Abstract
Aims
Bulking agents are a minimally invasive treatment option for women with stress urinary incontinence (SUI) or stress‐predominant mixed urinary incontinence (MUI). The aim of this study was to evaluate long‐term efficacy and safety following treatment with Bulkamid as a primary procedure for SUI or stress‐predominant MUI.
Methods
This was an Institutional Review Board‐approved single‐center retrospective study of female patients with SUI or stress‐predominant MUI who had undergone injection with Bulkamid since 2005 and had completed 7 years of follow up. The primary endpoint was patient satisfaction measured on a four‐point scale as cured, improved, unchanged, or worse. Secondary outcomes included the number of incontinence pads used, International Consultation on Incontinence Questionnaire‐Short Form (ICIQ‐UI SF) scores, Visual Analog Scale Quality of Life (VAS QoL), reinjection rates, and perioperative and postoperative complications.
Results
A total of 1,200 patients were treated with Bulkamid since 2005 and of these, 388 (32.3%) had completed 7 years of follow‐up. A total of 67.1% of the patients reported feeling cured or improved if Bulkamid was a primary procedure, 11.1% reported no change, and 2.3% reported worsening of incontinence. A total of 19.5% of patients received a subsequent other incontinence procedure. The ICIQ‐UI SF was reduced by 8.6 points. VAS QoL improved by a mean of 4.3 points. Postoperative complications were transient. Prolonged bladder emptying time was reported in 15.3% of patients and urinary tract infection in 3.5%.
Conclusions
Bulkamid injections are an effective and safe first‐line treatment option for women with SUI or stress‐predominant MUI providing durable outcomes at 7 years.
Keywords: Bulkamid, bulking agents, long‐term follow‐up, mixed urinary incontinence, stress urinary incontinence
1. INTRODUCTION
Stress urinary incontinence (SUI) is defined as the involuntary loss of urine on effort, physical exertion, or on sneezing or coughing. 1 Urgency urinary incontinence (UUI) occurs when there is a sudden urge to urinate at the same time as the bladder contracts, causing a leakage of urine. 1 Mixed urinary incontinence (MUI) is very common and occurs when symptoms of both SUI and UUI are present. First‐line treatments currently recommended for SUI in the American Urological Association (AUA) Guidelines on Surgical Treatment of Female Stress Urinary Incontinence include low risk measures such as continence pessaries, vaginal inserts, and pelvic floor exercises. 2 Surgical options for SUI include the synthetic midurethral sling, autologous fascia pubovaginal sling, Burch colposuspension, and bulking agents.
Bulking agents are an option for the treatment of women with SUI or stress‐predominant MUI who would prefer a less invasive procedure, with a lower side‐effect profile. 2 Bulking agents can be injected either periurethrally or transurethrally into the submucosa of the urethra. They work by improving coaptation of the urethra during the storage phase of the micturition cycle and when abdominal pressure is increased. In addition, the bulking material may function as additional central filler volume, which increases the length of the muscle fibers and thereby the power of the urethral sphincter. 3 Ideally the bulking agent should be non‐resorbable, nonimmunogenic, nonallergenic, and biocompatible to reduce the risk of inflammation and fibrosis. 4 A number of bulking agents have been used in the treatment of SUI, although several have been withdrawn due to safety and efficacy issues. Currently, two different types of bulking agents are used. The first type contains particles that cause inflammation, and this reaction gives support around the urethra. 5 The second type of bulking agent is a homogenous gel without particles.
Bulkamid® (Contura International A/S) is a homogenous gel without particles and was recently granted Food & Drug Administration approval for use in the United States as a urethral bulking agent. It is comprised of a polyacrylamide hydrogel (PAHG; 2.5% polyacrylamide and 97.5% water) that is nonbiodegradable. 4 The volume of material injected into the urethra provides the bulking effect while a network of fine fibers are formed to anchor the gel in situ. 6 A number of 12‐month studies have been completed demonstrating the efficacy and safety of this bulking agent 4 , 7 , 8 , 9 , 10 but few long‐term outcome studies have been performed. The primary study objective was to evaluate the long‐term effectiveness of Bulkamid injection as a primary procedure in women with SUI or stress‐predominant MUI as measured by patient satisfaction. The secondary objectives were to demonstrate the long‐term subjective effectiveness and long‐term safety of Bulkamid injection as a primary procedure in women with SUI or stress‐predominant MUI.
2. METHODS
This was an Institutional Review Board‐approved retrospective study of female patients who had previously undergone injection with Bulkamid for the treatment of SUI or stress‐predominant MUI. Since 2005 all women treated at the DRK Hospital Chemnitz‐Rabenstein Women's Clinic, Germany, were asked to consent to have anonymized data related to their treatment(s) stored and used for scientific research. The patients were regularly followed up and this investigation is a review of the 7‐year outcome data for patients who were treated with Bulkamid for SUI or stress‐predominant MUI.
All patients underwent a standardized history that included evaluation for SUI or stress‐predominant MUI, had a physical examination including a confirmatory diagnostic stress test, uroflowmetry, and postvoid residual urine testing. Exclusion criteria included those with signs of infection, with a uroflow less than 10 ml/s or residual urine ≥50 ml. The degrees of urinary incontinence were divided into three grades according to the Stamey classification. 11 Stamey Grade I is defined as urinary incontinence that occurs with general increase in abdominal pressure (coughing, sneezing, laughing, etc.). Grade II is more severe and caused by a mild increase in abdominal pressure (walking, standing up, sitting up in bed, etc.). Regardless of the change in one's position, Grade III is the most severe and defined as urinary incontinence with no relation to physical activity.
Before performing PAHG injections on patients, training on injection models and/or porcine bladders took place. To reduce the learning curve, multiple patients were treated during each session. All procedures were performed by two surgeons using a standardized technique and conducted under local or general anesthetic. All patients received antibiotic prophylaxis comprised of a single dose of 2 g Cefuroxime and 500 mg Metronidazole 30 min before Bulkamid injection. When local anesthesia was used, 10 ml 5% lidocaine was injected into the wall of the urethra at 3 and 9 o'clock positions. The women were placed in the lithotomy position and Bulkamid was injected transurethrally into the submucosa under cystoscopic control using a 23G × 120‐mm needle. A three‐point injection plan at 2, 6, and 10 o'clock positions was followed with material (0.2–0.8 ml per injection site) placed 0.5–1 cm distal to the bladder neck. The material was injected until the deposit visually reached the midline of the urethra. Following postoperative voiding urine residual was measured by ultrasound. If the postvoid residual urine was more than 150 ml, patients underwent a single catheterization using an 8‐Fr catheter. In certain patients where the benefits of Bulkamid had worn off after a period of some years, a second top‐up was offered. No third injection was offered to patients who did not benefit from the second treatment/first top up. In patients requiring a second or third treatment, different injection sites to the primary treatment sites were used.
The primary study objective was to evaluate the long‐term effectiveness of Bulkamid injection as a primary procedure in women with SUI or stress‐predominant MUI as demonstrated by patient satisfaction. This was measured on a four‐point scale as cured, improved, unchanged, or worse. The secondary objectives were to evaluate the long‐term effectiveness and safety of Bulkamid injection in women with SUI or stress‐predominant MUI. These outcomes were determined through the following: number of incontinence pads used; International Consultation on Incontinence Questionnaire‐Short Form (ICIQ‐UI SF) score 12 ; Visual Analog Scale Quality of Life (VAS QoL) 13 ; percentage of subjects requiring reinjection; perioperative complications; and postoperative complications. The wording of the VAS QoL was: “with regard to the impact your bladder condition has on your life, how would you describe your current quality of life?” with answers ranging from 0 (pleased) to 10 (terrible). In terms of safety, complications including urinary retention, urinary tract infection (UTI), hematuria, and worsened bladder symptoms were recorded.
The investigation was approved by the responsible Ethics Committee of Saxony State Medical Association in Germany and conducted in accordance with the ethical principles contained in the Declaration of Helsinki, in compliance with Good Clinical Practice, and in compliance with any applicable legal and regulatory requirements, as appropriate.
Numerical data are presented in summary tables by a number of subjects, arithmetic mean (geometric mean and coefficient of variation where applicable), median, SD, minimum, and maximum. Categorical data are presented by the number and percentage of subjects as well as the number of events (where applicable). The ICIQ‐UI SF score, number of pads, and VAS baseline and follow‐up values were compared using a paired t test. Data were analyzed with statistical package R version 3.6.1. p < .05 was considered statistically significant.
3. RESULTS
A total of 1,200 patients were treated with Bulkamid and of these, 553 patients had a 7‐year follow‐up. A total of 97 women were uncontactable, 24 were unable to respond, and 44 had died leaving a dataset of 388 (32.3%) patients (Figure 1). The mean (SD) follow‐up was 7.1 (0.1) years and the median (range) 7.1 (7.1–7.2). Characteristics of the 388 patients are shown in Table 1. The majority of patients (261 [67.3%]) were undergoing Bulkamid as a primary treatment. A second (“top‐up”) procedure of Bulkamid was conducted in 125 (32.2%) patients at a median of 9 months after the initial injection. The median (range) volume of Bulkamid injected at first injection was 1.6 (0.6–3.0) ml and at second injection 1.8 (0.7–2.9) ml.
Figure 1.
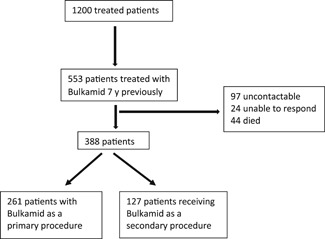
Study flow chart
Table 1.
Patient characteristics (n = 388)
| Parameter | |
|---|---|
| Age; mean (SD); y | 65.7 (10.4) |
| Body mass index; mean (SD) | 29.2 (5.3) |
| Indication | |
| Mixed urinary incontinence | 119 (30.7%) |
| Stress urinary incontinence: Stamey grade | |
| I | 14 (3.6%) |
| II | 230 (59.3%) |
| III | 25 (6.4%) |
| Prior surgery for incontinence | |
| Yes | 127 (32.7%) |
| Colposuspension | 19 (4.9%) |
| Bulking agent | 2 (0.5%) |
| Midurethral sling | 100 (25.8%) |
| Other | 6 (1.5%) |
| No | 261 (67.3%) |
| No. of Bulkamid procedures | |
| 1 | 388 (100%) |
| 2 | 125 (32.2%) |
| 3 | 17 (4.4%) |
| Prior surgeries/treatments | |
| Hysterectomy | 192 (50.5%) |
| Prolapse | 189 (48.7%) |
| Cancer therapy | 21 (5.4%) |
| Radiotherapy | 15 (3.9%) |
When Bulkamid was performed as a primary procedure, 67.1% reported feeling cured or improved, compared with 65.2% in the overall patient group and 61.5% in patients undergoing Bulkamid as a secondary procedure. Primary endpoint data are shown in Table 2. A total of 74 patients (19.1%) subsequently underwent a secondary procedure for persistent incontinence following Bulkamid treatment over the study period of whom 29 (39.2%) women were improved with Bulkamid but not fully satisfied with the treatment outcome.
Table 2.
Primary endpoint: patient satisfaction
| All patients | Patients receiving Bulkamid as a primary procedure | Patients receiving Bulkamid as a secondary procedure | |
|---|---|---|---|
| (n = 388) | (n = 261) | (n = 127) | |
| Cured/Improved | 253 (65.2%) | 175 (67.1%) | 78 (61.4%) |
| Cured | 62 (16.0%) | 43 (16.5%) | 19 (15.0%) |
| Improved | 191 (49.2%) | 132 (50.6%) | 59 (46.5%) |
| No change | 52 (13.4%) | 29 (11.1%) | 23 (18.1%) |
| Worse | 9 (2.3%) | 6 (2.3%) | 3 (2.4%) |
| Subsequently received other incontinence surgery(ies) | 74 (19.1%) | 51 (19.5%) | 23 (18.1%) |
Improvements were reported in all three secondary efficacy measures assessed (Table 3). In the overall patient group, VAS QoL improved by a mean of 4.4 points compared with 4.3 and 4.5 in patients undergoing Bulkamid as a primary or secondary procedure, respectively. Mean daily pad usage was reduced from 4.2 to 1.8 in patients receiving Bulkamid as a primary treatment and from 4.4 to 2.0 in those receiving it as a secondary procedure. The ICIQ‐UI SF was improved by 8.4 points in the overall patient group and by 8.6 and 8.1 in patients undergoing Bulkamid as a primary or secondary procedure, respectively. For all three secondary endpoints, the results were statistical significant with p of less than .0001. No perioperative complications were reported. Postoperative complications are shown in Table 4. Postoperative complications were transient, with the most common being prolonged bladder emptying time, which was subjectively reported in 15.3% of patients. UTI occurred in 3.5%.
Table 3.
Secondary endpoints at baseline and 7 years follow‐up expressed as mean (SD) and median (range)
| Total patient group | Patients receiving Bulkamid as a primary treatment | Patients receiving Bulkamid as a secondary treatment | |
|---|---|---|---|
| (n = 388) | (n = 261) | (n = 127) | |
| Pad usage | |||
| Baseline | 4.3 (2.3); 4.0 (1.0–15.0) | 4.2 (2.3); 4.0 (1.0–15.0) | 4.4 (2.4); 4.0 (1.0–14.0) |
| 7 years | 1.9 (1.7); 1.0 (0–9.0) | 1.8 (1.7); 1.0 (0–9.0) | 2.0 (1.7); 2.0 (0–9.0) |
| VAS QoL | |||
| Baseline | 7.9 (1.7); 8.0 (2.0–10.0) | 7.8 (1.8); 8.0 (2.0–10.0) | 8.0 (1.6); 8.0 (2.0–10.0) |
| 7 years | 3.5 (2.2); 3.0 (1.0–10.0) | 3.5 (2.2); 3.0 (1.0–10.0) | 3.5 (2.1); 3.0 (1.0–10.0) |
| ICIQ‐UI SF | |||
| Baseline | 15.5 (2.5); 16.0 (5.0–21.0) | 15.2 (2.4); 15.0 (5.0–21.0) | 16.1 (2.6); 16.0 (10.0–21.0) |
| 7 years | 7.1 (5.4); 8.0 (0–20.0) | 6.6 (5.3); 7.0 (0–19.0) | 8.0 (5.4); 9.0 (0–20.0) |
Note: All results were statistical significant with p < .0001.
Abbreviations: ICIQ‐UI SF, International Consultation on Incontinence Questionnaire‐Short Form; VAS QoL, Visual Analog Scale Quality of Life.
Table 4.
Postoperative complications in 388 patients
| Complication | N (%) |
|---|---|
| Urinary tract infection | 11 (3.5%) |
| Transient prolonged emptying time | 48 (15.3%) |
| Nocturia | 27 (8.6%) |
| Residual urine >50 ml/s | 1 (0.3%) |
| Persistent dysuria | 1 (0.3%) |
| Frequent urination | 30 (9.6%) |
4. DISCUSSION
To the best of our knowledge, this is the largest long‐term outcome study of Bulkamid injections for the treatment of SUI or stress‐predominant MUI to date. The majority of patients in this study underwent Bulkamid injections as a primary procedure for incontinence and 67.1% reported feeling either cured or improved 7 years after initial injection. Even as a secondary therapy following prior treatment for SUI or stress‐predominant MUI, the majority of patients reported durable long‐term outcomes. A previous long‐term study reported on 25 women with SUI injected with Bulkamid 24 were contactable at 8 years; 15 had no further treatment, seven underwent subsequent placement of a midurethral sling, and two were reinjected with Bulkamid. A cure/much‐improved rate of 44% was reported.
Several studies have reported promising Bulkamid outcomes over the short‐term. A study of 135 patients reported cured/improved rates of 66% at 12 months. 7 The responder rate was 58% among women treated at a low volume center (<15 injections), and 69% among those treated at a high volume center (≥15 injections), suggesting that clinician experience can improve outcomes. Leone Roberti Maggiore et al. 8 reported a cure rate of 74.4% at 12 months in a study of 82 patients with SUI, while Sokol et al. 9 reported that 77.1% of 188 were cured/improved at 12 months. Other reports include a cured/improved rate of 83.6% in a study of 60 patients previously failing MUS treatment 10 and cured rates of 25% in 24 patients previously treated with external beam radiation versus 36.4% in those not previously irradiated. 14 The findings from the present study indicate favorable efficacy rates compared with previous studies and the durability of Bulkamid injections for the treatment of SUI and stress‐predominant MUI.
One long‐term study of Bulkamid in 256 women showed an initial 82% rate of cure or significant improvement which was sustained at a median follow up 38 months. 15 Another long‐term study of bulking agents in general as a treatment in 63 women with SUI reported a cured/improved rate of 43% at a mean (SD) follow‐up of 8.3 (3.5) years. 16 Comparisons can be made with other bulking agents. A 5‐year study of Macroplastique in 21 women demonstrated a cured/improved rate of 80% 17 while a 3‐year study reported a cure rate of 47% in 85 patients. 18 Another medium‐term study showed cure rates of 40% in 25 Durasphere‐treated patients and 14% in 21 Contigen‐treated patients at follow‐ups of 2.6 and 2.8 years, respectively. 19 Two‐year outcomes have been reported for Urolastic in two separate studies. Zajda and Farag 20 reported an improvement in continence status in 66% of 18 women, while Futyma et al. 21 showed cured/improved rates of 32.7% in 49 patients. Overall, the outcomes from our study of the homogenous Bulkamid hydrogel suggest favorable improvement rates and durability compared to older particle‐based bulking agents.
For the ICIQ‐UI SF, the minimum important difference is an estimate of the minimum degree of change in an instrument's score that correlates with a patient's subjective sense of improvement and has been determined to be −5 at 12 months and −4 at 24 months. 22 In the current study, the change in the ICIQ‐UI SF of −8.4 at 7 years can be classified as clinically important, suggesting a long‐term improvement in the incontinence‐specific quality of life in patients treated with Bulkamid. Reductions of 50% in pad usage and the VAS QoL can also be considered clinically relevant.
This study also suggests a favorable long‐term safety profile for Bulkamid injections and corroborates the findings of prior studies of Bulkamid, with low rates of UTIs, nocturia, and dysuria. 7 , 8 , 9 , 10 , 14 Of note, in the current study, no cases of acute urinary retention, urgency/de novo urgency, injection site pain, and hematuria were reported. A systematic review of studies reporting on 777 patients treated with Bulkamid and 351 patients treated with Macroplastique showed similarly low rates of adverse events, 23 with the most common being UTIs, implantation site pain, acute urinary retention, persistent urge incontinence, and hematuria. The safety of Bulkamid has also been established in a 1‐year comparative study involving 224 women with primary SUI randomized to treatment with Bulkamid or tension‐free vaginal tape (TVT). 24 Perioperative complications were greater in patients treated with the tape compared with Bulkamid and all six reoperations due to complications were associated with TVT. High patient satisfaction and cure rates were reported in both groups, although these were higher in the TVT group.
Limitations of this study include the retrospective design and the fact that all procedures were performed at a single high‐volume specialty center, which could suggest better outcomes than might be realized at lower volume centers. However, with adequate training and similar procedure volumes, similar durable outcomes with Bulkamid may be achievable. The subjective nature of the primary and endpoints could be subject to bias, as patients may be reluctant to report bad results to their treating physicians. Also, data from this study cannot be extended to the particle‐based bulking agents given the different means by which they induce coaptation. 5 However, a large number of patients with long‐term follow‐up and the use of validated QoL questionnaires to measure patient‐reported outcomes suggest that the majority of patients enjoyed favorable results after treatment with Bulkamid, even as an initial first‐line procedural therapy.
5. CONCLUSION
With durable outcomes at 7 years, Bulkamid has been shown to be an effective and safe long‐term primary treatment option for women suffering from SUI or stress‐predominant MUI.
ACKNOWLEDGMENTS
The authors would like to acknowledge the valuable contributions of Mr. Philip Toozs Hobson in reviewing the manuscript. They would also like to thank statistician Søren Lophaven, PhD, Omicron ApS, Denmark, for conducting the statistical analyses and for contributing to the interpretation of the analyses. Editorial support was provided by Dr Christine McKillop.
Brosche T, Kuhn A, Lobodasch K, Sokol ER. Seven‐year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourology and Urodynamics. 2021;40:502−508. 10.1002/nau.24589
REFERENCES
- 1. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5−26. [DOI] [PubMed] [Google Scholar]
- 2. Surgical Treatment of Female Stress Urinary Incontinence (SUI): AUA/SUFU Guideline . 2017. https://www.auanet.org/guidelines/stress-urinary-incontinence-(sui)-guideline Accessed June 2020.
- 3. Klarskov N, Lose G. Urethral injection therapy: what is the mechanism of action? Neurourol Urodyn. 2008;27(8):789−792. [DOI] [PubMed] [Google Scholar]
- 4. Lose G, Mouritsen L, Nielsen JB. A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int. 2006;98(1):100−104. [DOI] [PubMed] [Google Scholar]
- 5. Chapple C, Dmochowski R. Particulate versus non‐particulate bulking agents in the treatment of stress urinary incontinence. Res Rep Urol. 2019;12(11):299−310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen LH, Nielsen JB, Mouritsen L, Sørensen M, Lose G. Tissue integration of polyacrylamide hydrogel: an experimental study of periurethral, perivesical, and mammary gland tissue in the pig. Dermatol Surg. 2008;34(Suppl 1):S68−S77. [DOI] [PubMed] [Google Scholar]
- 7. Lose G, Sørensen HC, Axelsen SM, Falconer C, Lobodasch K, Safwat T. An open multicenter study of polyacrylamide hydrogel (Bulkamid®) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21(12):1471−1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leone Roberti Maggiore U, Alessandri F, Medica M, Gabelli M, Venturini PL, Ferrero S. Outpatient periurethral injections of polyacrylamide hydrogel for the treatment of female stress urinary incontinence: effectiveness and safety. Arch Gynecol Obstet. 2013;288(1):131−137. [DOI] [PubMed] [Google Scholar]
- 9. Sokol ER, Karram MM, Dmochowski R. Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol. 2014;192(3):843−849. [DOI] [PubMed] [Google Scholar]
- 10. Zivanovic I, Rautenberg O, Lobodasch K, von Bünau G, Walser C, Viereck V. Urethral bulking for recurrent stress urinary incontinence after midurethral sling failure. Neurourol Urodyn. 2017;36(3):722−726. [DOI] [PubMed] [Google Scholar]
- 11. Stamey TA. Endoscopic suspension of the vesical neck for urinary incontinence. Surg Gynecol Obstet. 1973;136:547−554. [PubMed] [Google Scholar]
- 12. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322−330. [DOI] [PubMed] [Google Scholar]
- 13. Carroll TF, Christie A, Foreman M, Khatri G, Zimmern PE. Macroplastique for women with stress urinary incontinence secondary to intrinsic sphincter deficiency. Int Braz J Urol. 2019;45(5):989−998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krhut J, Martan A, Jurakova M, Nemec D, Masata J, Zvara P. Treatment of stress urinary incontinence using polyacrylamide hydrogel in women after radiotherapy: 1‐year follow‐up. Int Urogynecol J. 2016;27(2):301−305. [DOI] [PubMed] [Google Scholar]
- 15. Pai A, Al‐Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid(®)) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015;68(4):428−433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plotti F, Montera R, Terranova C, et al. Long‐term follow‐up of bulking agents for stress urinary incontinence in older patients. Menopause. 2018;25(6):663−667. [DOI] [PubMed] [Google Scholar]
- 17. Tamanini JT, D'Ancona CA, Netto NR. Macroplastique implantation system for female stress urinary incontinence: long‐term follow‐up. J Endourol. 2006;20:1082−1086. [DOI] [PubMed] [Google Scholar]
- 18. Serati M, Soligo M, Braga A, Cantaluppi S, Coluccia AC, Di Dedda MC. Efficacy and safety of polydimethylsiloxane injection (Macroplastique®) for the treatment of female stress urinary incontinence: results of a series of 85 patients with ≥3 years of follow‐up. BJU Int. 2019;123(2):353−359. [DOI] [PubMed] [Google Scholar]
- 19. Andersen RC. Long‐term follow‐up comparison of Durasphere and Contigen in the treatment of stress urinary incontinence. J Low Genit Tract Dis. 2002;6(4):239−243. [DOI] [PubMed] [Google Scholar]
- 20. Zajda J, Farag F. Urolastic for the treatment of women with stress urinary incontinence: 24‐month follow‐up. Cent European J Urol. 2015;68(3):334−338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Futyma K, Nowakowski Ł, Gałczyński K, Miotła P, Rechberger T. Nonabsorbable urethral bulking agent ‐ clinical effectiveness and late complications rates in the treatment of recurrent stress urinary incontinence after 2 years of follow‐up. Eur J Obstet Gynecol Reprod Biol. 2016;207:68−72. [DOI] [PubMed] [Google Scholar]
- 22. Sirls LT, Tennstedt S, Brubaker L, et al. The minimum important difference for the International Consultation on Incontinence Questionnaire ‐ Urinary Incontinence Short Form in women with stress urinary incontinence. Neurourol Urodyn. 2015;34(2):183−187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siddiqui ZA, Abboudi H, Crawford R, Shah S. Intraurethral bulking agents for the management of female stress urinary incontinence: a systematic review. Int Urogynecol J. 2017;28(9):1275−1284. [DOI] [PubMed] [Google Scholar]
- 24. Itkonen Freitas AM, Mentula M, Rahkola‐Soisalo P, Tulokas S, Mikkola TS. Tension‐free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203(2):372−378. [DOI] [PubMed] [Google Scholar]


