Asymptomatic infection seems to be a notable feature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but the prevalence is uncertain. This review summarizes available evidence to estimate the proportion of persons infected with SARS-CoV-2 who never develop symptoms.
Abstract
Background:
Asymptomatic infection seems to be a notable feature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes coronavirus disease 2019 (COVID-19), but the prevalence is uncertain.
Purpose:
To estimate the proportion of persons infected with SARS-CoV-2 who never develop symptoms.
Data Sources:
Searches of Google News, Google Scholar, medRxiv, and PubMed using the keywords antibodies, asymptomatic, coronavirus, COVID-19, PCR, seroprevalence, and SARS-CoV-2.
Study Selection:
Observational, descriptive studies and reports of mass screening for SARS-CoV-2 that were either cross-sectional or longitudinal in design; were published through 17 November 2020; and involved SARS-CoV-2 nucleic acid or antibody testing of a target population, regardless of current symptomatic status, over a defined period.
Data Extraction:
The authors collaboratively extracted data on the study design, type of testing performed, number of participants, criteria for determining symptomatic status, testing results, and setting.
Data Synthesis:
Sixty-one eligible studies and reports were identified, of which 43 used polymerase chain reaction (PCR) testing of nasopharyngeal swabs to detect current SARS-CoV-2 infection and 18 used antibody testing to detect current or prior infection. In the 14 studies with longitudinal data that reported information on the evolution of symptomatic status, nearly three quarters of persons who tested positive but had no symptoms at the time of testing remained asymptomatic. The highest-quality evidence comes from nationwide, representative serosurveys of England (n = 365 104) and Spain (n = 61 075), which suggest that at least one third of SARS-CoV-2 infections are asymptomatic.
Limitation:
For PCR-based studies, data are limited to distinguish presymptomatic from asymptomatic infection. Heterogeneity precluded formal quantitative syntheses.
Conclusion:
Available data suggest that at least one third of SARS-CoV-2 infections are asymptomatic. Longitudinal studies suggest that nearly three quarters of persons who receive a positive PCR test result but have no symptoms at the time of testing will remain asymptomatic. Control strategies for COVID-19 should be altered, taking into account the prevalence and transmission risk of asymptomatic SARS-CoV-2 infection.
Primary Funding Source:
National Institutes of Health.
The asymptomatic fraction of infection is the proportion of infected persons who never develop, perceive, and report symptoms (1). Among common pathogens, the asymptomatic fraction varies widely. For example, an asymptomatic carrier state has not been documented for measles virus infection (2), whereas a significant proportion of persons with cytomegalovirus or poliovirus infection have no symptoms and are unaware of infection (3, 4). The asymptomatic fraction of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection seems to be sizable (5). The range of severity of illness associated with SARS-CoV-2 infection is noteworthy because it spans asymptomatic infection; mild illness; and severe, life-threatening illness.
Perhaps because of this broad spectrum of presentation, the topic of asymptomatic SARS-CoV-2 infection has generated some controversy (6). Imprecise use of the term “asymptomatic” is partly to blame. “Asymptomatic” should be reserved for persons who never develop symptoms, whereas “presymptomatic” is a better description of those who have no symptoms when they receive a positive test result but who eventually develop symptoms. We know for certain who is asymptomatic only in retrospect. On the basis of our current knowledge of the natural history of coronavirus disease 2019 (COVID-19), after a person is infected with SARS-CoV-2, we must wait approximately 14 days to determine whether symptoms have developed (7). Infection without symptoms, whether presymptomatic or asymptomatic, is important because infected persons can transmit the virus to others even if they have no symptoms (8, 9).
In June 2020, we published a review of the limited data then available on the prevalence of asymptomatic SARS-CoV-2 infection (5). Since then, considerable new data have become available. The present review summarizes currently available data that might allow us to estimate the proportion of persons infected with SARS-CoV-2 who are asymptomatic.
Methods
Data Sources, Search Terms, and Study Selection
Using the keywords antibodies, asymptomatic, coronavirus, COVID-19, PCR, seroprevalence, and SARS-CoV-2, we periodically searched Google News, Google Scholar, medRxiv, and PubMed for observational, descriptive studies and reports of mass screening for SARS-CoV-2 that were either cross-sectional or longitudinal in design; were published through 17 November 2020; and involved SARS-CoV-2 nucleic acid or antibody testing of a target population, regardless of current symptomatic status, over a defined period.
Data Extraction and Quality Assessment
We recorded the total number of persons tested, the number that tested positive, the number of positive cases without symptoms, the criteria for determining symptomatic status, whether the data were cross-sectional or longitudinal in nature, whether random selection techniques were used to achieve a representative sample of a target population, and whether the testing involved polymerase chain reaction (PCR) analysis of a nasopharyngeal swab or serologic analysis of antibodies in a blood sample. For longitudinal studies that provided information on the evolution of symptomatic status, we recorded the proportion of persons who tested positive but had no symptoms at the time of testing and who then remained asymptomatic during a follow-up period. In addition, we flagged studies that required clarification of ambiguous details.
Studies or reports that are based on PCR results and include only cross-sectional data do not make it possible to distinguish between presymptomatic and asymptomatic SARS-CoV-2 infection because symptomatic status is observed on only 1 occasion, which may occur before the development of symptoms, if any. In contrast, we can distinguish between presymptomatic and asymptomatic infection with either antibody-based studies, in which an interview or questionnaire gathers information about symptoms reported at the time a blood sample is taken and during a prior period, or PCR-based studies that include longitudinal data.
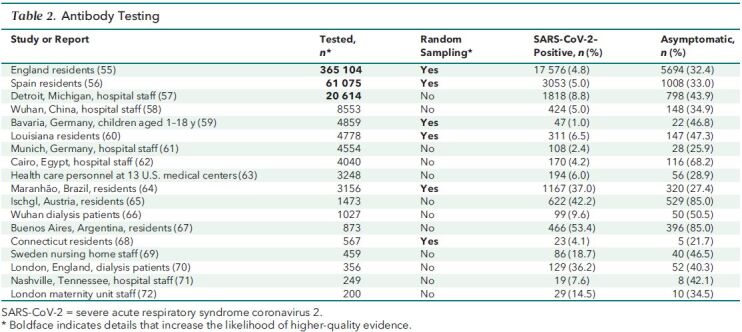
In assessing quality, we put the greatest emphasis on random selection of participants to achieve a representative sample of a regional or national population, a large number of study participants (n > 10 000), and study designs that make it possible to distinguish between presymptomatic and asymptomatic infection. Evaluated in this manner, the highest-quality evidence comes from large-scale, national studies with representative samples that include data from either antibody or longitudinal PCR testing. In Tables 1 and 2, we show in boldface the details that increase a study's likelihood of providing higher-quality evidence.
Table 1. Nucleic Acid PCR Testing.
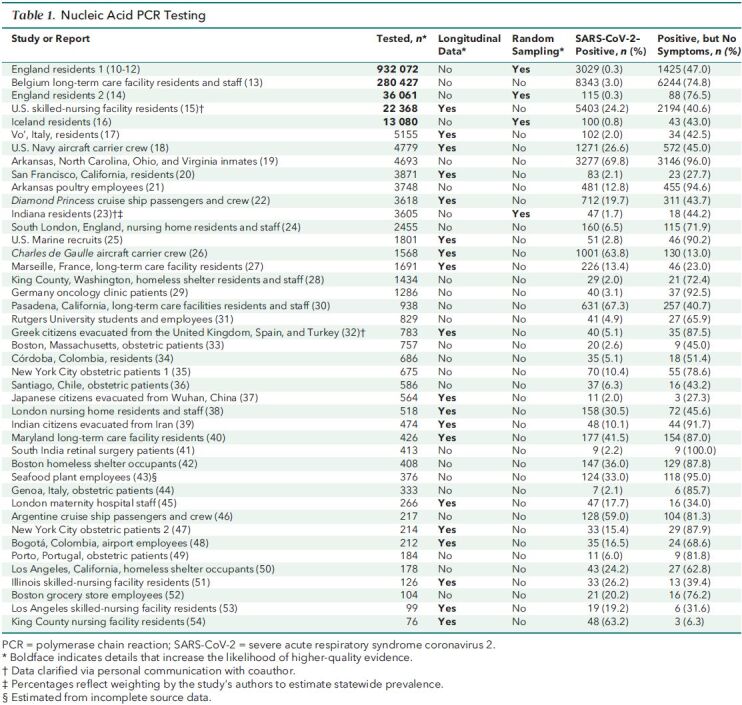
Table 2. Antibody Testing.
Data Synthesis and Analysis
We synthesized evidence qualitatively by evaluating study design, including whether data were collected longitudinally; testing methods; number of participants; and setting. We compared the range and consistency of estimates of the proportion of persons who tested positive but had no symptoms at the time of testing.
Role of the Funding Source
The National Institutes of Health played no role in the design, conduct, or analysis of this review or in the decision to submit the manuscript for publication.
Results
We identified 61 studies or reports that met eligibility criteria. Table 1 summarizes data from the 43 that used PCR testing, and Table 2 summarizes data from the 18 that used antibody testing. The heterogeneity of the studies—in particular, disparate settings and populations—precluded quantitative summaries using meta-analysis. We summarize the evidence in terms of the number of studies and the range, median, and interquartile range (IQR) for persons who tested positive but had no symptoms at the time of PCR testing or who reported having had no symptoms before or at the time of antibody testing. Thirty of the studies included a list of specific symptoms, independent of signs, used to determine symptomatic status (10–14, 17, 18, 22–28, 35, 36, 38, 42, 49, 51, 55–57, 60–62, 64). Many of the remaining studies used some variation of the catch-all phrase “symptoms compatible with COVID-19.”
Nucleic Acid PCR Testing
Among the 43 studies using PCR testing (10–41-42–54), the proportion of persons who tested positive but had no symptoms at the time of testing ranged from 6.3% to 100%, with a median of 65.9% (IQR, 42.8% to 87.0%).
Nineteen of the PCR-based studies collected data on symptoms longitudinally after testing, making it possible to distinguish between presymptomatic and asymptomatic infection (15, 17, 18, 20, 22, 25, 26, 27, 32, 37–40, 45, 47, 48, 51, 53, 54). The follow-up period in these studies ranged from 2 to 70 days, with a median of 14 days (IQR, 14.0 to 15.8 days). The proportion of persons who tested positive and remained asymptomatic ranged from 6.3% to 91.7%, with a median of 42.5% (IQR, 29.6% to 77.8%).
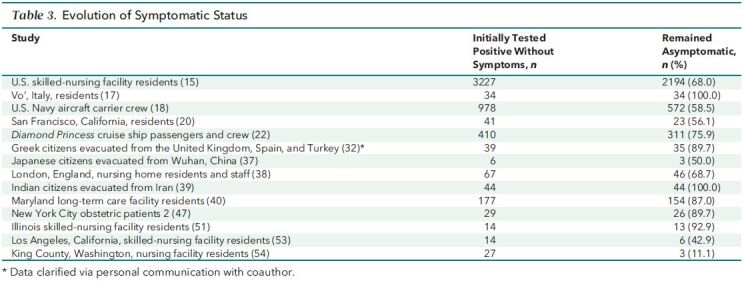
Of the 19 longitudinal studies, 14 provided information on the evolution of symptomatic status (Table 3) (15, 17, 18, 20, 22, 32, 37–40, 47, 51, 53, 54). Among persons who tested positive but had no symptoms at the time of testing, the proportion who remained asymptomatic during a follow-up period ranged from 11.1% to 100%, with a median of 72.3% (IQR, 56.7% to 89.7%).
Table 3. Evolution of Symptomatic Status.
Of the 43 studies that used PCR testing, 24 collected cross-sectional data and reported only the symptomatic status at the time of testing, so we could not distinguish between presymptomatic and asymptomatic cases (10–14, 16, 19, 21, 23, 24, 28–31, 33–36, 41–44, 46, 49, 50, 52). In these studies, the proportion of persons who tested positive but had no symptoms at the time of testing ranged from 40.7% to 100%, with a median of 75.5% (IQR, 50.3% to 86.2%).
Of the 43 studies that used PCR testing, 4 used random selection of participants to achieve a representative sample of their target population: residents of England (10–12, 14), Iceland (16), or Indiana (23). Proportions of persons who tested positive but had no symptoms at the time of testing ranged from 43.0% to 76.5%, with a median of 45.6% (IQR, 43.6% to 61.8%). None of the PCR testing studies that used random selection of participants collected longitudinal data on symptoms, so we could not distinguish between presymptomatic and asymptomatic cases.
The largest of the representative data sets, and the largest study identified in our search, was from the REACT (Real-time Assessment of Community Transmission) program. REACT has implemented nationwide nucleic acid and antibody testing (discussed later) for SARS-CoV-2 of persons in England aged 5 years and older in multiple phases since May 2020 (10–12). In Table 1, we have combined the results of 6 phases of nucleic acid testing from REACT, yielding data for 932 072 persons (England residents 1). At the time of testing, 1425 of 3029 persons (47.0%) who tested positive had no symptoms. The study did not collect longitudinal data on symptoms, so we could not distinguish between presymptomatic and asymptomatic cases.
The second largest of the representative studies was also from England; it included 36 061 persons tested between 26 April and 27 June 2020 (14). The proportion of persons who tested positive was 0.3%, identical to that reported by REACT, but the proportion of persons who tested positive but had no symptoms at the time of testing was 74.8%, much larger than in the REACT study. The study did not collect longitudinal data on symptoms, so we could not distinguish between presymptomatic and asymptomatic cases.
In the cross-sectional study of Belgian long-term care facilities (n = 280 427), age did not seem to affect the proportion of persons who tested positive but had no symptoms at the time of testing (13). The study tested 138 327 staff and 142 100 residents. Median age was 42 years for staff and 85 years for residents; despite this considerable difference, the proportion of those who tested positive without symptoms was 74.0% for staff and 75.3% for residents. This finding is consonant with the finding of a longitudinal study from Vo', Italy, in which more than 85% of the town's 3275 residents were tested: “Among confirmed SARS-CoV-2 infections, we did not observe significant differences in the frequency of asymptomatic infection between age groups” (17).
Of the 43 studies that used PCR testing, 21 involved high-density living or working environments, such as nursing homes and factories (13, 15, 18, 19, 21, 22, 24–28, 30, 38, 40, 42, 46, 50, 51, 53, 54). The settings with the highest proportion of persons who tested positive without symptoms included prisons (19) and poultry processing plants (21). Yet, the data seem to be insufficient to conclude that setting was a causative factor. In the 21 studies of high-density environments, the proportion of persons who tested positive but had no symptoms at the time of testing ranged from 6.3% to 96.0%, with a median of 62.8% (IQR, 40.6% to 87.0%). In the remaining 22 studies that did not involve such high-density environments, the proportion ranged from 27.3% to 100%, with a median of 67.2% (IQR, 43.5% to 84.7%).
Antibody Testing
In the 18 studies based on antibody testing (Table 2) (55–72), the proportion of persons who tested positive but did not report having had symptoms ranged from 21.7% to 85.0%, with a median of 41.2% (IQR, 32.6% to 48.1%).
Among the 18 antibody testing studies, 6 used random selection of participants to achieve a representative sample of their target population: residents of England (55); Spain (56); Bavaria, Germany (59); Louisiana (60); Maranhão, Brazil (64); or Connecticut (68). In these antibody studies with representative samples, the proportion of persons who tested positive but did not report having had symptoms ranged from 21.7% to 47.3%, with a median of 32.7% (IQR, 28.7% to 43.4%).
The 2 largest studies based on antibody testing were nationwide serosurveys from England (55) and Spain (56), both designed to achieve representative samples of community-dwelling persons. The English data, from the REACT program described earlier, were collected during 3 rounds of testing from June through September 2020 and include 365 104 persons. The Spanish data were collected 27 April to 11 May 2020 and include 61 075 persons. The proportion of persons who tested positive but did not report having had symptoms was 32.4% in England and 33.0% in Spain.
Discussion
Symptom detection relies on the subjective reports of patients (73). For example, anosmia has turned out to be a distinctive symptom of COVID-19 (74), and we depend on patients to perceive and report a diminution, however slight, of their normal olfactory abilities. But such self-reports are influenced by many factors, including variability in the ability to recall symptoms and idiosyncratic awareness of bodily sensations.
Current data suggest that infected persons without symptoms—including both presymptomatic and asymptomatic persons—account for more than 40% of all SARS-CoV-2 transmission (75–77). The proportion of new infections caused by asymptomatic persons alone is uncertain, but when researchers in Wanzhou, China, analyzed epidemiologic data for “183 confirmed COVID-19 cases and their close contacts from five generations of transmission,” they determined that the asymptomatic cases, which made up 32.8% of infected persons, caused 19.3% of infections (78).
The 61 studies and reports that we have collected provide compelling evidence that the asymptomatic fraction of SARS-CoV-2 infection is sizable. These data enable us to make reasonable inferences about the proportion of SARS-CoV-2 infections that are asymptomatic.
Studies designed to achieve representative samples of large populations provide useful data because they may accurately reflect human populations in general. Four of the PCR-based studies are in this category, with target populations of England (10–12, 14), Iceland (16), and Indiana (23). The proportion of persons who tested positive but had no symptoms at the time of testing ranged from 43.0% to 76.5%, with a median of 45.6% (IQR, 43.6% to 61.8%). However, these studies fall short of providing the highest-quality evidence because they collected only cross-sectional data. As a result, we cannot distinguish between presymptomatic and asymptomatic cases.
In 14 longitudinal studies that reported information on the evolution of symptomatic status, a median of 72.3% of persons who tested positive but had no symptoms at the time of testing remained asymptomatic during a follow-up period (15, 17, 18, 20, 22, 32, 37–40, 47, 51, 53, 54). If a similar proportion remained asymptomatic in the 4 large, representative, PCR-based studies, in which the median was 45.6%, the asymptomatic fraction of SARS-CoV-2 infection would be 33.0%.
Among the data that we have assembled here, the highest-quality evidence comes from the large-scale studies using antibody testing that were designed to achieve representative samples of nationwide populations in England (n = 365 104) (55) and Spain (n = 61 075) (56). It is remarkable that these independently conducted serosurveys yielded nearly identical proportions of asymptomatic SARS-CoV-2 infections: 32.4% in England and 33.0% in Spain.
We may infer that persons who receive positive antibody test results can be classified accurately as asymptomatic because such results are likely to occur only after the onset of symptoms, if any. In a study of 222 hospitalized patients in Wuhan, China, IgM and IgG antibodies to SARS-CoV-2 were first detected 3 and 4 days, respectively, after symptomatic onset of COVID-19 (79). In a study of 109 health care workers and 64 hospitalized patients in Zurich, Switzerland, the severity of illness seemed to affect how quickly SARS-CoV-2 antibodies appeared (80). Patients with severe COVID-19 had detectable SARS-CoV-2 antibody titers after symptom onset, but those with mild cases “remained negative or became positive [for SARS-CoV-2 antibodies] 12 to 14 days after symptom onset” (80). These data suggest that positive antibody test results are unlikely to occur during the period when it is uncertain whether an infected person is presymptomatic or asymptomatic.
However, serosurveys do have significant limitations for the purpose of estimating the asymptomatic fraction. Not all persons who are believed to have been infected with SARS-CoV-2 later have a positive result for SARS-CoV-2 antibodies (81). The reasons may include a false-positive result on the initial PCR test; a false-negative result on the antibody test; or the absence of detectable antibodies, perhaps because the infection was cleared without requiring adaptive immunity. In addition, the role of mucosal immunity in clearing SARS-CoV-2 infection has not yet been fully elucidated (82), and a nasal wash to detect the IgA antibodies active in mucosal immunity is not part of standard testing practice. Persons who clear SARS-CoV-2 infection through innate or mucosal immunity might be more likely to be asymptomatic but would not be categorized as such in a serosurvey, possibly contributing to an underestimate of the asymptomatic fraction.
Another limitation of serosurveys is the requirement that an interview or questionnaire about symptomatic status accompany the blood sample. The onus is on the study participant to accurately recall symptoms, if any, from weeks or even months earlier. In the midst of a pandemic that has transformed everyday life around the globe, it seems reasonable to hypothesize that awareness of and memory for symptoms possibly related to COVID-19 are heightened. This might result in a greater likelihood of noticing and reporting symptoms that would otherwise be missed or ignored, thereby leading to a lower estimate of the asymptomatic fraction. For these reasons, we have evaluated serosurveys in the context of other results and found them to be concordant.
When estimates from large-scale, cross-sectional, PCR-based studies with representative samples; longitudinal PCR-based studies; and nationwide serosurveys with representative samples are combined, it seems that the asymptomatic fraction of SARS-CoV-2 infection is at least one third. To confirm this estimate, large-scale longitudinal studies using PCR testing with representative samples of national populations would be useful. As SARS-CoV-2 vaccination campaigns are implemented worldwide, though, the window for such research may be closing.
In light of the data presented here, we believe that COVID-19 control strategies must be altered, taking into account the prevalence and transmission risk of asymptomatic SARS-CoV-2 infection. Frequent, inexpensive, rapid home tests (83) to identify and contain presymptomatic or asymptomatic cases—along with government programs that provide financial assistance and, if necessary, housing to enable infected persons to isolate themselves (84)—may be a viable option. And as the first generation of SARS-CoV-2 vaccines is deployed, more research will be needed to determine their efficacy in preventing asymptomatic infection (85).
Footnotes
This article was published at Annals.org on 22 January 2021
References
- 1. Champredon D , Moghadas SM . Quantifying the contribution of asymptomatic infection to the cumulative incidence. Epidemiol Infect. 2017;145:1256-1258. [PMID: ] doi: 10.1017/S0950268817000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Measles. Accessed at www.cdc.gov/vaccines/pubs/pinkbook/meas.html on 24 November 2020.
- 3. Centers for Disease Control and Prevention. About cytomegalovirus (CMV). Accessed at www.cdc.gov/cmv/overview.html on 24 November 2020.
- 4. European Center for Disease Prevention and Control Disease factsheet about poliomyelitis. Updated 11 July 2018. Accessed at www.ecdc.europa.eu/en/poliomyelitis/facts on 3 October 2020.
- 5. Oran DP , Topol EJ . Prevalence of asymptomatic SARS-CoV-2 infection. A narrative review. Ann Intern Med. 2020;173:362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apuzzo M, Gebrekidan S, Kirkpatrick DD. How the world missed COVID-19's silent spread. New York Times. 27 June 2020. Accessed at www.nytimes.com/2020/06/27/world/europe/coronavirus-spread-asymptomatic.html on 1 October 2020.
- 7. Lauer SA , Grantz KH , Bi Q , et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577-582. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 10 September 2020. Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html on 4 October 2020.
- 9. Furukawa NW , Brooks JT , Sobel J . Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26. [PMID: ] doi: 10.3201/eid2607.201595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riley S, Ainslie KEC, Eales O, et al. High prevalence of SARS-CoV-2 swab positivity in England during September 2020: interim report of round 5 of REACT-1 study. medRxiv. Preprint posted online 2 October 2020. doi:10.1101/2020.09.30.20204727
- 11. Riley S, Ainslie KEC, Eales O, et al. High and increasing prevalence of SARS-CoV-2 swab positivity in England during end September beginning October 2020: REACT-1 round 5 updated report. medRxiv. Preprint posted online 14 October 2020. doi:10.1101/2020.10.12.20211227
- 12. Riley S, Ainslie KEC, Eales O, et al. REACT-1 round 6 updated report: high prevalence of SARS-CoV-2 swab positivity with reduced rate of growth in England at the start of November 2020. 12 November 2020. Accessed at https://spiral.imperial.ac.uk/handle/10044/1/83912 on 26 November 2020.
- 13. Hoxha A , Wyndham-Thomas C , Klamer S , et al. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities [Letter]. Lancet Infect Dis. 2020. [PMID: ] doi: 10.1016/S1473-3099(20)30560-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen I , Phillips A . Three quarters of people with SARS-CoV-2 infection are asymptomatic: analysis of English household survey data. Clin Epidemiol. 2020;12:1039-1043. [PMID: ] doi: 10.2147/CLEP.S276825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White EM , Santostefano CM , Feifer RA , et al. Asymptomatic and presymptomatic severe acute respiratory syndrome coronavirus 2 infection rates in a multistate sample of skilled nursing facilities. JAMA Intern Med. 2020;180:1709-1711. [PMID: ] doi: 10.1001/jamainternmed.2020.5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gudbjartsson DF , Helgason A , Jonsson H , et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302-2315. [PMID: ] doi: 10.1056/NEJMoa2006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavezzo E , Franchin E , Ciavarella C , et al; Imperial College COVID-19 Response Team. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo'. Nature. 2020;584:425-429. [PMID: ] doi: 10.1038/s41586-020-2488-1 [DOI] [PubMed] [Google Scholar]
- 18. Kasper MR , Geibe JR , Sears CL , et al. An outbreak of Covid-19 on an aircraft carrier. N Engl J Med. 2020;383:2417-2426. [PMID: ] doi: 10.1056/NEJMoa2019375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. So L, Smith G. In four U.S. state prisons, nearly 3,300 inmates test positive for coronavirus — 96% without symptoms. Reuters. 25 April 2020. Accessed at www.reuters.com/article/us-health-coronavirus-prisons-testing-in-idUSKCN2270RX on 26 April 2020.
- 20. Chamie G , Marquez C , Crawford E , et al; CLIAhub Consortium. SARS-CoV-2 community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lush T. Hundreds test positive at Tyson Foods plant in Arkansas. Associated Press. 21 June 2020. Accessed at https://apnews.com/article/9b2b24d3b3ed981041a7fe6ab499100c on 6 October 2020.
- 22. Sakurai A , Sasaki T , Kato S , et al. Natural history of asymptomatic SARS-CoV-2 infection [Letter]. N Engl J Med. 2020;383:885-886. [PMID: ] doi: 10.1056/NEJMc2013020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menachemi N , Yiannoutsos CT , Dixon BE , et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample — Indiana, April 25-29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:960-964. [PMID: ] doi: 10.15585/mmwr.mm6929e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marossy A , Rakowicz S , Bhan A , et al. A study of universal SARS-CoV-2 RNA testing of residents and staff in a large group of care homes in South London. J Infect Dis. 2020. [PMID: ] doi: 10.1093/infdis/jiaa565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Letizia AG , Ramos I , Obla A , et al. SARS-CoV-2 transmission among Marine recruits during quarantine. N Engl J Med. 2020;383:2407-2416. [PMID: ] doi: 10.1056/NEJMoa2029717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centre d'Épidémiologie et de Santé Publique des Armées. Investigation de l'épidémie de COVID-19 au sein du Groupe Aéronaval: 21 janvier – 13 avril 2020. 4 May 2020. Accessed at www.defense.gouv.fr/content/download/583466/9938746/file/20200405_929_ARM_SSA_CESPA_rapport_epidemie_covid19_Gan_VEXP.pdf on 4 June 2020.
- 27. Ly TDA , Zanini D , Laforge V , et al. Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int J Antimicrob Agents. 2020;56:106219. [PMID: ] doi: 10.1016/j.ijantimicag.2020.106219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers JH , Link AC , McCulloch D , et al. Characteristics of COVID-19 in homeless shelters: a community-based surveillance study. Ann Intern Med. 2020. doi: 10.7326/M20-3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hempel D, Milani V, Kleespies A, et al. SARS-CoV-2 infections in outpatients with cancer: most infected patients are asymptomatic carriers without impact on chemotherapy [Abstract]. Ann Oncol. 2020;31(Suppl 4):S995. Abstract no. 1680P. doi:10.1016/j.annonc.2020.08.1744 [DOI] [PMC free article] [PubMed]
- 30. Feaster M , Goh YY . High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis. 2020;26:2416-2419. [PMID: ] doi: 10.3201/eid2610.202694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrett ES , Horton DB , Roy J , et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. med. Rxiv. 2020. [PMID: ] doi: 10.1101/2020.04.20.20072470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lytras T , Dellis G , Flountzi A , et al. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27. [PMID: ] doi: 10.1093/jtm/taaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldfarb IT , Diouf K , Barth WH , et al. Universal SARS-CoV-2 testing on admission to the labor and delivery unit: low prevalence among asymptomatic obstetric patients. Infect Control Hosp Epidemiol. 2020;41:1095-1096. [PMID: ] doi: 10.1017/ice.2020.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattar S, Martinez-Bravo C, Rivero R, et al. Epidemiological and viral features of a cohort of SARS-CoV-2 symptomatic and asymptomatic individuals in an area of the Colombian Caribbean. Research Square. Preprint posted online 30 October 2020. doi:10.21203/rs.3.rs-57254/v3 [DOI] [PMC free article] [PubMed]
- 35. Prabhu M , Cagino K , Matthews KC , et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556. [PMID: ] doi: 10.1111/1471-0528.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Díaz-Corvillón P , Mönckeberg M , Barros A , et al. Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS One. 2020;15:e0239887. [PMID: ] doi: 10.1371/journal.pone.0239887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kutsuna S , Suzuki T , Hayakawa K , et al. SARS-CoV-2 screening test for Japanese returnees from Wuhan, China, January 2020. Open Forum Infect Dis. 2020;7:ofaa243. [PMID: ] doi: 10.1093/ofid/ofaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ladhani SN , Chow JY , Janarthanan R , et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26:100533. [PMID: ] doi: 10.1016/j.eclinm.2020.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saurabh S , Kumar R , Kumar N , et al. Dynamics of SARS-CoV-2 transmission among Indian nationals evacuated from Iran. Disaster Med Public Health Prep. 2020:1-7. [PMID: ] doi: 10.1017/dmp.2020.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bigelow BF , Tang O , Barshick B , et al. Outcomes of universal COVID-19 testing following detection of incident cases in 11 long-term care facilities. JAMA Intern Med. 2021;181:127-129. [PMID: ] doi: 10.1001/jamainternmed.2020.3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kannan NB , Sen S , Reddy H , et al. Preoperative COVID-19 testing for elective vitreoretinal surgeries: experience from a major tertiary care institute in South India. Indian J Ophthalmol. 2020;68:2373-2377. [PMID: ] doi: 10.4103/ijo.IJO_2870_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baggett TP, Keyes H, Sporn N, et al. COVID-19 outbreak at a large homeless shelter in Boston: implications for universal testing. medRxiv. Preprint posted online 15 April 2020. doi:10.1101/2020.04.12.20059618
- 43. Cline S. Cases at seafood plant cause spike in Oregon COVID numbers. Associated Press. 9 June 2020. Accessed at https://apnews.com/article/4b9d38f206db9ce5267a5898ac24f238 on 6 October 2020.
- 44. Massarotti C , Adriano M , Cagnacci A , et al. Asymptomatic SARS-CoV-2 infections in pregnant patients in an Italian city during the complete lockdown. J Med Virol. 2020. [PMID: ] doi: 10.1002/jmv.26458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khalil A , Hill R , Ladhani S , et al. COVID-19 screening of health-care workers in a London maternity hospital [Letter]. Lancet Infect Dis. 2021;21:23-24. [PMID: ] doi: 10.1016/S1473-3099(20)30403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ing AJ , Cocks C , Green JP . COVID-19: in the footsteps of Ernest Shackleton. Thorax. 2020;75:693-694. [PMID: ] doi: 10.1136/thoraxjnl-2020-215091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sutton D , Fuchs K , D'Alton M , et al. Universal screening for SARS-CoV-2 in women admitted for delivery [Letter]. N Engl J Med. 2020;382:2163-2164. [PMID: ] doi: 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malagón-Rojas J , Gómez-Rendón C , Parra EL , et al. SARS-CoV-2 and RT-PCR in asymptomatic patients: results of a cohort of workers at El Dorado International Airport in Bogotá, 2020. Biomedica. 2020;40:166-172. [PMID: ] doi: 10.7705/biomedica.5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Figueiredo R , Tavares S , Moucho M , et al. Systematic screening for SARS-CoV-2 in pregnant women admitted for delivery in a Portuguese maternity. J Perinat Med. 2020;48:977-980. [PMID: ] doi: 10.1515/jpm-2020-0387 [DOI] [PubMed] [Google Scholar]
- 50. Chou E. Dozens positive for coronavirus at LA's Skid Row homeless shelter, after all residents tested. Los Angeles Daily News. 21 April 2020. Updated 22 April 2020. Accessed at www.dailynews.com/2020/04/21/dozens-positive-for-coronavirus-at-las-skid-row-homeless-shelter-after-all-residents-tested on 23 April 2020.
- 51. Patel MC , Chaisson LH , Borgetti S , et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020;71:2920-2926. [PMID: ] doi: 10.1093/cid/ciaa763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lan FY , Suharlim C , Kales SN , et al. Association between SARS-CoV-2 infection, exposure risk and mental health among a cohort of essential retail workers in the USA. Occup Environ Med. 2020. [PMID: ] doi: 10.1136/oemed-2020-106774 [DOI] [PubMed] [Google Scholar]
- 53. Dora AV , Winnett A , Jatt LP , et al. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans — Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:651-655. [PMID: ] doi: 10.15585/mmwr.mm6921e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arons MM , Hatfield KM , Reddy SC , et al; Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081-2090. [PMID: ] doi: 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ward H, Cooke G, Atchison C, et al. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. Posted online 27 October 2020. doi:10.1101/2020.10.26.20219725
- 56. Pollán M , Pérez-Gómez B , Pastor-Barriuso R , et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535-544. [PMID: ] doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sims MD , Maine GN , Childers KL , et al; BLAST COVID-19 Study Group. COVID-19 seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang S , Guo M , Wu F , et al. Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: a multicentre retrospective cohort study. Clin Microbiol Infect. 2020;26:1670-1675. [PMID: ] doi: 10.1016/j.cmi.2020.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hippich M , Holthaus L , Assfalg R , et al. Public health antibody screening indicates a six-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med (N Y). 2020. [PMID: ] doi: 10.1016/j.medj.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feehan A, Fort D, Garcia-Diaz J, et al. Frequency of symptoms and asymptomatic SARS-CoV-2 infection in Louisiana, USA. SSRN. Preprint posted online 2 October 2020. doi:10.2139/ssrn.3702933
- 61. Erber J, Kappler V, Haller B, et al. Strategies for infection control and prevalence of anti-SARS-CoV-2 IgG antibodies in 4,554 employees of a university hospital in Munich, Germany. SSRN. Preprint posted online 13 October 2020. doi:10.2139/ssrn.3678599
- 62. Mostafa A , Kandil S , El-Sayed MH , et al. Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: an Egyptian pilot model in a university with 12 public hospitals and medical centers. Int J Epidemiol. 2020. [PMID: ] doi: 10.1093/ije/dyaa173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Self WH , Tenforde MW , Stubblefield WB , et al; CDC COVID-19 Response Team. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network — 13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221-1226. [PMID: ] doi: 10.15585/mmwr.mm6935e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Silva AMA, Lima Neto LG, Azevedo CMPS, et al. Population-based seroprevalence of SARS-CoV-2 is more than halfway through the herd immunity threshold in the State of Maranhao, Brazil. medRxiv. Preprint posted online 1 September 2020. doi:10.1101/2020.08.28.20180463
- 65. Österreichischer Rundfunk. Ischgl: 42,4 prozent haben antikörper. 25 June 2020. Accessed at https://tirol.orf.at/stories/3054826 on 5 October 2020.
- 66. Tang H , Tian JB , Dong JW , et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis. 2020;76:490-499.e1. [PMID: ] doi: 10.1053/j.ajkd.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Figar S, Pagotto V, Luna L, et al. Community-level SARS-CoV-2 seroprevalence survey in urban slum dwellers of Buenos Aires City, Argentina: a participatory research. medRxiv. Preprint posted online 18 July 2020. doi:10.1101/2020.07.14.20153858
- 68. Mahajan S , Srinivasan R , Redlich CA , et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies among adults living in Connecticut: post-infection prevalence (PIP) study. Am J Med. 2020. [PMID: ] doi: 10.1016/j.amjmed.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lindahl JF , Hoffman T , Esmaeilzadeh M , et al. High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden. Infect Ecol Epidemiol. 2020;10:1789036. [PMID: ] doi: 10.1080/20008686.2020.1789036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clarke C , Prendecki M , Dhutia A , et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969-1975. [PMID: ] doi: 10.1681/ASN.2020060827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stubblefield WB , Talbot HK , Feldstein L , et al; Influenza Vaccine Effectiveness in the Critically Ill (IVY) Investigators. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients—Nashville, Tennessee. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bampoe S , Lucas DN , Neall G , et al. A cross-sectional study of immune seroconversion to SARS-CoV-2 in frontline maternity health professionals. Anaesthesia. 2020;75:1614-1619. [PMID: ] doi: 10.1111/anae.15229 [DOI] [PubMed] [Google Scholar]
- 73. Knapp TR . Symptom: cause, effect, both, or neither? [Editorial]. Clin Nurs Res. 2018;27:391-394. [PMID: ] doi: 10.1177/1054773818761904 [DOI] [PubMed] [Google Scholar]
- 74. Aziz M , Goyal H , Haghbin H , et al. The association of “Loss of smell” to COVID-19: a systematic review and meta-nnalysis. Am J Med Sci. 2020. [PMID: ] doi: 10.1016/j.amjms.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. He X , Lau EHY , Wu P , et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672-675. [PMID: ] doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 76. Ma S , Zhang J , Zeng M , et al. Epidemiological parameters of COVID-19: case series study. J Med Internet Res. 2020;22:e19994. [PMID: ] doi: 10.2196/19994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang H , Hong C , Zheng Q , et al. A multi-family cluster of COVID-19 associated with asymptomatic and pre-symptomatic transmission in Jixi City, Heilongjiang, China, 2020 [Letter]. Emerg Microbes Infect. 2020;9:2509-2514. [PMID: ] doi: 10.1080/22221751.2020.1837015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shi Q , Hu Y , Peng B , et al. Effective control of SARS-CoV-2 transmission in Wanzhou, China. Nat Med. 2020. [PMID: ] doi: 10.1038/s41591-020-01178-5 [DOI] [PubMed] [Google Scholar]
- 79. Zhang B , Zhou X , Zhu C , et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. [PMID: ] doi: 10.3389/fmolb.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cervia C , Nilsson J , Zurbuchen Y , et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2020. [PMID: ] doi: 10.1016/j.jaci.2020.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang C , Wang Y , Hu M , et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Transl Immunology. 2020;9:e1182. [PMID: ] doi: 10.1002/cti2.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Russell MW , Moldoveanu Z , Ogra PL , et al. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11:611337. [PMID: ] doi: 10.3389/fimmu.2020.611337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mina MJ , Parker R , Larremore DB . Rethinking Covid-19 test sensitivity — a strategy for containment. N Engl J Med. 2020;383:e120. [PMID: ] doi: 10.1056/NEJMp2025631 [DOI] [PubMed] [Google Scholar]
- 84. Stieg C. Could you get paid to quarantine during the Covid-19 pandemic? Some local governments are already doing it. CNBC. 2 September 2020. Accessed at www.cnbc.com/2020/09/02/getting-paid-to-quarantine-during-the-covid-19-pandemic.html on 1 December 2020.
- 85. Bleier BS , Ramanathan M Jr , Lane AP . COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. 2020:194599820982633. [PMID: ] doi: 10.1177/0194599820982633 [DOI] [PubMed] [Google Scholar]


