1. Introduction
In the recent context of the Coronavirus disease 2019 (COVID-19) pandemic, secondary fungal infections, such as invasive pulmonary aspergillosis, have been reported for about 30% of the cases admitted to the ICU, mostly in patients in whom the European organisation for the research and treatment of cancer: Mycoses study group education and research consortium (EORTC/MSGERC) host factors were absent [1], [2], [3]. At the university hospital of Besançon (northeastern France), while a similar proportion of putative invasive pulmonary aspergillosis was observed, we also had one case of mixed-mold infection displaying both Aspergillus fumigatus and Rhizopus microsporus isolated in the respiratory samples of an immunocompromised patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We propose here to report and discuss this rare occurrence.
2. Case
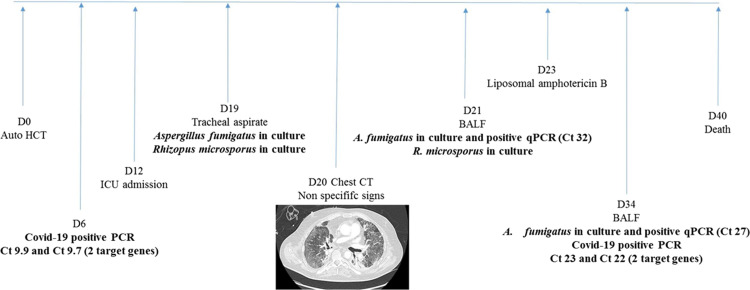
A 55-year-old man was diagnosed in 2017 with follicular lymphoma, which recurred in 2018. After autologous hematopoietic stem cell transplantation (auto-HCT) was planned, the patient tested positive for Influenza B virus (Day –20). Prior to auto-HCT, a SARS-CoV-2 Real-time polymerase chain reaction (RT–PCR) test [4] was performed from a nasopharyngeal sample on Day –7 and was negative. Auto-HCT was performed on Day 0 (D0). Over the following days, the patient became feverish while in aplasia despite antibiotic treatment, and a new SARS-CoV-2 RT–PCR test from a nasopharyngeal swab came back positive with a very high respiratory viral load (D6; cycle threshold (Ct) value = 9.9 and 9.7 for the 2 target genes). The patient was then transferred to the infectious diseases department. His aplasia period ended at D10. A strong inflammatory response was observed with D-Dimer at 3710 ng/mL, fibrinogen at 8.93 g/L and C-reactive protein (CRP) reaching 552 mg/L at D11. At D12, the respiratory status of the patient abruptly worsened and the patient was admitted to an intensive care unit (ICU) to receive mechanical ventilation. On D19 and D21, respiratory samples (tracheal aspirate and broncho-alveolar lavage fluid [BALF]) were positive in culture for both Aspergillus fumigatus and Rhizopus microsporus (Fig. 1 ). On D34, a 2nd BALF was positive in culture for A. fumigatus (Fig. 1). The parasitology–mycology department of the university hospital of Besançon developed fungal qPCR in-house techniques, targeting A. fumigatus and Mucorales that are routinely used to screen for invasive mold disease (IMD) [5], [6]. Fungal qPCR was performed on BALF at D21 and D34 and found positive for A. fumigatus, with Ct ranging from 27 to 32 (Fig. 1). Fungal qPCR was performed on serum at D17 and D21 and found negative, as was the galactomannan (GM) antigen in serum (D17 and D21). Due to the patient's positive COVID-19 status, which represented an exposure risk for the technicians, GM measurement in BALF was not performed in our laboratory. Susceptibility testing was carried out by E-test, and both strains were wild types. CT–thorax imagery was performed at D20 and showed non-specific bilateral ground glass opacities, presumably due primarily to the SARS-CoV-2 infection (Fig. 1). Treatment with liposomal amphotericin B began at D23 (5 mg/kg). Despite its administration, pejorative evolution was observed, with development of pulmonary fibrosis. Another secondary infection was sought; the patient was tested negative three times for Pneumocystis jiroveci. While no secondary bacterial infection was documented, CMV and HHV6 viremia were observed. SARS-CoV-2 respiratory viral loads remain high on BALF at D13 (Ct = 17.60 and 15.15) and D34 (Ct = 23.07 and 21.93). The patient died at D40.
Fig. 1.
Chronology of the positivity of culture and qPCRs for the patient.
3. Discussion
While a certain number of invasive pulmonary aspergillosis (IPA) cases in SARS-CoV-2 infected patients have been published [1], [2], [7], the fungal infection, known as mucormycosis, has seldom been reported. In the context of the COVID-19 epidemic, most IPA cases have occurred in patients without the EORCT/MSGERC host factors, and they necessitated new case definitions to guide a decision to initiate anti-fungal treatment [2], [7]. The criteria required to confirm the existence of a “putative” CAPA in the presence of non-specific radiology signs are two or more positives across different test types, or multiple positives within one test type among the following: positive culture from BALF, positive GM in BALF (≥ 1.0), positive GM in serum (≥ 0.5), positive qPCR in BALF or blood, positive beta-D glucan in serum/plasma [7].
In the present case, the patient displayed the EORCT/MSGERC host factors [3] and non-specific radiology signs of IMD. He had two positive cultures and two positive A. fumigatus qPCR in BALF (Fig. 1), which meant that the criteria required to confirm the existence of a putative IPA, as defined by White et al., had been fulfilled [7].
The originality of this IMD case consisted in the association of A. fumigatus with a Mucorales species. Several cases of mucormycosis in SARS-CoV-2 infected patients have been reported recently, describing varied clinical forms in those without EORCT/MSGERC host factors [8]. The hypothesis put forward to explain these rare occurrences were:
-
•
that the steroids used to treat the SARS-CoV-2 infection may favour the development of molds, providing that the latter were pre-existing and/or colonising the patient and;
-
•
that the SARS-CoV-2 infection itself may induce an immunosuppressive state exposing the patient to IMD [8].
In the present case, from day 6 to day 34, the SARS-CoV-2 viral load in respiratory samples remained high, with a Ct value < Ct 25 [9], [10], and it was associated with pronounced elevation of inflammatory markers. The slow decline of SARS-CoV-2 viral load could reflect failed triggering of an effective innate immune response against the virus. A recent study showed that a hematologic malignancy was independently associated with high SARS-CoV-2 viral load (Ct < 25) upon admission [9]; moreover, high SARS-CoV-2 viral load (Ct < 25) has been shown to be independently associated with an outcome of death [9], [10].
This patient had two major risk factors: SARS-CoV-2 infection and severe immunosuppression. Even though it was not possible, in our case, to determine which of the two was more damaging, it may have provided some insight into the interactions between molds, such as A. fumigatus and Mucorales, and the SARS-CoV-2 infected lung [2].
4. Conclusion
This reported case of secondary fungal infection in an immunocompromised SARS-CoV-2 infected patient is a rare occurrence due to the mixed presence of A. fumigatus and a Mucorales species.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., Van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verweij P.E., Gangneux J.P., Bassetti M., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1(2):e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organisation for research and treatment of cancer and the Mycoses study group education and research consortium. Clin Infect Dis. 2019;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biguenet A., Bouiller K., Marty-Quinternet S., Brunel A.S., Chirouze C., Lepiller Q. SARS-CoV-2 respiratory viral loads and association with clinical and biological features. J Med Virol. 2020 doi: 10.1002/jmv.26489. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Bellanger A.P., Millon L., Berceanu A., Grenouillet F., Grenouillet F.E., Larosa F., et al. Combining aspergillus mitochondrial and ribosomal QPCR, in addition to galactomannan assay, for early diagnosis of invasive aspergillosis in hematology patients. Med Mycol. 2015;53(7):760–764. doi: 10.1093/mmy/myv051. [DOI] [PubMed] [Google Scholar]
- 6.Millon L., Herbrecht R., Grenouillet F., Morio F., Alanio A., Letscher-Bru V., et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French surveillance network of invasive fungal infections (RESSIF) Clin Microbiol Infect. 2016;22(9):810e1–810e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 7.White P.L., Dhillon R., Cordey A., et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. [ciaa1298] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faíco-Filho K.S., Passarelli V.C., Bellei N. Is higher viral load in SARS-CoV-2 associated with death? Am J Trop Med Hyg. 2020;103(5):2019–2021. doi: 10.4269/ajtmh.20-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westblade L.F., Brar, Pinhiro G.L.C., Paidoussis D., Rajan M., Martin P., et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalised with COVID-19. Cancer Cell. 2020;38(5):661e2–671e2. doi: 10.1016/j.ccell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]