Abstract
Aims
To understand patient preferences for once‐daily oral versus once‐weekly injectable type 2 diabetes mellitus (T2DM) medication administration profiles, and reasons for their preferences.
Materials and methods
The REVISE study, a cross‐sectional online survey of 600 participants with T2DM (United Kingdom, n = 300; United States, n = 300), elicited general preferences for once‐daily oral versus once‐weekly injectable diabetes medications, and reasons for the preference. Participants then viewed two videos describing the administration procedures for injectable dulaglutide and oral semaglutide, based on the product instructions for use. Thereafter, participants indicated their preference for a once‐weekly injectable or a once‐daily oral medication based on the video descriptions. Participants who switched preferences were asked to identify the reasons influencing their decision.
Results
The participants were predominantly male (n = 349; 58.2%), with a mean (SD) age of 64 (11.3) years. Nearly all (n = 557; 92.8%) were taking an oral T2DM medication, and 158 (26.3%) were using an injectable. Initially, 76.5% (n = 459; 95% confidence interval [CI] 73.1–79.9) preferred a once‐daily oral and 23.5% a once‐weekly injectable (n = 141; 95% CI 20.1–26.9; P < 0.0001). After viewing the videos describing the product‐specific administration, the proportions of participants preferring each option were not statistically different (oral semaglutide administration description (n = 315; 52.5%; 95% CI 48.5–56.5; dulaglutide administration description (n = 285; 47.5%; 95% CI 43.5–51.5; NS, P = 0.2207). The most common reason for switching preferences was the timing and steps of administration.
Conclusion
Several treatment‐related characteristics, including route, frequency and complexity of the treatment, play a role in patients' preferences for T2DM treatments and should be considered during treatment selection.
Keywords: daily oral, preferences, type 2 diabetes, United Kingdom, United States, weekly injectable
1. INTRODUCTION
It is estimated that 30.3 million people in the United States and 4.6 million people in the United Kingdom are living with diabetes, approximately 90.0% of whom have type 2 diabetes mellitus (T2DM). 1 , 2 The principal aim of T2DM treatment is to maintain blood glucose levels within acceptable limits, in turn, reducing the risk of long‐term complications associated with hyperglycaemia. First‐line T2DM therapy is metformin, along with lifestyle modifications, such as diet, exercise, and weight management. 3 For patients who are unable to control blood glucose levels with metformin therapy and lifestyle changes, physicians may consider pharmacological treatment such as a glucagon‐like peptide 1 receptor agonists (GLP‐1RAs). 4 For patients who are at risk of or who have established atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure, a GLP‐1RA may be considered as part of the glucose‐lowering regimen, independent of glycated haemoglobin (HbA1c) level. 4
Dulaglutide and semaglutide are both GLP‐1RA treatments. Dulaglutide is available as a once‐weekly subcutaneous injection that can be administered at any time of the day, independent of food or other medications. 5 Semaglutide is available as a once‐weekly injectable and also as a daily oral formulation. However, oral semaglutide has specific administration requirements; oral semaglutide must be taken on an empty stomach with no more than half a glass (4 oz or 120 mL) of water, and patients must wait at least 30 minutes after taking oral semaglutide before eating, drinking, or taking any other medications. Failure to follow these procedures when taking oral semaglutide can diminish the treatment effect by lowering absorption. 6
Understanding patients' preferences for once‐daily oral versus once‐weekly injectable medication administration profiles may be useful for clinicians and other agencies interested in evaluating healthcare options for T2DM. 4 , 7 The existing literature provides evidence that patients often prefer less frequent administration schedules (eg, weekly over daily) as they perceive them to be more convenient, to interfere less with work and social life, and to improve adherence. 8 , 9 , 10 A patient preference study comparing attributes of daily versus weekly injectable T2DM therapies found that injection frequency was the most important attribute compared to type of device, needle size and pain, refrigeration, and injection‐site reactions, with participants strongly preferring less frequent administration. 11 Additionally, patients often prefer oral over injectable medications, preferring to avoid injectable medications, in part due to embarrassment and inconvenience. 9 In a previous study of patients with T2DM, 65.0% of patients preferred vildagliptin (oral) over liraglutide (injectable) and the route of administration was ranked by 71.0% of those choosing the oral medication as either important or very important. 12 A separate study of patients with T2DM found that 81.9% of participants preferred a once‐daily oral treatment over a once‐daily injectable, with 57.5% ranking the route of administration as the most important factor driving their preference. 13 Across several studies, reasons provided for an oral preference included cultural factors, 10 fear of injections/needles, fear of consequences of incorrect administration, fear of pain of injection, and concerns about stigma around injectable medication use. 14 , 15 In a previous study, reasons provided by US‐based patients for discontinuing injectable diabetes treatments included cost (53.1%), inconvenience (35.7%), a general preference for oral medications (34.2%), pain (25.4%), concerns about needle size (23.4%), and injection‐site reactions (5.0%). 16 However, to date, no known published research has directly compared a once‐daily oral medication to once‐weekly injectable administration. Further, among patients who do prefer weekly injectable medications over daily oral medications, little is known about the rationale for their preference.
Patient preferences are influenced by a wide range of factors beyond route of administration and dosing frequency. For example, patients with diabetes often cite inflexible treatment regimens, such as those that require coordination with meals, to be burdensome. 9 In addition to the complexity and flexibility of the treatment regimens, patient preferences for medications may be impacted by their overall health, existing treatment regimens, and specific life circumstances. Often, physicians' perceptions of patient preferences are different from patients' actual preferences. 17 Therefore, having a better understanding of patients' preferences for diabetes treatment and the reasons for these preferences might assist physicians in shared decision‐making for a diabetes treatment that is best for the patient. 17
The purpose of the present study was to understand the preferences of people with T2DM for once‐daily oral versus once‐weekly injectable medication administration profiles, using both general and product‐specific preference questions, and with oral semaglutide and injectable dulaglutide administration profiles used as examples. Additional objectives were to understand why patients prefer once‐daily oral or once‐weekly injectable T2DM treatments, as well as aspects of treatments that might change their preferences.
2. MATERIALS AND METHODS
2.1. Overall design and sample
The REVISE study, a cross‐sectional online survey with 600 participants, was conducted among patients with T2DM (n = 300 from the United Kingdom; n = 300 from the United States). Participants included in the study were residents of the United States or United Kingdom, aged at least 18 years, who had been diagnosed with T2DM by a medical doctor for at least 6 months, and who were currently treating their diabetes with medication (oral and/or injectable). Individuals were excluded from the study if they had a cognitive disability, hearing difficulty, type 1 diabetes, gestational diabetes or latent autoimmune disease in adults, or if they were employed in the pharmaceutical industry or in a position with a direct role in treating patients with diabetes. The study protocol was reviewed by the Advarra Institutional Review Board and was determined to be exempt from institutional review board oversight (study number Pro:00040684; determination date: February 13, 2020). Informed consent was obtained from participants prior to data collection.
Participants were recruited through online patient panels and were invited to participate via email and study advertisements accessible only to panelists. These online patient panels require panelists to complete extensive screening surveys, consisting of sociodemographic and healthcare data that characterize panelists to identify eligible participants for studies. Participants completed a series of online screening questions based on the inclusion/exclusion criteria to confirm eligibility, and if eligible, they provided informed consent before data collection commenced. All participants were recruited in February and March 2020. Participants were remunerated for their participation.
2.2. Survey
As a preliminary step, a qualitative study was conducted to evaluate patients' preferences for either a once‐weekly subcutaneous injectable or a once‐daily oral T2DM treatment, both before and after learning about the administration procedures of the medications, and to identify the reasons for these preferences. Individual interviews were conducted with 25 people with T2DM from the United Kingdom and 25 from the United States as a separate sample from the present study, but also recruited through online patient panels. The results of the qualitative interviews informed the development of the online survey used in the REVISE study, particularly the content and wording of the questions and response options, including those that elicited the reasons for specific preferences.
The online survey used in the present study began by asking participants about their experience with diabetes. Next, participants were asked about their general preferences for a once‐daily oral diabetes medication versus a once‐weekly injectable medication (assuming both are similar in terms of the overall effectiveness, side effects, and cost). The general preference question read as follows:
“Imagine you had the choice between two medications for your type 2 diabetes. One is a non‐insulin injectable medication that you would self‐inject under your skin once a week and the other is a pill you take by mouth every day. Both choices are similar in terms of the overall effectiveness, side effects, and cost. Which one would you choose?
A weekly injectable (A non‐insulin injectable medication that you take once per week)
A daily pill (An oral medication that you take daily)”
The order of the response options was randomized, so approximately half of the participants were presented with the once‐weekly injectable as the first option and approximately half were presented with the once‐daily oral as the first option. After responding to this general preference question, participants were asked to provide the reasons for these preferences.
Participants were then shown two videos, each approximately 2 minutes in length, describing the administration procedures for injectable dulaglutide and oral semaglutide. These administration procedures were based on the published documents for each of the medications. 5 , 6 Neither product was mentioned by name in the videos or anywhere in the survey. Instead, the video referred to injectable dulaglutide as Product X and oral semaglutide as Product Y. The order in which the videos were presented was also randomized across participants. The survey was programmed such that participants were unable to fast‐forward through or advance past the videos.
After the videos were shown, participants were asked whether they would prefer a once‐weekly injectable medication such as the one shown in the video or a once‐daily oral medication similar to the oral medication described in the video. The question on product‐specific preferences asked post‐video read:
“Please think back to the videos you just viewed and consider your life and what works best for you. If you had the choice between these two medications to treat your type 2 diabetes, which one would you choose? Again, both choices are similar in terms of the overall effectiveness, side effects, and cost.” [select only one response]
[Response options shown to participants in randomized order]
The once‐weekly non‐insulin injectable medication administered under the skin into the thigh, upper arm, or abdomen via the pre‐filled pen that can be taken at any time of day.
The once‐daily oral pill that should be taken on an empty stomach when you first wake up with half glass of water (4 oz) and then wait for at least 30 minutes before eating, drinking, or taking any other medications.”
Participants whose post‐video product‐specific preferences were not aligned with their initial preferences (eg, participants who selected the dulaglutide profile after watching the videos, but had initially preferred the daily oral and vice versa) were additionally asked about the reasons for the change in preference. At the end of the survey, participants reported their sociodemographic and clinical characteristics.
2.3. Statistical methods
The planned sample size was a total of 600 participants, comprising 300 from each country. Descriptive statistics (eg, mean, median, SD and range for continuous variables, and frequency and percentages for categorical variables) were used to summarize participants' responses as well as to characterize the sample in terms of sociodemographic and clinical characteristics. Pooled and country‐specific analyses were completed. Chi‐squared tests and t‐tests were used to test for differences across countries. All statistical tests were two‐sided, using a significance level of 0.05. SAS statistical software version 9.4 (SAS Institute Inc., Cary, North Carolina) was used to conduct the analyses.
For the main outcomes, 95% confidence intervals (CIs) are provided, including the proportion of participants initially preferring the once‐daily oral or a once‐weekly injectable, the proportion of participants preferring the oral semaglutide product profile or the injectable dulaglutide product profile after learning about the administration of each, and the proportion of participants whose initial preferences did not match their product‐specific preferences.
3. RESULTS
3.1. Sample
The sociodemographic characteristics of the study sample are reported in Table 1. The majority of the sample was male (n = 349; 58.2%) and the mean (SD) age was 64 (11.3) years. In both countries, the vast majority of participants reported their race as White (United Kingdom: n = 289; 96.3%; United States: n = 271; 90.3%) and slightly more than half of the participants were retired (n = 342; 57.0%). There was a fairly even distribution of participants by education level and household income. While the US participants were well distributed across regions of the United States, the UK participants were primarily from England (n = 258; 86.0%).
TABLE 1.
Sociodemographic characteristics, overall and by country
| Overall sample, N = 600 | United Kingdom, n = 300 | United States, n = 300 | P a | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 251 (41.8) | 120 (40.0) | 131 (43.7) | 0.3626 |
| Male | 349 (58.2) | 180 (60.0) | 169 (56.3) | |
| Age, years | ||||
| N | 600 | 300 | 300 | |
| Mean (SD) | 64 (11.3) | 61 (11.1) | 66 (10.8) | <0.0001 |
| Median (min–max) | 66 (21–87) | 64 (27–84) | 70 (21–87) | |
| Age group, n (%) | ||||
| 18–29 years | 2 (0.3) | 1 (0.3) | 1 (0.3) | <0.0001 |
| 30–39 years | 19 (3.2) | 13 (4.3) | 6 (2.0) | |
| 40–49 years | 54 (9.0) | 35 (11.7) | 19 (6.3) | |
| 50–64 years | 181 (30.2) | 118 (39.3) | 63 (21.0) | |
| 65+ years | 344 (57.3) | 133 (44.3) | 211 (70.3) | |
| Race/Ethnicity b , n (%) | ||||
| White | 560 (93.3) | 289 (96.3) | 271 (90.3) | N/A |
| Black | 22 (3.6) | 2 (0.7) | 20 (6.7) | |
| Asian | 14 (2.3) | 8 (2.7) | 6 (2.0) | |
| American Indian or Alaska native | 3 (0.5) | N/A | 3 (1.0) | |
| Other | 4 (0.7) | 1 (0.3) | 3 (1.0) | |
| Ethnicity (US participants only), n (%) | ||||
| Hispanic or Latino | 7 (1.2) | N/A | 7 (2.3) | N/A |
| Employment status b , n (%) | ||||
| Full‐time (> 32 hours per week for pay) | 111 (18.5) | 65 (21.7) | 46 (15.3) | 0.0458 |
| Part‐time (<32 hours per week for pay) | 53 (8.8) | 29 (9.7) | 24 (8.0) | 0.4720 |
| Homemaker/housewife | 26 (4.3) | 17 (5.7) | 9 (3.0) | 0.1087 |
| Unemployed | 23 (3.8) | 14 (4.7) | 9 (3.0) | 0.2877 |
| Retired | 342 (57.0) | 141 (47.0) | 201 (67.0) | <0.0001 |
| Disabled | 53 (8.8) | 37 (12.3) | 16 (5.3) | 0.0025 |
| Other | 3 (0.5) | 3 (1.0) | 0 (0) | 0.0825 |
| Education, n (%) | ||||
| Less than high school/no formal qualifications | 32 (5.3) | 31 (10.3) | 1 (0.3) | N/A |
| Secondary/high school/GED/GCSE/ O' levels or equivalent | 148 (24.7) | 75 (25.0) | 73 (24.3) | |
| Associate degree, technical, or trade school/vocational/work‐based qualifications | 164 (27.3) | 60 (20.0) | 104 (34.7) | |
| A' level or equivalent | 39 (6.5) | 39 (13.0) | N/A | |
| College/university degree (BA, BSc) | 144 (24.0) | 62 (20.7) | 82 (27.3) | |
| Postgraduate degree (MA, MBA, PhD, PGCE) | 68 (11.3) | 29 (9.7) | 39 (13.0) | |
| Other | 5 (0.8) | 4 (1.3) | 1 (0.3) | |
| Household income (UK participants), n (%) | ||||
| Less than £15 599 | 67 (11.2) | 67 (22.3) | N/A | |
| £15 600 to £25 999 | 75 (12.5) | 75 (25.0) | N/A | |
| £26 000 to £36 399 | 50 (8.3) | 50 (16.7) | N/A | |
| £36 400 to £51 999 | 54 (9.0) | 54 (18.0) | N/A | |
| £52 000 or more | 31 (5.2) | 31 (10.3) | N/A | |
| Household income (US participants), n (%) | ||||
| Less than $29 999 | 70 (11.7) | N/A | 70 (23.3) | |
| $30 000 to $44 999 | 57 (9.5) | N/A | 57 (19.0) | |
| $45 000 to $59 999 | 51 (8.5) | N/A | 51 (17.0) | |
| $60 000 to $99 999 | 69 (11.5) | N/A | 69 (23.0) | |
| $100 000 or more | 48 (8.0) | N/A | 48 (16.0) | |
| Prefer not to answer | 28 (4.7) | 23 (7.7) | 5 (1.7) | |
| Residence (UK participants), n (%) | ||||
| England | 258 (86.0) | 258 (86.0) | N/A | |
| Northern Ireland | 9 (3.0) | 9 (3.0) | N/A | |
| Scotland | 26 (8.7) | 26 (8.7) | N/A | |
| Wales | 7 (2.3) | 7 (2.3) | N/A | |
| Residence (US participants), n (%) | ||||
| Northeast | 55 (18.3) | N/A | 55 (18.3) | |
| South | 111 (37.0) | N/A | 111 (37.0) | |
| Midwest | 73 (24.3) | N/A | 73 (24.3) | |
| West | 61 (20.3) | N/A | 61 (20.3) | |
P values represent chi‐squared tests for categorical variables and t‐tests for continuous variables to test for differences across country.
Not mutually exclusive.
Participants' self‐reported clinical characteristics are shown in Table 2. The mean (SD) participant body mass index was 31.9 (7.5) kg/m2. Most participants (n = 279; 46.5%) had been diagnosed with T2DM more than 10 years ago, while 149 (24.8%) and 162 (27.0%) reported that they had been diagnosed 6 to 10 years ago and 1 to 5 years ago, respectively. Nearly all participants (n = 557; 92.8%) were currently taking an oral T2DM medication. Overall, 158 participants (26.3%) reported the use of an injectable medication, and most commonly a daily injectable (n = 135; 22.5%). Of those reporting use of oral diabetes medication, 53.5% (n = 298) were taking one oral diabetes medication, while 29.4% (n = 164) were taking two, and 17.1% (n = 95) were taking three or more. When reporting the number of total oral medications they took each day (for diabetes or any other comorbid conditions combined), nearly half (n = 288; 48.0%) of the sample reported taking six or more medications daily.
TABLE 2.
Self‐reported clinical characteristics, overall and by country
| Overall sample N = 600 | United Kingdom, n = 300 | United States, n = 300 | p a | |
|---|---|---|---|---|
| BMI b , kg/m2 | ||||
| N | 600 | 300 | 300 | |
| Mean (SD) | 31.9 (7.5) | 32.1 (8.2) | 31.8 (6.8) | 0.7201 |
| Median(min–max) | 30.7 (14–78) | 30.3 (18–78) | 30.7 (14–63) | |
| Duration of T2DM, n (%) | ||||
| < 1 year | 10 (1.7) | 8 (2.7) | 2 (0.7) | 0.0024 |
| 1–5 years | 162 (27.0) | 97 (32.3) | 65 (21.7) | |
| 6–10 years | 149 (24.8) | 73 (24.3) | 76 (25.3) | |
| > 10 years | 279 (46.5) | 122 (40.7) | 157 (52.3) | |
| Current T2DM treatment c , n (%) | ||||
| Oral medication | 557 (92.8) | 291 (97.0) | 266 (88.7) | <0.0001 |
| Injectable medication | 158 (26.3) | 58 (19.3) | 100 (33.3) | <0.0001 |
| Weekly non‐insulin injectable medication | 41 (6.8) | 17 (5.7) | 24 (8.0) | 0.2574 |
| Daily injectable | 135 (22.5) | 50 (16.7) | 85 (28.3) | 0.0006 |
| Daily non‐insulin injectable medication | 15 (2.5) | 7 (2.3) | 8 (2.7) | 0.7937 |
| Daily injectable insulin | 109 (18.2) | 39 (13.0) | 70 (23.3) | 0.0010 |
| A non‐insulin and insulin combination therapy | 11 (1.8) | 4 (1.3%) | 7 (2.3%) | 0.3613 |
| How many oral diabetes medications if currently taking, n (%) | ||||
| 1 | 298 (53.5) | 139 (47.8) | 159 (59.8) | 0.0125 |
| 2 | 164 (29.4) | 93 (32.0) | 71 (26.7) | |
| ≥3 | 95 (17.1) | 59 (20.3) | 36 (13.5) | |
| How many total oral medications currently taking per day, n (%) | ||||
| 0 | 4 (0.7) | 1 (0.3) | 3 (1.0) | 0.4057 |
| 1 | 45 (7.5) | 23 (7.7) | 22 (7.3) | |
| 2 | 56 (9.3) | 26 (8.7) | 30 (10.0) | |
| 3 | 71 (11.8) | 32 (10.7) | 39 (13.0) | |
| 4 | 66 (11.0) | 34 (11.3) | 32 (10.7) | |
| 5 | 70 (11.7) | 34 (11.3) | 36 (12.0) | |
| 6 | 65 (10.8) | 27 (9.0) | 38 (12.7) | |
| 7 | 48 (8.0) | 23 (7.7) | 25 (8.3) | |
| 8 | 43 (7.2) | 20 (6.7) | 23 (7.7) | |
| 9 | 28 (4.7) | 17 (5.7) | 11 (3.7) | |
| 10 | 22 (3.7) | 11 (3.7) | 11 (3.7) | |
| > 10 | 82 (13.7) | 52 (17.3) | 30 (10.0) | |
| Ever used self‐injectable diabetes medication, n (%) | ||||
| Yes | 184 (30.7) | 70 (23.3) | 114 (38.0) | <0.0001 |
| No | 411 (68.5) | 225 (75.0) | 186 (62.0) | |
| Do not know | 5 (0.8) | 5 (1.7) | 0 (0) | |
| Ever used self‐injectable medication for any other condition, n (%) | ||||
| Yes | 55 (9.2) | 22 (7.3) | 33 (11.0) | 0.2212 |
| No | 539 (89.8) | 274 (91.3) | 265 (88.3) | |
| Do not know | 6 (1.0) | 4 (1.3) | 2 (0.7) | |
| Diabetes complications c , n (%) | ||||
| Kidney complications | 32 (5.3) | 14 (4.7) | 18 (6.0) | 0.4674 |
| Eye complications | 74 (12.3) | 40 (13.3) | 34 (11.3) | 0.4563 |
| Cardiovascular complications (eg, heart attack or stroke) | 49 (8.2) | 18 (6.0) | 31 (10.3) | 0.0526 |
| Foot complications (eg, non‐healing wounds, diabetic foot ulcer, or amputation) | 30 (5.0) | 17 (5.7) | 13 (4.3) | 0.4537 |
| Nerve complications (eg, numbness or pain in the feet or hands) | 150 (25.0) | 59 (19.7) | 91 (30.3) | 0.0026 |
| Other | 7 (1.2) | 3 (1.0) | 4 (1.3) | 0.7038 |
| None | 383 (63.8) | 205 (68.3) | 178 (59.3) | 0.0218 |
| Current HbA1c level, n (%) | ||||
| < 53 mmol/mol (< 7%) | 214 (35.7) | 66 (22.0) | 148 (49.3) | <0.0001 |
| 54–64 mmol/mol (7.1%–8%) | 163 (27.2) | 79 (26.3) | 84 (28.0) | |
| 65–75 mmol/mol (8.1%–9%) | 66 (11.0) | 36 (12.0) | 30 (10.0) | |
| > 75 mmol/mol (>9%) | 23 (3.8) | 17 (5.7) | 6 (2.0) | |
| Do not know | 134 (22.3) | 102 (34.0) | 32 (10.7) | |
| Comorbidities c , n (%) | ||||
| Alcohol abuse | 21 (3.5) | 8 (2.7) | 13 (4.3) | 0.2667 |
| Substance abuse | 5 (0.8) | 4 (1.3) | 1 (0.3) | 0.1779 |
| Angina | 29 (4.8) | 17 (5.7) | 12 (4.0) | 0.3412 |
| Anxiety | 108 (18.0) | 64 (21.3) | 44 (14.7) | 0.0336 |
| Arthritis | 204 (34.0) | 88 (29.3) | 116 (38.7) | 0.0158 |
| Chronic obstructive pulmonary disease/emphysema | 55 (9.2) | 23 (7.7) | 32 (10.7) | 0.2029 |
| Diabetic retinopathy | 34 (5.7) | 15 (5.0) | 19 (6.3) | 0.4800 |
| Heart attack or heart disease | 85 (14.2) | 33 (11.0) | 52 (17.3) | 0.0261 |
| Hypertension | 295 (49.2) | 110 (36.7) | 185 (61.7) | <0.0001 |
| Stroke | 21 (3.5) | 8 (2.7) | 13 (4.3) | 0.2667 |
| Depression | 128 (21.3) | 73 (24.3) | 55 (18.3) | 0.0728 |
| None | 142 (23.7) | 90 (30.0) | 52 (17.3) | 0.0003 |
Abbrevations: BMI, body mass index; T2DM, type 2 diabetes.
P values represent chi‐squared tests for categorical variables and t‐tests for continuous variables to test for differences across country.
The standard calculation formula: [weight in kg / (height in m × height in m)] was used to calculate BMI. US participants reported height in feet and inches, which was converted to cm by multiplying by 2.54. US participants reported weight in pounds and UK participants reported weight in stones and pounds, which was converted to kg by multiplying by 0.453592.
Not mutually exclusive.
Most participants had not experienced T2DM‐related complications (n = 383; 63.8%); however, among those who had, nerve (n = 150; 25.0%) and eye (n = 74; 12.3%) complications were the most common. Overall, 35.7% (n = 214) reported a current HbA1c level of below 53 mmol/mol, 27.2% (n = 163) reported an HbA1c of 54 mmol/mol to 64 mmol/mol, 11.0% (n = 66) reported an HbA1c of 65 mmol/mol and 75 mmol/mol, 3.8% (n = 23) reported a level higher than 75 mmol/mo, and 22.3% (n = 134) did not know their current HbA1c level. HbA1c levels and knowledge of current HbA1c levels varied significantly between the two countries (P < 0.0001).
3.2. Preference results
When asked the initial preference question (whether they would prefer a once‐weekly medication or a once‐daily oral medication for their T2DM without further information about administration), a significantly greater proportion of participants (76.5%) preferred the once‐daily oral medication (n = 459; 95% confidence interval [CI] 73.1–79.9) over the once‐weekly injectable (n = 141, 23.5%; 95% CI 20.1–26.9; P < 0.0001 [Figure 1; Table 3]). The most commonly selected reasons for preferring the once‐daily oral medication included “because it is taken orally instead of injected” (n = 329; 71.7%) and “because I am already taking oral medications and can add another oral medication” (n = 260; 56.6%; these response options were not mutually exclusive [Table 4]). Among participants who initially preferred the once‐weekly injectable medication (n = 141; 23.5%), commonly cited reasons for this preference included “because it is taken once a week instead of daily” (n = 126; 89.4%) and “because I am already taking oral medications and do not want another oral medication” (n = 71; 50.4%; not mutually exclusive).
FIGURE 1.
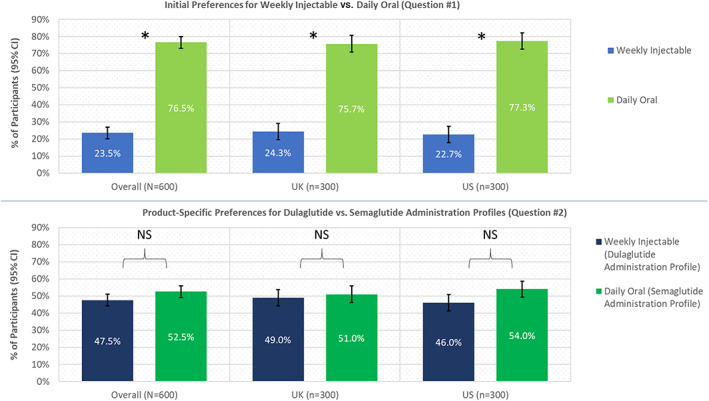
Initial and post‐video product‐specific preferences overall and by country. * P < 0.05 when comparing initial preference for daily oral versus weekly injectable. Abbreviations: CI, confidence interval; NS, not statistically significant
TABLE 3.
Preferences for daily oral versus weekly injectable medications before and after product‐specific information was provided to patients
| Product‐specific preference c | ||||
|---|---|---|---|---|
| Product X (weekly injectable dulaglutide), n (%) | Product Y (daily oral semaglutide), n (%) | Total, N (%) | ||
| Weekly injectable | 132 (22.0) | 9 (1.5) a | 141 (23.5) | |
| Initial preference b | Daily oral | 153 (25.5) a | 306 (51.0) | 459 (76.5) |
| Total | 285 (47.5) | 315 (52.5) | ||
Switchers.
P < 0.0001 for general preference for weekly injectable (95% confidence interval [CI] 20.1–26.9) vs. daily oral (95% CI 73.1–79.9) comparison.
P = 0.2207 for preference for Product X (95% CI 43.5–51.5) vs. Product Y (95% CI 48.5–56.5) comparison.
TABLE 4.
Reasons for preferences initially and after viewing daily oral semaglutide and weekly injectable dulaglutide product‐specific videos
| Overall sample N = 600 | United Kingdom, n = 300 | United States, n = 300 | ||||||
|---|---|---|---|---|---|---|---|---|
| Endorsing b , n (%) | Primary reason, n (%) | Endorsing b ,n (%) | Primary reason, n (%) | Endorsing b , n (%) | Primary reason, n (%) | P a | ||
| Reasons for initial preference | Reasons if initially preferred daily oral | n = 459 | n = 459 | n = 227 | n = 227 | n = 232 | n = 232 | |
| It is taken orally instead of injected | 329 (71.7) | 220 (47.9) | 154 (67.8) | 102 (44.9) | 175 (75.4) | 118 (50.9) | 0.0712 | |
| I am already taking oral medications and can add another oral medication | 260 (56.6) | 111 (24.2) | 117 (51.5) | 57 (25.1) | 143 (61.6) | 54 (23.3) | 0.0291 | |
| It is taken once a day instead of weekly | 138 (30.1) | 45 (9.8) | 68 (30.0) | 22 (9.7) | 70 (30.2) | 23 (9.9) | 0.9597 | |
| I am concerned about using an injectable medication | 124 (27.0) | 52 (11.3) | 57 (25.1) | 28 (12.3) | 67 (28.9) | 24 (10.3) | 0.3632 | |
| It fits better into my schedule | 105 (22.9) | 15 (3.3) | 46 (20.3) | 8 (3.5) | 59 (25.4) | 7 (3.0) | 0.1876 | |
| Other | 18 (3.9) | 16 (3.5) | 11 (4.8) | 10 (4.4) | 7 (3.0) | 6 (2.6) | 0.3129 | |
| Reasons if initially preferred weekly injectable | n = 141 | n = 141 | n = 73 | n = 73 | n = 68 | n = 68 | ||
| It is taken once a week instead of daily | 126 (89.4) | 110 (78.0) | 63 (86.3) | 55 (75.3) | 63 (92.6) | 55 (80.9) | 0.2220 | |
| I am already taking oral medications and do not want another oral medication | 71 (50.4) | 20 (14.2) | 43 (58.9) | 11 (15.1) | 28 (41.2) | 9 (13.2) | 0.0354 | |
| It is burdensome to have to carry pills | 35 (24.8) | 1 (0.7) | 22 (30.1) | 1 (1.4) | 13 (19.1) | 0 (0) | 0.1301 | |
| It fits better into my schedule | 28 (19.9) | 3 (2.1) | 15 (20.5) | 3 (4.1) | 13 (19.1) | 0 (0) | 0.8315 | |
| It is injected instead of taken orally | 14 (9.9) | 5 (3.5) | 10 (13.7) | 3 (4.1) | 4 (5.9) | 2 (2.9) | 0.1209 | |
| Other | 5 (3.5) | 2 (1.4) | 2 (2.7) | 0 (0) | 3 (4.4) | 2 (2.9) | 0.5917 | |
| Reasons if swiched preferences post‐video | Reasons considered among those switching from oral to injectable | Overall sample, N = 153 | United Kingdom, N = 76 | United States, N = 77 | ||||
| Timing/steps of taking the medication | 90 (58.8) | — | 40 (52.6) | — | 50 (64.9) | — | 0.1221 | |
| Convenience | 81 (52.9) | — | 41 (53.9) | — | 40 (51.9) | — | 0.8043 | |
| Frequency the medication is taken | 62 (40.5) | — | 33 (43.4) | — | 29 (37.7) | — | 0.4682 | |
| How the medication fits better with my schedule | 62 (40.5) | — | 28 (36.8) | — | 34 (44.2) | — | 0.3569 | |
| Injection device | 51 (33.3) | — | 31 (40.8) | — | 20 (26.0) | — | 0.0519 | |
| Concerns about properly administering the medication | 23 (15.0) | — | 9 (11.8) | — | 14 (18.2) | — | 0.2726 | |
| Past experience with injectable | 8 (5.2%) | — | 5 (6.6%) | — | 3 (3.9%) | — | 0.4561 | |
| Other | 7 (4.6%) | — | 3 (3.9%) | — | 4 (5.2%) | — | 0.7120 | |
| Reasons considered among those switching from injectable to oral | N = 9 | N = 2 | N = 7 | |||||
| Frequency the medication is taken | 2 (22.2) | — | 0 (0) | — | 2 (28.6) | — | — | |
| Injection device | 2 (22.2) | — | 0 (0) | — | 2 (28.6) | — | — | |
| Concerns about properly administering the medication | 2 (22.2) | — | 1 (50.0) | — | 1 (14.3) | — | — | |
| Convenience | 2 (22.2) | — | 0 (0) | — | 2 (28.6) | — | — | |
| How the medication fits better with my schedule | 1 (11.1) | — | 0 (0) | — | 1 (14.3) | — | — | |
| Timing/steps of taking the medication | 1 (11.1) | — | 1 (50.0) | — | 0 (0) | — | — | |
| Other | 1 (11.1) | — | 0 (0) | — | 1 (14.3) | — | — | |
P values represent chi‐squared tests for categorical variables and t‐tests for continuous variables to test for differences across country.
Not mutually exclusive.
Participants were asked to indicate which of their selected reasons was the main reason for their initial preference. The most commonly endorsed main reason for preferring the once‐daily oral medication was “because it is taken orally instead of injected” (n = 220; 47.9%). The majority of participants who initially preferred the once‐weekly injectable medication (n = 110; 78.0%) indicated that the main reason for this preference was “because it is taken once a week instead of daily.”
When answering the second question (after viewing the two videos describing the product‐specific administration characteristics), there was no significant difference (P = 0.2207) between the proportions of the total sample preferring the injectable dulaglutide product description (n = 285; 47.5%; 95% CI 43.5–51.5) and the oral semaglutide product description (n = 315; 52.5%; 95% CI 48.5–56.5 [Figure 1]). Patient preferences were consistent in both countries (United Kingdom: weekly injectable dulaglutide, n = 147 [49.0%; 95% CI 43.3–54.7]; daily oral semaglutide, n = 153 [51.0%; 95% CI 45.3–56.7] and United States: weekly injectable dulaglutide, n = 138 [46.0%; 95% CI 40.4–51.6]; daily oral semaglutide, n = 162 [54.0%; 95% CI 48.4–59.6]).
After learning about the product‐specific administration characteristics of daily oral semaglutide and once‐weekly injectable dulaglutide, participants were more likely to switch from preferring oral to injectable medications (25.5%), as compared with switching from preferring injectable to oral medications (1.5%; Table 3). The most common reason for switching from an initial preference for a daily oral to the weekly injectable dulaglutide profile was the timing and steps of intake (58.8%; Table 4). Before viewing the product administration information, more than half (56.6%) of those who preferred a daily oral medication indicated that one of the reasons for their preference was that they were already taking daily oral medications and felt they could add another, whereas half (50.4%) of those who preferred a weekly injectable medication indicated that because they were already on oral medications, they did not want to add another.
As a post‐hoc analysis designed to assess the potential impact that patients' experiences with injectable medications had on preferences, a sensitivity analysis was conducted to compare the initial and product‐specific administration profile preferences of those who were currently using an injectable diabetes medication to those who reported using only oral diabetes medications. Initially, a larger proportion of those taking only oral diabetes medications preferred the daily oral (87.6%; n = 387/442) compared to those using an injectable diabetes medication (45.6%; n = 72/158). After viewing the videos on the product‐specific administrations, similar proportions of participants from both groups switched from preferring the daily oral to preferring the dulaglutide administration profile (25.8% of those who were taking oral medications only and 24.7% of those who were using an injectable; see Table SS1).
4. DISCUSSION
Previously, the proportion of participants preferring a daily oral medication over a weekly injectable medication was unknown. Based on the results of the present study, patients generally prefer a daily oral T2DM medication over a weekly injectable medication by a ratio of approximately 3:1 (when provided with no additional information and assuming both products are similar in terms of overall effectiveness, side effects, and cost). However, the results of the present study also suggest that complex treatment regimens may have a substantial impact on these preferences, as was noted by several participants in the preliminary qualitative interview study. Additionally, participants in the qualitative interviews also commonly noted disliking needles. After learning about the specific administration procedures of injectable dulaglutide and oral semaglutide, there was no significant difference between the proportions of participants preferring the weekly injectable dulaglutide and daily oral semaglutide medication administration profiles.
The results of the present study support the need for clear and complete communication of medication requirements and administration procedures between prescribing physicians and their patients. In particular, this study suggests that some aspects of medication administration profiles, such as fasting and waiting prior to food, beverage, or other medication intake can be very important to patients and impact their stated preferred treatment. Furthermore, it is important for physicians to keep in mind that complexity and flexibility of treatment administration, such as the timing restrictions and steps of administration may be just as important to patients as mode and frequency of administration.
For example, one of the restrictions of oral semaglutide is that after taking it, patients must wait at least 30 minutes before taking any other oral medications. Nearly all (97%) people with T2DM have at least one comorbidity and 88.5% have at least two, 18 often resulting in patients with diabetes being prescribed multiple medications. Over 70% of participants in the REVISE study reported taking four or more oral medications daily. When asked for the reasons for their initial preferences, more than half of those who preferred a daily oral medication indicated that one of the reasons for their preference was that they were already taking daily oral medications so they felt that they could add another. Conversely, half of those who preferred a weekly injectable indicated that because they were already on oral medications, they did not want to add another. After learning about the administration of the products, 58.8% of participants who switched from an initial preference for a daily oral medication to a product‐specific preference for the injectable dulaglutide profile reported the timing and steps of taking the medication as a reason for switching. We hypothesize that informing participants that other oral medications should not be taken within 30 minutes of taking oral semaglutide may have had a significant impact on participants' preferences for oral semaglutide versus injectable dulaglutide. These findings highlight that it is important for physicians to consider how a new medication may impact patients' existing treatment regimens and to discuss this with them before deciding on a treatment. For example, the results of the present study suggest that the medication requirements may inconvenience patients by interrupting their daily schedules, in particular with regard to their typical eating times and the timing of their other oral medications. Previous research found that more than one third (35.7%) of US‐based patients cited inconvenience as a reason for discontinuing injectable medications. 16 We hypothesize that medication regimens that patients perceive to be “inconvenient” may also impact patients' adherence to oral T2DM medications. Additionally, it is important that physicians highlight the importance of adhering to medication administration requirements because not taking oral semaglutide as directed can reduce its effectiveness.
The results of this study should be interpreted with consideration for the following limitations. The sample population was predominantly White and the study was conducted only among patients with T2DM and therefore the results may not be generalizable to all people with T2DM or to other conditions and populations. Additionally, these findings may not be generalizable to other daily oral versus injectable products. For example, the needle of the dulaglutide injectable device is hidden, which may lessen any patient anxiety toward needles compared to other injectable devices with exposed needles. Future studies may utilize alternative study designs to assess related research questions, for example, studies conducted in different populations, therapeutic areas, with different product profiles, and using different methods of presenting the information to participants.
The product‐specific information presented was based on the US 'instruction for use' for injectable dulaglutide and the US patient medication guide for oral semaglutide. The US and European Union package inserts for oral semaglutide are almost identical, with minor wording differences related to the directions for patients to take the medication on an empty stomach. 19 It should also be noted that the initial preference question and the product‐specific preference question were worded differently, with the latter referencing information that was presented in the videos, as shown in the methods section.
In conclusion, the initial stated patient preference ratio for a once‐daily oral medication compared to a once‐weekly injectable medication was approximately 3:1. After learning about the specific treatment medication administration profiles of weekly injectable dulaglutide and daily oral semaglutide, participants were split approximately 50/50 in their preference. This study demonstrates that a one‐size‐fits‐all approach does not apply to patients' preferences for a T2DM treatment. Instead, preferences depend on several factors such as flexibility and complexity of the treatment regimen, the route and frequency of administration, and the patient and their lifestyle. It may be important to consider this finding in future patient preference studies, ensuring that the specifics of medication administration profiles, device characteristics, and so on, are described specifically and accurately to participants to ensure that studies yield meaningful patient preference data. Additionally, physicians should discuss with patients how complex treatment regimens will work with their lifestyles and their existing treatment regimens when deciding on a diabetes treatment that is best for the patient.
CONFLICT OF INTEREST
Melissa Ross and Heather Gelhorn – nothing to disclose. Reema Mody, Manige Konig, and Kristina Boye are minor shareholders and employees of Eli Lilly and Company.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14244.
Supporting information
Table S1. Sensitivity analysis of preferences for daily oral versus weekly injectable medications before and after product‐specific information is provided to patients, by current medication use.
Boye K, Ross M, Mody R, Konig M, Gelhorn H. Patients' preferences for once‐daily oral versus once‐weekly injectable diabetes medications: The REVISE study. Diabetes Obes Metab. 2021;23:508–519. 10.1111/dom.14244
Funding information Eli Lilly and Company
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Centers for Disease Control (CDC) Newsroom . New CDC Report: More than 100 million Americans have diabetes or prediabetes. [Press Release]. Juy 18, 2017. https://www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed May 18, 2020.
- 2. Diabetes UK . Key statistics on diabetes. Diabetes in the UK 2018. 2020; https://www.diabetes.org.uk/professionals/position-statements-reports/statistics. Accessed May 18, 2020.
- 3. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Assoication . Pharmacologic approaches to glycemic treatment: standards of medical Care in Diabetes—2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 5. Lilly Eli: Instructions for use: TRULICITY (TRU‐li‐si‐tee) (dulaglutide) injection, for subcutaneous use 0.75 mg/0.5 mL Single‐Dose Pen use 1 time each week (once weekly). 2020; https://uspl.lilly.com/trulicity/trulicity.html#ug. Accessed May 18, 2020.
- 6. Novo Nordisk Inc . Oral GLP‐1 RA Medication—RYBELSUS (semaglutide) tablets 7 mg or 14 mg. 2020a; https://www.rybelsuspro.com/. Accessed May 18, 2020.
- 7. Marsh K, van Til JA, Molsen‐David E, et al. Health preference research in Europe: a review of its use in marketing authorization, reimbursement, and pricing decisions—report of the ISPOR stated preference research special interest group. Value Health. 2020;23:831‐841. [DOI] [PubMed] [Google Scholar]
- 8. Bogelund M, Vilsboll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark ‐ a discrete choice experiment. Curr Med Res Opin. 2011;27(11):2175‐2183. [DOI] [PubMed] [Google Scholar]
- 9. Hayes RP, Bowman L, Monahan PO, Marrero DG, McHorney CA. Understanding diabetes medications from the perspective of patients with type 2 diabetes: prerequisite to medication concordance. Diabetes Educ. 2006;32(3):404‐414. [DOI] [PubMed] [Google Scholar]
- 10. Ryden A, Chen S, Flood E, Romero B, Grandy S. Discrete choice experiment attribute selection using a multinational interview study: treatment features important to patients with type 2 diabetes mellitus. Patient. 2017;10(4):475‐487. [DOI] [PubMed] [Google Scholar]
- 11. Hauber AB, Nguyen H, Posner J, Kalsekar I, Ruggles J. A discrete‐choice experiment to quantify patient preferences for frequency of glucagon‐like peptide‐1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin. 2016;32(2):251‐262. [DOI] [PubMed] [Google Scholar]
- 12. Ludemann J, Dutting ED, Dworak M. Patient preference and tolerability of a DPP‐4 inhibitor versus a GLP‐1 analog in patients with type 2 diabetes mellitus inadequately controlled with metformin: a 24‐week, randomized, multicenter, crossover study. Ther Adv Endocrinol Metab. 2015;6(4):141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dibonaventura MD, Wagner JS, Girman CJ, et al. Multinational internet‐based survey of patient preference for newer oral or injectable type 2 diabetes medication. Patient Prefer Adherence. 2010;4:397‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brundisini F, Vanstone M, Hulan D, DeJean D, Giacomini M. Type 2 diabetes patients' and providers' differing perspectives on medication nonadherence: a qualitative meta‐synthesis. BMC Health Serv Res. 2015;15:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spain CV, Wright JJ, Hahn RM, Wivel A, Martin AA. Self‐reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653‐1664. e1651. [DOI] [PubMed] [Google Scholar]
- 16. Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real‐world cross‐sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muhlbacher AC, Juhnke C. Patient preferences versus physicians' judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163‐180. [DOI] [PubMed] [Google Scholar]
- 18. Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co‐prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243‐1252. [DOI] [PubMed] [Google Scholar]
- 19. Novo Nordisk Inc . Rybelsus (semaglutide) [UKPackage Insert]. Information for the patient. 2020b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity analysis of preferences for daily oral versus weekly injectable medications before and after product‐specific information is provided to patients, by current medication use.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


