Abstract
Background
Debate continues regarding the usefulness and benefits of wide prescription of antibiotics in patients hospitalized with coronavirus disease 2019 (COVID-19).
Methods
All patients hospitalized in the Infectious Diseases Department, Dijon University Hospital, Dijon, France between 27 February and 30 April 2020 with confirmed COVID-19 were included in this study. Clinical, biological and radiological data were collected, as well as treatment and outcome data. An unfavourable outcome was defined as death or transfer to the intensive care unit. Patient characteristics and outcomes were compared between patients who did and did not receive antibiotic therapy using propensity score matching.
Findings
Among the 222 patients included, 174 (78%) received antibiotic therapy. The univariate analysis showed that patients who received antibiotic therapy were significantly older, frailer and had more severe presentation at admission compared with patients who did not receive antibiotic therapy. Unfavourable outcomes were more common in patients who received antibiotic therapy [hazard ratio (HR) 2.94, 95% confidence interval (CI) 1.07–8.11; P = 0.04]. Multi-variate analysis and propensity score matching indicated that antibiotic therapy was not significantly associated with outcome (HR 1.612, 95% CI 0.562–4.629; P = 0.37).
Conclusion
Antibiotics were frequently prescribed in this study and this was associated with more severe presentation at admission. However, antibiotic therapy was not associated with outcome, even after adjustment. In line with recent publications, such data support the need to streamline antibiotic therapy in patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Antibiotic therapy, Prognosis
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). SARS-Cov-2 is similar to SARS-CoV-1, which resulted in many deaths due to SARS in 2003. SARS-CoV-2 was discovered in December 2019 in China (Wu et al., 2020), and has since spread worldwide. The COVID-19 pandemic reached France in January 2020 (Lescure et al., 2020).
In many cases, the clinical presentation of COVID-19 is mild to moderate with flu-like symptoms (Guan et al., 2020, Lechien et al., 2020). At the beginning of the pandemic, nearly 60% of patients in France were hospitalized (French Public Health, 2020), particularly for the management of viral pneumonia requiring supplemental oxygen (Aggarwal et al., 2020). Nearly one-quarter of patients have been reported to develop acute respiratory distress syndrome (ARDS) and been admitted to intensive care units (ICU) in need of supplemental oxygen (Richardson et al., 2020, Wiersinga et al., 2020, Wu and McGoogan, 2020). The mortality rate is generally low, but has been reported to vary between 2% and 3% in China (Guan et al., 2020, Wu and McGoogan, 2020) and nearly 20% in US studies (Aggarwal et al., 2020). The mortality rate among non-hospitalized patients is not well known. The mortality rate may be higher in hospitalized patients, and higher still in patients requiring intensive care support.
The optimum way to manage patients hospitalized with COVID-19 remains to be determined. Although some treatments, such as corticosteroids, have shown good results, few therapeutic options have been shown to have proven efficacy and safety in clinical trials (Horby et al., 2020, Toniati et al., 2020). Thousands of clinical trials are currently underway to study this new disease, included its treatment. However, to the authors’ knowledge, there have been no clinical trials on the efficacy of antibiotics in patients with COVID-19.
Whether or not antibiotics are part of the optimal management of patients with COVID-19 has been debated since the beginning of the pandemic. Indeed, it was initially feared that, as for influenza, COVID-19 could be associated with bacterial co- or super-infection (especially with Staphylococcus aureus and Streptococcus pneumoniae) which could legitimize the use of antibiotics. However, no or few bacterial infections have been found to be associated with COVID-19 (Garcia-Vidal et al., 2020, Rawson et al., 2020), so many national guidelines do not recommend systematic empiric antibiotic therapy in patients hospitalized with COVID-19 (French Public Health Committee, 2020). Nevertheless, many patients are still treated with antibiotics (Rawson et al., 2020), and no clinical studies have assessed the potential usefulness of empiric antibiotic therapy in patients hospitalized with COVID-19.
As such, a retrospective study was conducted to compare the characteristics and outcomes of hospitalized patients who did and did not receive antibiotic therapy using propensity score matching.
Methods
Study design and participants
All patients (age ≥18 years) hospitalized in the Infectious Diseases Department, Dijon University Hospital, Dijon, France with confirmed COVID-19 between 27 February and 30 April 2020 (during the first peak of the pandemic in France) were included in this study.
A confirmed case of COVID-19 was defined as a patient with a positive result on real-time reverse transcription polymerase chain reaction (RT-PCR) assay from a nasopharyngeal swab or a sputum sample. Patients were excluded if they were admitted from an ICU.
Patient consent was not obtained given the retrospective nature of the study, as this is not mandatory under French law.
Data collection
For each patient, epidemiological, demographic, clinical, biological, radiological, treatment and outcome data were collected from medical records.
Comorbidities were collected systematically and are summarized in Table 1 ( Du et al., 2020). The Charlson Comorbidity Index, with and without age, was also assessed.
Table 1.
Comparison of characteristics of patients hospitalized with coronavirus disease 2019 (COVID-19) who did and did not receive antibiotic therapy.
| Antibiotic therapy (n = 174) | No antibiotic therapy (n = 48) | P-value | |
|---|---|---|---|
| Age, mean ± SD | 71.5 ± 15.8 | 65.3 ± 20.4 | 0.03 |
| Sex | 0.85 | ||
| Female, n (%) | 77 (44) | 22 (46) | |
| Male, n (%) | 97 (66) | 26 (54) | |
| Risk factors for COVID-19, n (%) | 144 (83) | 32 (67) | 0.02 |
| Age >75 years, n (%) | 76 (44) | 14 (29) | 0.07 |
| Diabetes mellitus, n (%) | 37 (21) | 14 (29) | 0.25 |
| Cardiovascular diseasea, n (%) | 111 (64) | 22 (46) | 0.03 |
| Chronic respiratory diseaseb, n (%) | 24 (14) | 6 (13) | 0.82 |
| BMI < 16 kg/m², n (%) | 1 (1) | 0 (0) | 0.60 |
| BMI ≥ 30 kg/m², n (%) | 29 (17) | 5 (11) | 0.28 |
| Pregnancy, n (%) | 2 (1) | 0 (0) | 0.46 |
| Immunodepressionc, n (%) | 15 (9) | 4 (9) | 0.98 |
| Cirrhosis, n (%) | 1 (1) | 0 (0) | 0.60 |
| Chronic kidney failured, n (%) | 16 (9) | 2 (4) | 0.26 |
| Other past medical history | |||
| Current smoker, n (%) | 4 (3) | 4 (11) | 0.03 |
| Chronic alcoholism, n (%) | 11 (9) | 2 (6) | 0.66 |
| Depression, n (%) | 23 (13) | 9 (19) | 0.33 |
| Dementia, n (%) | 38 (22) | 10 (21) | 0.85 |
| CCI score, median (IQR) | 4.0 (2.0–6.0) | 3.0 (1.5–6.0) | 0.24 |
| CCI score without age, median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.61 |
SD, standard deviation; BMI, body mass index; CCI, Charlson Comorbidity Index; IQR, interquartile range.
Any of the following cardiovascular diseases with or without heart failure: high blood pressure, valvular disease, rhythmic disease and coronaropathy.
Any of the following chronic respiratory diseases with or without respiratory failure: chronic obstructive pulmonary disease, emphysema and asthma.
Any type of immunodepression.
Defined as creatinine clearance <60 mL/min.
Data on clinical signs at hospital admission were collected systematically and are summarized in Table 2. The following biological data were collected at hospital admission: neutrophils, lymphocytes, fibrinogen, C-reactive protein, albumin, pre-albumin, creatinine, glomerular filtration rate, urea and alanine aminotransferase. Radiological data [computer tomography (CT) scan] were classified based on severity, as follows: non-evocative, 0%; minimal, 1–15%; moderate, 15–25%; extensive, 25–50%; severe, 50–75%; and critical, >75%.
Table 2.
Comparison of clinical, biological and radiological characteristics of patients hospitalized with coronavirus disease 2019 (COVID-19) who did and did not receive antibiotic therapy.
| Antibiotic therapy (n = 174) | No antibiotic therapy (n = 48) | P-value | |
|---|---|---|---|
| Time between symptom onset and hospital admission (days), median (IQR) | 6.0 (3.0–9.0) | 5.0 (2.0–9.0) | 0.19 |
| Clinical signs before admission | |||
| Fevera, n (%) | 143 (82) | 26 (54) | <0.001 |
| Cough, n (%) | 120 (69) | 26 (54) | 0.06 |
| Dyspnoea, n (%) | 118 (68) | 19 (40) | <0.001 |
| Flu-likesyndrome, n (%) | 59 (34) | 23 (48) | 0.08 |
| Digestive disorders, n (%) | 53 (30) | 13 (27) | 0.65 |
| Confusion, n (%) | 32 (18) | 5 (10) | 0.19 |
| Anosmia, n (%) | 10 (6) | 3 (6) | 0.90 |
| Dysgeusia, n (%) | 17 (10) | 0 (0) | 0.02 |
| NEWS at D0, median (IQR) | 6.0 (4.0–8.0) | 2.0 (1.0–5.0) | <0.001 |
| Biological features at admission | |||
| Neutrophils, g/L, median (IQR) | 4.8 (3.5–7.3) | 4.2 (2.8–6.0) | 0.06 |
| Lymphocytes, g/L, median (IQR) | 0.9 (0.6–1.2) | 1.1 (0.8–1.8) | 0.02 |
| Fibrinogen, g/L, median (IQR) | 6.0 (5.3–6.0) | 5.5 (4.8–6.3) | 0.04 |
| C-reactive protein, mg/L, median (IQR) | 94.0 (52.5–135.0) | 29.1 (9.2–73.0) | <0.001 |
| Thoracic CT scanb | 0.01 | ||
| Non-evocative of COVID-19, n (%) | 11 (8) | 6 (24) | |
| Minimal, n (%) | 13 (9) | 6 (24) | |
| Moderate, n (%) | 45 (32) | 8 (32) | |
| Extensive, n (%) | 45 (32) | 3 (12) | |
| Severe, n (%) | 21 (15) | 1 (4) | |
| Critical, n (%) | 4 (3) | 1 (4) |
IQR, interquartile range; NEWS2, National Early Warning Score 2; D0, Day 0 (i.e. day of hospital admission); CT, computer tomography.
Defined as temperature >38 °C.
Degree of lesions on CT scan: non-evocative (0%), minimal (1–15%), moderate (15–25%), extensive (25–50%), severe (50–75%) and critical (>75%).
For each patient, three clinical scores were calculated: the National Early Warning Score 2 (NEWS2), the Quick Sepsis-related Organ Failure Assessment (qSOFA) score (Singer et al., 2016, Goulden et al., 2018), and the CRB65 (confusion, respiratory rate, blood pressure, 65 years old) score (Brabrand and Henriksen, 2018). The three scores were defined at different times following hospital admission (when possible): Day (D) 0 (D0), D7, D10, D14, D21 and D28.
For antibiotic therapy, data on first- and second-line (if any) treatments were collected systematically for all patients. Antibiotics were classified as: amoxicillin, amoxicillin + clavulanate, third-generation cephalosporin, third-generation cephalosporin + macrolide, piperacillin + tazobactam, and other. Patients who were not prescribed any antibiotics throughout their hospital stay were classified as receiving no antibiotic therapy.
Outcomes
The following outcomes were collected for each patient up to D28: discharged alive, died or transferred to ICU. An unfavourable outcome was defined as death or transfer to ICU during the 28 days following hospital admission.
Statistical analysis
Univariate analysis was performed to compare patients who did and did not receive antibiotic therapy. Survival curves and hazard ratios (HRs) were calculated using log rank tests.
Subsequently, multi-variate analysis (primary analysis) was performed on complete data using the Cox model. The analysis was adjusted for baseline variables that were clinically relevant or statistically linked to the principal outcome. A step-by-step descending model was used. Figure 1 shows the adjustment variables. HRs with 95% confidence intervals (95% CI) were calculated.
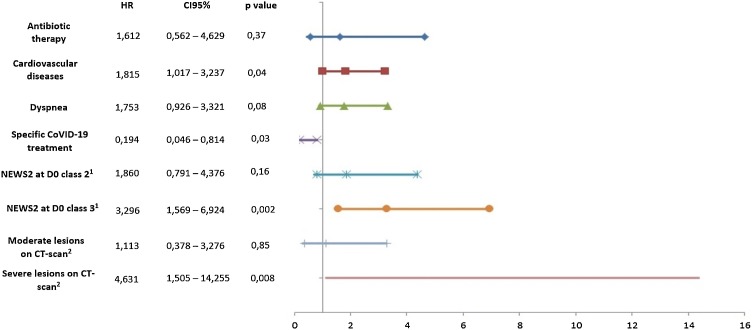
Figure 1.
Factors associated with death or transfer to intensive care unit in 28 days following admission in patients hospitalized with coronavirus disease 2019 (COVID-19) who did and did not receive antibiotic therapy according to multi-variate regression analysis (Cox method). Propension matching score was adjusted for: sex, age (< or ≥75 years), diabetes mellitus, cardiovascular disease, respiratory disease, obesity, immunosuppression, cirrhosis, chronic kidney disease, place of residence (home or other), depression, fever, dyspnoea, flu syndrome, confusion, anosmia, prescription of specific COVID-19 treatment, prescription of corticosteroid, degree of lesions on computed tomography (CT) scan lesions (reference: no lesions2), National Early Warning Score 2 (NEWS2) class on Day 0 (D0) (reference: Class 11), Quick Sepsis-related Organ Failure Assessment (qSOFA) score at D0, and CRB65 (confusion, respiratory rate, blood pressure, 65 years old) score at D0. HR, hazard ratio; CI, confidence interval.
To analyse sensitivity, a second Cox model was computed with propensity score matching. The propensity score was calculated using the mean of a logistic regression model including antibiotic prescription as the dependent variable, and all relevant clinical and biological variables as independent variables. When biological data were missing, data imputation using the multiple imputation method was performed to estimate the propensity score. Next, Cox regression was undertaken using complete data weighted by 1-propensity score (a patient with a score of 0.1 is weighted 0.9 and a patient with a score of 0.9 is weighted 0.1). The model included the same covariates as used in the primary analysis.
Statistical analyses were performed using SAS software. P < 0.05 was considered to indicate statistical significance.
Results
In total, 222 patients were included in this study without loss of follow-up to D28. Patients who were transferred from the ICU were excluded.
Among the 222 patients, 174 (78%) received antibiotic therapy. The characteristics of patients receiving antibiotic therapy are shown in Table 1, Table 2. The median [interquartile range (IQR)] interval between the onset of symptoms and hospitalization was 6 (IQR 3–9) days. The most common clinical signs were fever [n = 143 (82%)], cough [n = 120 (69%)] and dyspnoea [n = 118 (68%)]. Most patients receiving antibiotic therapy had moderate or extensive lesions on CT scan [n = 45 (32%) and 45 (32%), respectively]. Severe lesions and critical lesions were found in 21 patients (15%) and four patients (3%), respectively.
In terms of first-line antibiotic therapy, the most common antibiotic therapy was amoxicillin + clavulanate [n = 95 (55%)], followed by a third-generation cephalosporin with or without a macrolide [n = 25 (14%) and 27 (16%), respectively] (Table 3 ).
Table 3.
Treatments prescribed in management of patients with coronavirus disease 2019 (COVID-19).
| Overall (n = 222) | |
|---|---|
| Specific treatment studied for COVID-19 | 15 (7) |
| Hydroxychloroquine, n (%) | 6(40) |
| Remdesivir, n (%) | 4(27) |
| Lopinavir/ritonavir/interferon beta, n (%) | 3(20) |
| Lopinavir/ritonavir, n (%) | 2(13) |
| Corticosteroids, n (%) | 7 (3) |
| Antibiotics, n (%) | 174 (78) |
| First-line antibiotic therapy | |
| Amoxicillin, n (%) | 7 (4) |
| Amoxicillin + clavulanate, n (%) | 95 (55) |
| Third-generation cephalosporina, n (%) | 25 (14) |
| Third-generation cephalosporin + macrolideb, n (%) | 27 (16) |
| Piperacillin + tazobactam, n (%) | 9 (5) |
| Otherc, n (%) | 11 (6) |
| Second-line antibiotic therapy | |
| Amoxicillin, n (%) | 1 (3) |
| Amoxicillin + clavulanate, n (%) | 10 (29) |
| Third-generation cephalosporina, n (%) | 6 (18) |
| Third-generation cephalosporin + macrolideb, n (%) | 4 (12) |
| Piperacillin + tazobactam, n (%) | 6 (18) |
| Otherc, n (%) | 7 (21) |
Cefotaxime or ceftriaxone.
Cefotaxime associated with rovamycine.
Other antibiotics used: meropenem, levofloxacin, pristinamycin, linezolid, teicoplanin and metronidazole.
A unfavourable event (death or transfer to ICU) was observed for 60 patients (34%) in the antibiotic therapy group, compared with four patients (8%) who did not receive antibiotic therapy (HR 2.94, 95% CI 1.07–8.11; P = 0.04).
On Cox multi-variate regression, antibiotic therapy was not associated with outcome (HR 1.612, 95% CI 0.562–4.629; P = 0.37) after adjustment for cardiovascular disease, dyspnoea, prescription of a specific COVID-19 treatment, NEWS2 class at D0, and degree of lesions on CT scan (Figure 1).
Using the propensity score, the following variables were found to be associated with outcome: presence of previous cardiovascular disease (HR 2.136, 95% CI 1.269–3.593; P = 0.004), dyspnoea at hospital admission (HR 1.941, 95% CI 1.045–3.605; P = 0.04), class 3 NEWS2 at hospital admission (HR 3.397, 95% CI 1.748–6.600; P < 0.001), severe lesions on CT scan (HR 7.070, 95% CI 2.721–18.370; P < 0.001) and prescription of specific COVID-19 treatment (HR 0.170, 95% CI 0.041–0.708; P = 0.01). No significant association was found between antibiotic therapy and outcome after propensity score matching (HR 1.238, 95% CI 0.77–2.00; P = 0.37).
Discussion
As for influenza, antibiotics have been hypothesized to be necessary in the management of COVID-19. Fear of bacterial co- or super-infection with high mortality may have been the main reason behind the frequent prescription of antibiotics, especially at the beginning of the pandemic. This retrospective study found that three-quarters of the study population received antibiotic therapy, in agreement with many other studies published worldwide (Rawson et al., 2020). The present study also found that antibiotic prescription was more common in certain populations, such as older patients and patients with cardiovascular disease; those presenting with higher rates of fever, dyspnoea and requirement for supplemental oxygen; and those with extensive lesions on CT scan. Therefore, patients who received antibiotic therapy during hospitalization were frailer and had more severe presentation at admission. This correlates with the management of patients who present to emergency departments with lower respiratory tract symptoms during influenza epidemics. The higher the risk of mortality, the higher the rate of antibiotic prescription.
Currently, the rationale for antibiotic prescription was based on other viral infections, especially influenza virus. Indeed, in patients with influenza, there is a high rate of bacterial (or fungal with aspergillosis) co- or super-infection (Metersky et al., 2012, Klein et al., 2016). S. aureus and S. pneumoniae are the most common bacteria found in these patients (Metersky et al., 2012). The prognosis of patients with co-infection is poor, and elderly patients are the main population affected by co-infection (Metersky et al., 2012).
However, in the few cohorts dealing with this topic, the rate of bacterial co-infection was very low or zero (Garcia-Vidal et al., 2020, Rawson et al., 2020). No cases of bacterial infection were documented in the present study (including secondary bloodstream infection), but this was were not searched for systematically in all hospitalized patients. Only blood cultures were collected systematically. Sputum samples were collected in cases with poor evolution during hospitalization and cases with strong suspicion of bacterial infection.
Another potential benefit of certain antibiotics is their anti-inflammatory properties, as the potential severity of COVID-19 is linked to a systemic inflammatory response (Mangalmurti and Hunter, 2020, Ragab et al., 2020). Macrolides, such as azithromycin, are known to downregulate pro-inflammatory cytokines (Kuo et al., 2019, Ulrich and Pillat, 2020), which has potential clinical benefit in patients with influenza (Lee et al., 2017) and patients with ARDS (Kawamura et al., 2018). However, this benefit was not observed in patients infected with Middle East respiratory syndrome coronavirus (Arabi et al., 2019). No clear specific effect could be demonstrated in the COVID-19 context (Rizk et al., 2020) and, if any, could be counterbalanced by the weight of side effects (Ray et al., 2012).
This study found that antibiotic therapy was not associated with a better outcome, with similar results found using two different multi-variate analyses (Cox model and propensity score matching). These two approaches should allow overrunning the negative impact of a more severe baseline presentation in patients on antibiotics. The characteristics usually associated with poorer outcomes in other studies (Du et al., 2020) were also found in this study, namely pre-existing cardiovascular disease, higher NEWS2 score at hospital admission, and more severe lesions on CT scan. Treatments prescribed to treat COVID-19 specifically were associated with a better prognosis. Most of these treatments were given as part of therapeutic research protocols, which may represent selection bias. However, it could be advocated that such a bias should have limited impact in the multi-variate analysis. A benefit of these treatments considered as a whole would be surprising as some of them were shown individually to have no effect or a mild effect on the evolution of COVID-19. It is also unlikely that corticosteroids, recently reported to have a positive impact on survival (Horby et al., 2020), would have affected the observed results as very few of the study patients were on such adjunctive therapy.
This study had some limitations. First, only patients with COVID-19 hospitalized on a medical ward were included, and those in the ICU were excluded. The possible impact of wide antibiotic use in this context cannot be excluded. Second, microbiological documentation was often missing, as antibiotics were often given early in patient management. In cases of undocumented genuine bacterial co-infection, it could be speculated that incorrect antibiotics were used, although they cover the main bacterial pathogens seen in the context of viral superinfection. Finally, this study likely suffered from a lack of statistical power. Nevertheless, and to the best of the authors’ knowledge, this is one of the first studies on antibiotic prescription for the management of COVID-19 in a whole cohort with near-complete and assessable data.
In conclusion, in the study population, a high proportion of patients with COVID-19 were treated with antibiotics although no bacterial co-infection was documented (note: screening for bacterial co-infection was not systematic). The likelihood of antibiotic prescription was correlated with the severity of COVID-19 and/or the frailty of the patient. However, wide prescription of antibiotics did not appear to have a significant impact on the prognosis of patients hospitalized with COVID-19 in medical wards without evidence of bacterial co-infection. According to these data, antibiotic therapy should not be prescribed systematically for patients with COVID-19, but should only be used in cases of proven (or strongly suspected) bacterial infection. Confirmation of these results from clinical trials is required.
Funding
None.
Ethical approval
Not required.
Conflict of interest
None declared.
Acknowledgements
The authors wish to thank all the doctors, residents and medical students of the Infectious Diseases Department, Dijon University Hospital for their help and work during the pandemic. The authors also wish to thank the Virology Laboratory for performing SARS-CoV-2 RT-PCR assays, the patients, and Dr Claire Breniaux (Doctoral Researcher in British Society and Politics and Teaching Fellow at the University of Burgundy) for linguistic support.
References
- Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagn Berl Ger. 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., Deeb A.M., Al-Hameed F., Mandourah Y., Almekhlafi G.A., Sindi A.A. Macrolides in critically ill patients with Middle East respiratory syndrome. Int J Infect Dis. 2019;81:184–190. doi: 10.1016/j.ijid.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabrand M., Henriksen D.P. CURB-65 score is equal to NEWS for identifying mortality risk of pneumonia patients: an observational study. Lung. 2018;196:359–361. doi: 10.1007/s00408-018-0105-y. [DOI] [PubMed] [Google Scholar]
- Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French Public Health . 2020. COVID-19: Epidemiologic Review of 17 July 2020. [Google Scholar]
- French Public Health Committee . French Public Health Committee; 2020. Guidelines Concerning the Use of Antibiotics for the Management of COVID-19. [Google Scholar]
- Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M. Incidence of co-infections and superinfections in hospitalised patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden R., Hoyle M.-C., Monis J., Railton D., Riley V., Martin P. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35:345–349. doi: 10.1136/emermed-2017-207120. [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y.u., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. Infect Dis (except HIV/AIDS) 2020 [Google Scholar]
- Kawamura K., Ichikado K., Takaki M., Eguchi Y., Anan K., Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int J Antimicrob Agents. 2018;51:918–924. doi: 10.1016/j.ijantimicag.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.-H. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.-H., Lee M.-S., Kuo H.-F., Lin Y.-C., Hung C.-H. Azithromycin suppresses Th1- and Th2-related chemokines IP-10/MDC in human monocytic cell line. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2019;52:872–879. doi: 10.1016/j.jmii.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa‐Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Wong C.-K., Chan M.C.W., Yeung E.S.L., Tam W.W.S., Tsang O.T.Y. Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48–56. doi: 10.1016/j.antiviral.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metersky M.L., Masterton R.G., Lode H., File T.M., Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16:e321–e331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm: what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020:80. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;4324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]