Q. Guo, Z.Q. Zhu, Y. Ling, L. Zhang, M.L. Gao performed research. H.C. Shi, Y.R. Xing, and J. He analyzed data. L.J. Zhang, H.Z. Lu, and E.Q Mao contributed new reagents/analytical tools.
Funding
This study was funded by National Science and Technology Program during the Thirteenth Five-year Plan Period (China grant, no. 2017ZX09304027), Shanghai Innovative treatment of novel coronavirus pneumonia (20411950200), and Shanghai Research and development of emergency drugs against new coronavirus pneumonia (20431900103).
Declaration of Competing Interest
All authors declared no potential conflicts.
Acknowledgment
We thank the experts from Zhongshan and Shanghai Sixth People's Hospital for their clinical therapy, and the patients involved in this observational study. We thank Shandong Yingsheng Biology CO., LTD for technical and material support. We also thank professor Tiefu Liu from Fudan university and Editage (http://wkauthorservices.editage.com/) for revising the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jpba.2021.113927.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Du W.D., Yuan Z.R., Sun J., Tang J.X., Cheng A.Q., Shen D.M., Huang C.J., Song X.H., Yu X.F., Zheng S.B. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J. Gastroenterol. 2003;9:2565–2569. doi: 10.3748/wjg.v9.i11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrein K., Oudemans-van Straaten H.M., Berger M.M. Vitamin therapy in critically ill patients: focus on thiamine, vitamin C, and vitamin D. Intensive Care Med. 2018;44:1940–1944. doi: 10.1007/s00134-018-5107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Gorkom G.N.Y., Klein Wolterink R.G.J., Van Elssen C., Wieten L., Germeraad W.T.V., Bos G.M.J. Influence of vitamin C on lymphocytes: an overview. Antioxidants Basel (Basel) 2018;7 doi: 10.3390/antiox7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston C.S., Barkyoumb G.M., Schumacher S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: a randomized controlled trial. Nutrients. 2014;6:2572–2583. doi: 10.3390/nu6072572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J., Azmaiparashvili Z., Patarroyo Aponte G. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev. Anti. Ther. 2020;18:1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B., Ling Y., Li J., Peng Y., Huang J., Wang Y., Qu H., Gao Y., Li Y., Hu B., Lu S., Lu H., Zhang W., Mao E. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann. Palliat. Med. 2020 doi: 10.21037/apm-20-1387. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.J., Byun J.S., Kwon H.S., Kim D.Y. Cellular toxicity driven by high-dose vitamin C on normal and cancer stem cells. Biochem. Biophys. Res. Commun. 2018;497:347–353. doi: 10.1016/j.bbrc.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 11.Massey L.K., Liebman M., Kynast-Gales S.A. Ascorbate increases human oxaluria and kidney stone risk. J. Nutr. 2005;135:1673–1677. doi: 10.1093/jn/135.7.1673. [DOI] [PubMed] [Google Scholar]
- 12.Verrax J., Calderon P.B. The controversial place of vitamin C in cancer treatment. Biochem. Pharmacol. 2008;76:1644–1652. doi: 10.1016/j.bcp.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 13.D’Costa M.R., Winkler N.S., Milliner D.S., Norby S.M., Hickson L.J., Lieske J.C. Oxalosis associated with high-dose vitamin C ingestion in a peritoneal Dialysis patient. Am. J. Kidney Dis. 2019;74:417–420. doi: 10.1053/j.ajkd.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine M., Wang Y., Rumsey S.C. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999;299:65–76. doi: 10.1016/s0076-6879(99)99009-2. [DOI] [PubMed] [Google Scholar]
- 15.Tangney C.C. Analyses of vitamin C in biological samples with an emphasis on recent chromatographic techniques. Prog. Clin. Biol. Res. 1988;259:331–362. [PubMed] [Google Scholar]
- 16.Szultka M., Buszewska-Forajta M., Kaliszan R., Buszewski B. Determination of ascorbic acid and its degradation products by high-performance liquid chromatography-triple quadrupole mass spectrometry. Electrophoresis. 2014;35:585–592. doi: 10.1002/elps.201300439. [DOI] [PubMed] [Google Scholar]
- 17.Jansson P.J., Jung H.R., Lindqvist C., Nordstrom T. Oxidative decomposition of vitamin C in drinking water. Free Radic. Res. 2004;38:855–860. doi: 10.1080/10715760410001700497. [DOI] [PubMed] [Google Scholar]
- 18.Kayashima T., Katayama T. Oxalic acid is available as a natural antioxidant in some systems. Biochim. Biophys. Acta. 2002;1573:1–3. doi: 10.1016/s0304-4165(02)00338-0. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J., Chen F. Degradation of ascorbic acid in aqueous solution. J. Agric. Food Chem. 1998;46:5078–5082. [Google Scholar]
- 20.FAD; USA: 2018. Administration, Bioanalytical Method Validation Guidance for Industry. [Google Scholar]
- 21.Beal S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 22.Padayatty S.J., Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22:463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hampl J.S., Taylor C.A., Johnston C.S. Vitamin C deficiency and depletion in the United States: the third national health and nutrition examination survey, 1988 to 1994. Am. J. Public Health. 2004;94:870–875. doi: 10.2105/ajph.94.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleicher R.L., Carroll M.D., Ford E.S., Lacher D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES) Am. J. Clin. Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 26.Lindblad M., Tveden-Nyborg P., Lykkesfeldt J. Regulation of vitamin C homeostasis during deficiency. Nutrients. 2013;5:2860–2879. doi: 10.3390/nu5082860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosdol A., Erens B., Brunner E.J. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J. Public Health Oxf. (Oxf) 2008;30:456–460. doi: 10.1093/pubmed/fdn076. [DOI] [PubMed] [Google Scholar]
- 28.Hornig D. Metabolism and requirements of ascorbic acid in man. S. Afr. Med. J. 1981;60:818–823. [PubMed] [Google Scholar]
- 29.Wilson R., Willis J., Gearry R., Skidmore P., Fleming E., Frampton C., Carr A. Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: associations with glycaemic control, obesity, and smoking. Nutrients. 2017;9 doi: 10.3390/nu9090997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun W., Wang X., Tian W., Li M., Chen J., Wang L., Zhang J., Liu H. The clinical application of determination of ascorbic acid concentration in leukemia patient plasma by HPLC-MS/MS. Int. J. Lab. Med. Res. 2019;40:798–802. [Google Scholar]
- 31.Karlsen A., Blomhoff R., Gundersen T.E. Stability of whole blood and plasma ascorbic acid. Eur. J. Clin. Nutr. 2007;61:1233–1236. doi: 10.1038/sj.ejcn.1602655. [DOI] [PubMed] [Google Scholar]
- 32.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R., Park J.B., Lazarev A., Graumlich J.F., King J., Cantilena L.R. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechtersbach L., Cigic B. Reduction of dehydroascorbic acid at low pH. J. Biochem. Biophys. Methods. 2007;70:767–772. doi: 10.1016/j.jbbm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Salminen I., Alfthan G. Plasma ascorbic acid preparation and storage for epidemiological studies using TCA precipitation. Clin. Biochem. 2008;41:723–727. doi: 10.1016/j.clinbiochem.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Pullar J.M., Bayer S., Carr A.C. Appropriate Handling, Processing and Analysis of Blood Samples Is Essential to Avoid Oxidation of Vitamin C to Dehydroascorbic Acid. Antioxidants (Basel) 2018;7 doi: 10.3390/antiox7020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.