Abstract
Essentials.
There is a need for improved tools to predict persistent and chronic immune thrombocytopenia (ITP).
We developed and validated a clinical prediction model for recovery from newly diagnosed ITP.
The Childhood ITP Recovery Score predicts transient vs. persistent ITP and response to intravenous immunoglobulins.
The score may serve as a useful tool for clinicians to individualize patient care.
Abstract
Background
Childhood immune thrombocytopenia (ITP) is an autoimmune bleeding disorder. The prognosis (transient, persistent, or chronic ITP) remains difficult to predict. The morbidity is most pronounced in children with persistent and chronic ITP. Clinical characteristics are associated with ITP outcomes, but there are no validated multivariate prediction models.
Objective
Development and external validatation of the Childhood ITP Recovery Score to predict transient versus persistent ITP in children with newly diagnosed ITP.
Methods
Patients with a diagnosis platelet count ≤ 20 × 109/L and age below 16 years were included from two prospective multicenter studies (NOPHO ITP study, N = 377 [development cohort]; TIKI trial, N = 194 [external validation]). The primary outcome was transient ITP (complete recovery with platelets ≥100 × 109/L 3 months after diagnosis) versus persistent ITP. Age, sex, mucosal bleeding, preceding infection/vaccination, insidious onset, and diagnosis platelet count were used as predictors.
Results
In external validation, the score predicted transient versus persistent ITP at 3 months follow‐up with an area under the receiver operating characteristic curve of 0.71. In patients predicted to have a high chance of recovery, we observed 85%, 90%, and 95% recovered 3, 6, and 12 months after the diagnosis. For patients predicted to have a low chance of recovery, this was 32%, 46%, and 71%. The score also predicted cessation of bleeding symptoms and the response to intravenous immunoglobulins (IVIg).
Conclusion
The Childhood ITP Recovery Score predicts prognosis and may be useful to individualize clinical management. In future research, the additional predictive value of biomarkers can be compared to this score. A risk calculator is available (http://www.itprecoveryscore.org).
Keywords: decision support techniques, immune thrombocytopenia, intravenous immunoglobulins, prognosis
1. INTRODUCTION
Childhood immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disease characterized by low platelet counts (≤100 × 109/L). 1 , 2 , 3 The yearly incidence is 2 to 6 per 100 000 children. 4 Most children with newly diagnosed ITP have self‐limiting disease courses and recover spontaneously, yet 40% of children exhibit thrombocytopenia and bleeding beyond 3 months (persistent ITP), and 10% to 20% percent 1 year after the diagnosis (chronic ITP). 5 , 6 , 7 The morbidity of childhood ITP is most significant among the children with persistent and chronic thrombocytopenia. 8 , 9 To predict such persistent and chronic ITP disease courses, a systematic review and meta‐analysis identified from the literature the following clinical characteristics: female gender, older age, the absence of a preceding infection or vaccination, insidious disease onset, and a relatively higher platelet count at diagnosis. 10 A recent analysis in the Intercontinental Cooperative ITP Study Group (ICIS) Registry II also showed age and bleeding as potential predictors, but did not find effects of platelet count or sex, albeit with fewer observations than in the previous meta‐analysis. 11 Notably, these predictors correlate with each other, in particular with age (Schmidt et al; medRxiv pre‐print https://doi.org/10.1101/2020.06.09.20125385). Moreover, how well these characteristics actually predict ITP outcomes is not yet known (in terms of predictive ability, discrimination, calibration), as no multivariate clinical prediction models have been developed.
Clinical prediction models can assist clinicians to identify patients with a particular prognosis. 12 , 13 In the case of childhood ITP, this concerns the identification of children that can be safely followed by watchful waiting and minimal monitoring, and children with a high chance of persistent and chronic ITP. Importantly, the error rates and clinical utility of prediction models can be evaluated. Moreover, it can be evaluated how the extension of a model by further clinical predictors or biomarkers can improve prediction. 14 In 2007, Edslev et al proposed a clinical prediction score for transient ITP, using insidious onset, age <10 years, a preceding infection, a platelet count <5 × 109L, the presence of wet purpura, and male sex. 15 This simple Bayesian score has some drawbacks: individualized risk estimates cannot be obtained, multivariate effects are not considered, and information on risk differentiation from continuous variables is lost due to dichotomization. 16 Moreover, this score has not been prospectively validated in an external cohort, but two retrospective studies indicated that patients with the highest risk scores showed more often transient disease courses. 17 , 18
The objective of this study was to develop a novel clinical prediction tool to predict transient versus persistent ITP disease courses in children with newly diagnosed ITP at the time of diagnosis. We used individual patient data of 577 patients from two large prospective childhood ITP studies. 7 , 15 The data from the observational NOPHO (Nordic Pediatric Hematology‐Oncology) ITP study were used to develop the score. By validating the score in the TIKI (Treatment With or Without Intravenous Immunoglobulins in Kids with ITP) randomized trial, we were able to test the usefulness of the new score in predicting disease outcomes in untreated children, as well as children treated with intravenous immunoglobulins (IVIg).
2. METHODS
2.1. Study design, data sources, and ethical considerations
The study is reported according to the TRIPOD (transparent reporting of a multivariate prediction model for individual prognosis or diagnosis) checklist for prediction model development and validation. 19 Two cohorts were included in this study to develop and validate the clinical prediction model. The NOPHO ITP study included children with newly diagnosed ITP in a prospective registry cohort in five Nordic countries between 1998 to 2000. 5 , 15 Patients with a diagnosis of ITP consistent with clinical criteria and absent clinical or laboratory evidence of alternative causes for thrombocytopenia were recruited at pediatric departments. Data was recorded at diagnosis. Children with an age below 15 years and a platelet count ≤20 × 109/L and available 6‐month follow‐up were included (Figure 1A; N = 377). The study was approved by ethical review committees and authorities in each participating country. A second cohort of children with newly diagnosed ITP was obtained from the TIKI trial, 7 a multicenter randomized controlled trial in the Netherlands that enrolled children between 2009 and 2015 (Figure 1B; N = 194). Inclusion criteria were an age between 3 months and 16 years and a platelet count ≤20 × 109/L. Patients were excluded when severe or life‐threatening bleeding was present (Buchanan score ≥ 4), immunomodulatory drugs were given within 1 month of diagnosis, or contraindications to IVIg therapy were present. Clinical characteristics were obtained at diagnosis and registered in standardized clinical report forms. Enrolled patients were randomized to receive careful observation or a single dose of 0.8g/kg IVIg. During follow‐up, patients were routinely assessed according to a pre‐defined schedule at 1 and 4 weeks, as well as 3, 6, and 12 months after the diagnosis. The study was approved by the Institutional Review Board of University Medical Center Utrecht. Patients (≥12 years) and parents gave written informed for study participation. All analyses were performed on deidentified, coded data. Of note, in both studies, patients were not eligible for participation when there were indications for other underlying conditions (secondary ITP) from the patient history, physical exam, or laboratory tests.
FIGURE 1.
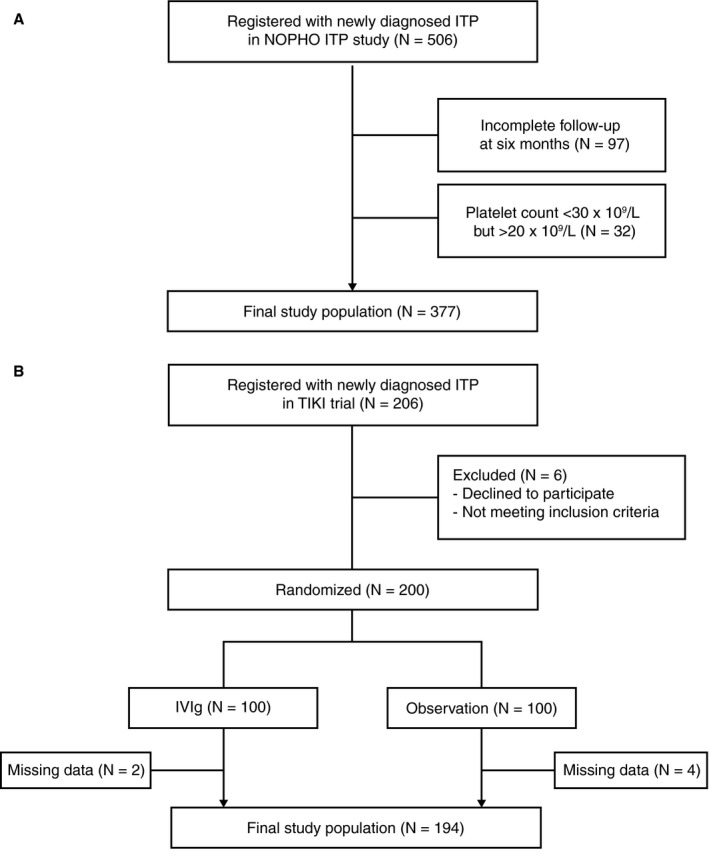
Flow chart of included study cohorts. A, from the NOPHO ITP study and B, TIKI trial.
2.2. Clinical outcome definitions
Recovery was assessed at each follow‐up, according to standardized outcome criteria by the International Working Group. 20 Complete recovery was defined as a platelet count ≥100 × 109/L. Transient ITP was defined as a complete recovery 3 months after diagnosis. In the NOPHO study, transient ITP was defined as a platelet count ≥150 × 109/L 3 months after diagnosis. Persistent ITP was defined as the absence of transient ITP, 20 and includes here both children with recovery after 3 months and with disease lasting for more than 1 year.
Rescue treatment with corticosteroids or IVIg between 1 and 3 months after the diagnosis was administered to three patients (NOPHO) and seven patients (TIKI). A further eleven patients of the NOPHO study received rescue treatment, but no exact date was recorded. Following rescue treatment, one patient of the TIKI study and none in the NOPHO study exhibited a complete recovery at the time of assessment of the clinical outcome.
2.3. Predictor definitions
The following predictors were included: age (continuous, years), sex (male/female), self‐reported history of a preceding infection or preceding vaccination within 28 days (TIKI) or 1 month (NOPHO) of diagnosis (two variables, dichotomous), insidious disease onset (self‐reported symptom duration ≥14 days), and platelet count at the time of diagnosis (continuous, ×109/L). Mucosal bleeding was defined as presence of “wet purpura/mucosal oozing” (NOPHO ITP study) or a modified Buchanan score ≥ 3 (TIKI study). 5 , 7 , 21 A linear effect term in the prediction model was judged to sufficiently approximate the association of age and platelet count with the probability of transient ITP (Figure S1 in supporting information).
2.4. Model development
The model was developed in the NOPHO ITP study. To predict transient versus persistent ITP, a binomial logistic regression model was fitted with ridge regression using glmnet. 22 , 23 Ridge regression is a preferred shrinkage technique when variable selection is not required. 24 Ridge regression also deals well with collinearity of predictors and prevents overfitting. 25 , 26 , 27 All considered clinical predictors on which data was collected (see above) were included in the final model, irrespective of their predictive ability, as ridge regression shrinks effects toward zero for irrelevant predictors, and there is no cost in obtaining the parameters clinically. The optimal shrinkage parameter was determined by 10‐fold cross‐validation.
2.5. Model validation and performance
The prediction model was validated in the study population of the TIKI trial. Discriminative ability was assessed by the receiver operating characteristic (ROC) area under the curve. Calibration was evaluated by calculating mean predicted and observed proportions of complete recovery 3 months after diagnosis (ie, transient versus persistent ITP).
2.6. Statistical analyses
Statistical analyses were performed in R (version 3.6.0). No sample size calculation was performed. Six patients from the TIKI trial were excluded because of a missing clinical characteristic; data were not imputed. Penalized regression reduces the variance of regression estimators; confidence intervals are therefore not displayed. 28 Longitudinal recovery rates (first complete recovery) were visualized with Kaplan‐Meier curves. Effects of the score on recovery were quantified with a Cox proportional hazard model with the score as a continuous predictor. Effect estimates obtained during the model validation are unbiased due to the independent development and validation steps.
3. RESULTS
3.1. Baseline characteristics and incidence of transient ITP in NOPHO and TIKI cohorts
The final study population included 571 patients for model development and validation (Figure 1). In the NOPHO ITP study, the outcome was missing in 97/506 (19%) of patients. The clinical characteristics of both study cohorts were similar at the diagnosis (Table 1). Moreover, the pre‐test probability of a favorable disease outcome, ie, the incidence of transient ITP 3 months after diagnosis, was also similar (68% and 73% for NOPHO and TIKI, respectively; Table 1). Thus, despite differences in the geographical location and the inclusion period and the exclusion of severe bleeding patients in the TIKI trial, the patient characteristics were homogeneous between the cohorts.
TABLE 1.
Baseline characteristics of patients with ITP in the development and validation cohort
| Development (NOPHO) N = 377 | Validation (TIKI) N = 194 | |
|---|---|---|
| Age, median (IQR) | 4.0 (2.0; 7.0) | 4.0 (2.4; 7.5) |
| Platelet count, ×109/L, median (IQR) | 7 (4; 12) | 6 (3; 10) |
| Female | 162 (43%) | 88 (45%) |
| Preceding infection | 217 (58%) | 108 (56%) |
| Preceding vaccination | 27 (7%) | 7 (4%) |
| Mucosal bleeding | 165 (44%) | 79 (41%) |
| Insidious disease onset | 84 (22%) | 28 (15%) |
| Transient ITP (3 months) | 255 (68%) | 142 (73%) |
Data are N (% of cohort) unless stated otherwise. Preceding infection was defined ≤1 month prior to onset of disease. Insidious disease onset, symptoms ≥14 days at diagnosis.
Abbreviations: IQR, interquartile range; ITP, immune thrombocytopenia; NOPHO, Nordic Pediatric Hematology‐Oncology ITP study; TIKI, Treatment With or Without Intravenous Immunoglobulins in Kids with ITP trial.
3.2. Development of the Childhood ITP Recovery Score for transient ITP
For model development (Figure 2A), 255 events of transient ITP were predicted in 377 patients, using clinical characteristics obtained at the diagnosis. A penalized regression model was fit to predict transient versus persistent ITP at 3 months follow‐up (Figure 2B). The association of the absence of an insidious disease onset with transient ITP was the most pronounced of all predictors. Coefficients of the model (the Childhood ITP Recovery Score) are given in Table 2. The predicted probabilities obtained from the model were similarly distributed between the NOPHO and TIKI cohorts with an approximate bimodal pattern (Figure 2C). In particular, we observed a large proportion of patients with transient ITP who exhibited scores above ~0.5, corresponding to a ~62% posterior probability (the predicted probability by the model to have transient ITP after consideration of all predictors). Compared to the NOPHO ITP score, 15 the Childhood ITP Recovery Score showed additional differentiation in patients with intermediate NOPHO scores between 4 and 12, and overall a good correlation (Spearman rho 0.82; P < .0001). The Childhood ITP Recovery Score is available as an online calculator (http://www.itprecoveryscore.org); an example calculation of the score “by hand” is given in Appendix S1 in the Supporting Information.
FIGURE 2.
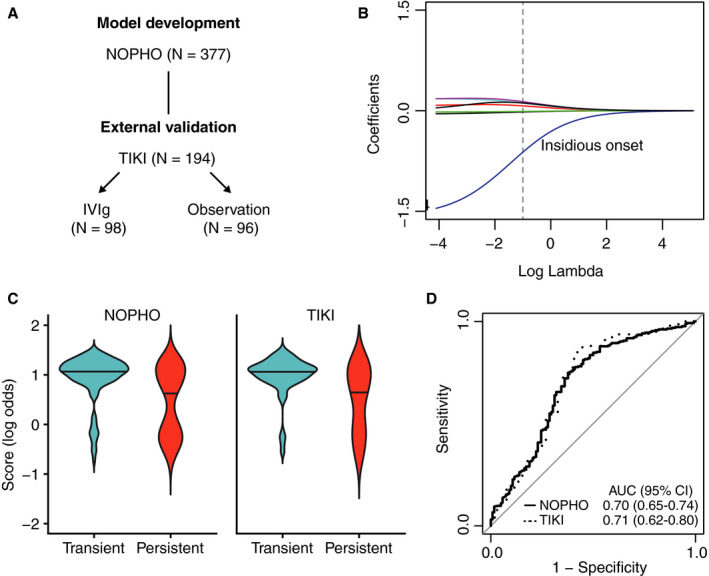
Development of the Childhood ITP Recovery Score and discrimination of disease courses. A, The score was developed in the NOPHO (Nordic Pediatric Hematology‐Oncology) ITPstudyby multivariate penalized logistic regression and evaluated in the TIKI (Treatment With or Without Intravenous Immunoglobulins in Kids with ITP) randomized controlled trial. B, Ridge regression coefficients (each line is one predictor), based on different penalty factors (lambda). The optimal lambda value (dashed line) was chosen by 10‐fold cross‐validation using binomial deviance. The largest coefficient was observed for insidious disease onset. C, Linear predictor of the Childhood ITP Recovery Score for transient versus persistent ITP disease courses in the derivation and external validation cohorts NOPHO and TIKI, respectively. A similar score distribution is observed. D, Receiver‐operating characteristic area under the curve for the Childhood ITP Recovery Score. A similar discrimination is observed between the derivation and external validation cohort, illustrating the robust generalizability of the Childhood ITP Recovery Score, as developed with penalized regression.
TABLE 2.
Regression coefficients and odds ratios of the association between the predictors and the occurrence of transient versus prolonged ITP disease courses
| Coefficient | Odds ratio | |
|---|---|---|
| Intercept | 1.11 | |
| Age, per year | −0.03 | 0.97 |
| Platelet count, ×109/L (steps of 1) | −0.02 | 0.98 |
| Male | 0.09 | 1.10 |
| Preceding infection | 0.18 | 1.20 |
| Preceding vaccination | 0.11 | 1.12 |
| Mucosal bleeding | 0.17 | 1.18 |
| Insidious disease onset | −1.15 | 0.32 |
Variables are for presence (categorical variable) with the usual dummy coding (0, absence; 1, presence) or a one‐unit increase (continuous variable) of the respective predictor. Coefficients are for the linear predictor for logit (transient ITP). The odds ratio is given for transient ITP. Confidence intervals or p‐values are not obtained for penalized regression (see Methods).
Abbreviation: ITP, immune thrombocytopenia.
3.3. Model performance and validation of the childhood ITP recovery score in the TIKI cohort
The transient and persistent ITP disease courses were discriminated with a receiver operating characteristic area under the curve (ROC AUC) of 0.70 (95% confidence interval [CI], 0.65‐0.74) in the NOPHO evaluation and 0.71 (95% CI, 0.62‐0.80) in the TIKI external validation (Figure 2D). A ROC AUC of 0.50 indicates random classification of the patients, and 1.00 perfect classification. In the TIKI cohort we observed an equal discrimination of disease courses in patients randomized to careful observation (ROC AUC 0.71, 95% CI 0.58‐0.83) and IVIg treatment (ROC AUC 0.69, 95% CI 0.54‐0.85). The assessment of the model calibration showed a clear relationship between predicted and observed probabilities of complete recovery (Figure 3A‐B). Furthermore, the calibration was similar in the development and validation cohorts.
FIGURE 3.
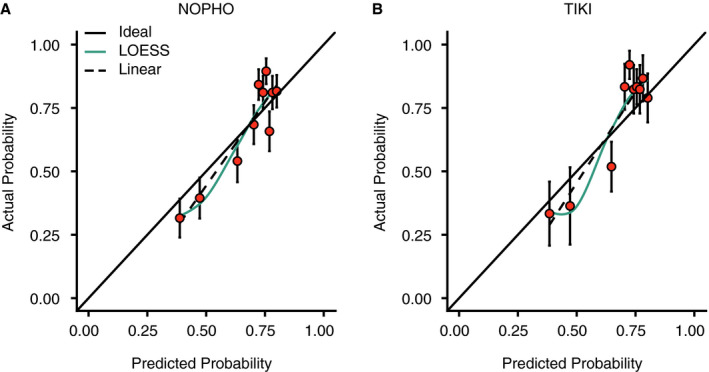
Calibration of the Childhood ITP Recovery Score. Data are shown for A, the development cohort (NOPHO [Nordic Pediatric Hematology‐Oncology]) and B,the external validation cohort (TIKI [Treatment With or Without Intravenous Immunoglobulins in Kids with ITs]). Probabilities were grouped in deciles. Points represent mean ± standard error. Solid line, ideal fit; dashed line, linear fit of observed versus predicted probabilities; colored solid line, locally estimated scatterplot smoothing (LOESS) fit
3.4. Differentiation of disease courses during long‐term follow‐up and after IVIg treatment
The Childhood ITP Recovery Score also differentiated the complete recovery rates at 6‐ and 12‐months follow‐up, with comparable rates between both cohorts (Table 3). Using data from the TIKI trial, we also assessed the predictive ability of the Childhood ITP Recovery Score for longitudinal disease courses during 1‐year follow‐up, both for patients randomized to observation or IVIg treatment. Inspecting Kaplan‐Meier curves for three terciles of scores, the group with the lowest Childhood ITP Recovery Score was clearly associated with a reduced chance for complete recovery during follow‐up, and included a high proportion of patients with chronic ITP 1 year after diagnosis (Figure 4). Notably, we focused on a first observed recovery, whereas a small proportion of children showed recurrent thrombocytopenia with platelets below 30 × 109/L after this: two patients in the observation cohort and six in the IVIg cohort (Figure S3 in Supporting Information). The spontaneous recovery in untreated patients and responses to IVIg were similarly associated with the Childhood ITP Recovery Score (Figure 4), where the group of patients with the lowest scores showed enrichment of persistent and chronic ITP disease courses. Of note, the administration of IVIg treatment to patients with the lowest scores still resulted in a 50% response rate to IVIg (Figure 4B). During the full 1‐year follow‐up, an increase of one unit in the Childhood ITP Recovery Score corresponded with a hazard ratio for complete recovery of 2.59 (95% CI, 1.66‐4.04) in the observation cohort and 1.56 (95% CI, 1.01‐2.42) in the IVIg cohort, where a hazard ratio of 1.00 would indicate no difference in recovery. The Childhood ITP Recovery Score also correlated with bleeding symptoms as assessed by the Buchanan score throughout follow‐up (Table 4).
TABLE 3.
Proportion of patients from the NOPHO ITP study and TIKI trial who exhibited complete recovery or chronic ITP during 1‐year follow‐up, according to the Childhood ITP Recovery Score prediction of the chance of recovery at 3‐month follow‐up. The score was split as outlined in the main text
| Low chance of recovery | Intermediate chance of recovery | High chance of recovery | |
|---|---|---|---|
| NOPHO (N = 377) | |||
| N (% of total) | 84 (22%) | 70 (19%) | 223 (59%) |
| Month 3 | 0.37 (0.27‐0.47) | 0.64 (0.53‐0.76) | 0.80 (0.75‐0.85) |
| Month 6 | 0.45 (0.35‐0.56) | 0.73 (0.62‐0.83) | 0.87 (0.82‐0.91) |
| Chronic ITP (old definition) | 0.55 | 0.27 | 0.13 |
| TIKI (N = 194) | |||
| N (% of total) | 28 (14%) | 45 (23%) | 121 (63%) |
| Month 3 | 0.32 (0.15‐0.49) | 0.69 (0.55‐0.82) | 0.85 (0.79‐0.91) |
| Month 6 | 0.46 (0.28‐0.65) | 0.82 (0.71‐0.93) | 0.90 (0.85‐0.95) |
| Chronic ITP (old definition) | 0.54 | 0.18 | 0.10 |
| Month 12 | 0.71 (0.55‐0.88) | 0.87 (0.77‐0.97) | 0.95 (0.91‐0.99) |
| Chronic ITP | 0.29 | 0.13 | 0.05 |
Data are the proportion (95% CI) of patients experiencing a complete recovery at the indicated timepoint. The definition of chronic ITP was changed in 2009 from ITP lasting more than 6 months after the diagnosis to 12 months after the diagnosis. 20
Abbreviations: CI, confidence interval; ITP, immune thrombocytopenia; NOPHO, Nordic Pediatric Hematology‐Oncology ITP study; TIKI, Treatment With or Without Intravenous Immunoglobulins in Kids with ITP trial.
FIGURE 4.
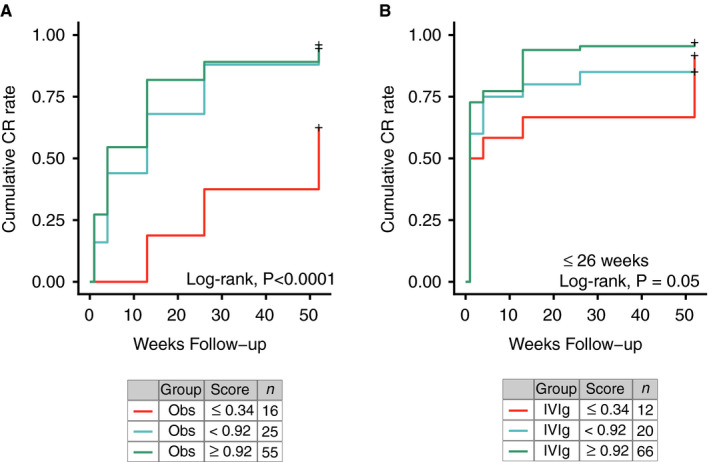
Longitudinal recovery from immune thrombocytopenia (ITP) based on the Childhood ITP Recovery Score. Differentiation of disease courses in the TIKI (Treatment With or Without Intravenous Immunoglobulins in Kids with ITP) trial for patients randomized to the (A) observation and (B) intravenous immunoglobulin (IVIg) cohort. A first complete recovery is considered as event. Statistics are given for a log‐rank test for the null hypothesis that differences in complete recovery in all three groups are due to chance
TABLE 4.
Proportion of patients in the TIKI trial with bleeding events during 1‐year follow‐up, stratified by the Childhood ITP Recovery Score at the diagnosis
| Low chance of recovery N = 28 | Intermediate chance of recovery N = 45 | High chance of recovery N = 121 | |
|---|---|---|---|
| At least extensive petechiae and/or large bruises (Buchanan score > 1) | |||
| Week 1 | 0.75 ± 0.08 | 0.87 ± 0.05 | 0.62 ± 0.05 |
| Month 1 | 0.79 ± 0.08 | 0.58 ± 0.07 | 0.41 ± 0.05 |
| Month 3 | 0.75 ± 0.08 | 0.42 ± 0.08 | 0.29 ± 0.04 |
| Month 6 | 0.69 ± 0.09 | 0.51 ± 0.08 | 0.33 ± 0.05 |
| Month 12 | 0.45 ± 0.11 | 0.20 ± 0.06 | 0.15 ± 0.04 |
| At least mucosal bleeding (Buchanan score > 2) | |||
| Week 1 | 0.43 ± 0.09 | 0.49 ± 0.07 | 0.33 ± 0.04 |
| Month 1 | 0.50 ± 0.09 | 0.18 ± 0.06 | 0.22 ± 0.04 |
| Month 3 | 0.36 ± 0.09 | 0.19 ± 0.06 | 0.10 ± 0.03 |
| Month 6 | 0.27 ± 0.09 | 0.10 ± 0.05 | 0.08 ± 0.03 |
| Month 12 | 0.14 ± 0.07 | 0.08 ± 0.04 | 0.04 ± 0.09 |
Observed proportion of patients ± standard error. Data are the proportion of patients experiencing the indicated modified Buchanan score at the indicated follow‐up timepoint.
Abbreviations: ITP, immune thrombocytopenia; TIKI, Treatment With or Without Intravenous Immunoglobulins in Kids with ITP.
3.5. Clinical scenarios
To illustrate the use of the prediction model to derive post‐test probabilities, we first consider the case of a boy with a diagnosis platelet count of 8 × 109/L, a preceding infection, no vaccination history, mucosal bleeding, and an abrupt disease onset. At the age of 2, 8, and 14 years, the Childhood ITP Recovery Score gives a probability for transient ITP of 79%, 76%, and 73% (all grouped as “high”), respectively. Thus, age marginally impacted the boy's chance for recovery, and the score suggests that the child will have a high chance of a transient disease course.
Next we consider the case of a girl of 4 years of age. She has a diagnosis platelet count of 8 x 109/L, no history of a preceding infection or vaccination, and skin bleeding only, and an insidious disease onset. The Childhood ITP Recovery Score probability for transient ITP is 42%. If the girl was 14 years instead, the probability of recovery would be 35% (both “low”). The presence of a preceding infection, for the two ages, would adjust the probability to 47% and 39% (both “low”), respectively. Thus, one might consider anticipating a longer duration of disease with more morbidity, irrespective of age and a preceding infection.
Together, these cases display how the use of the presented clinical prediction score allows evidence‐based weighing of complex clinical characteristics.
4. DISCUSSION
In the present study, we have developed and externally validated a clinical prediction score to determine course of disease in newly diagnosed childhood ITP. All predictors are easily available at diagnosis. The score is developed in the NOPHO cohort and externally validated in the TIKI trial, and shows differentiation of disease courses and bleeding symptoms, both for patients randomized to careful observation as well as treatment with IVIg. Thus, the clinical parameters included in the Childhood ITP Recovery Score are associated with ITP disease courses, irrespective of treatment. The cohorts were independent with regard to time and geography. The Childhood ITP Recovery Score is available as an online calculator that provides an individualized probability for transient ITP (http://www.itprecoveryscore.org).
4.1. Strengths and limitations
Our study combines two large European prospective multicenter studies conducted in childhood ITP to date, providing a relatively large sample size for a study in a rare disease. A major strength is that these cohorts were independent with regard to time and geography. Moreover, the Nordic and Dutch study populations showed similar clinical characteristics and disease outcomes. The key improvements over the previously introduced score by Edslev et al 15 are the development of a multivariate score, the larger derivation cohort, and the integration of additional information by inclusion of previously dichotomized predictors as continuous variables. 16 Multivariate ridge regression does not require removal of predictors, and there is no clinical cost to obtaining any of the included variables. Statistically, the similar ROC AUC between the development and validation cohort illustrates the strength of ridge regression for shrinkage of coefficients by cross‐validation, thereby preventing overfitting.
An initial concern for our study was that 19% of the patients included in our development cohort 5 did not have baseline and follow‐up data available, which might have been due to selective loss to follow‐up. Although we cannot exclude this possibility, we were reassured by the similar clinical characteristics and disease outcomes between the NOPHO and TIKI population. Moreover, the external validation of the prediction model in the TIKI trial showed robust results. The generalizability of our study is limited to the inclusion criteria, ie, children between 6 months and 16 years of age and a diagnosis platelet count below 20 × 109/L. Because the NOPHO ITP study defined the outcome at 150 × 109/L, instead of 100 × 109/L in TIKI, the NOPHO recovery frequency was marginally lower during follow‐up. Another limitation is that we did not fit interaction effects in the prediction model, but this may be considered with larger available sample sizes (eg, age, sex). It is unlikely that the inclusion of mucosal bleeding in the prediction model had a large effect on our score's relationship with bleeding during follow‐up, because in the TIKI cohort, bleeding symptoms at the diagnosis alone do not predict bleeding during follow‐up well (data not shown), and the effect size of bleeding in our multivariate prediction model is small. The self‐reporting of the symptom duration (disease onset) and a preceding infection implies potential recall bias. However, this was no strict limitation for the aim of prediction, and we observed that such self‐reported variables obtained in routine clinical care can be good predictors of disease courses.
4.2. Findings of others
We have previously systematically reviewed clinical and laboratory predictors of chronic ITP. 10 Most of the included studies in this systematic review only assessed a single or small selection of predictors and incompletely reported other data. Clearly, some predictors of disease courses may correlate (eg, a low platelet count and more severe bleeding symptoms; female sex and an older age at diagnosis), yet multivariate analyses have rarely been performed. Moreover, it remained unclear whether the findings of small studies are generalizable to other populations. The multivariate effect sizes included in the presented prediction model require careful comparison to historical data, given the penalization effects in ridge regression. However, even when no penalty was subjected to the regression coefficients (λ → 0; Figure 2), the variables gender, the presence of a preceding infection or vaccination, an insidious disease onset, and mucosal bleeding all had significantly reduced effect sizes, 10 as may be expected from a multivariate model. An important consideration is that these predictors, except for bleeding, are strongly associated with age at presentation (Schmidt et al; medRxiv pre‐print https://doi.org/10.1101/2020.06.09.20125385). Overall, the most important predictor for transient ITP was the absence of an insidious disease onset, which is in line with the very large effect size identified in our systematic review (odds ratio for chronic ITP, 11.3 [95% CI, 6.3‐20.3] 10 ). Of note, the dichotomization of continuous variables may lead to type I errors and produce inflated effect sizes; 16 , 29 this was prevented in our model development by the inclusion of age and platelet count as continuous variables. The effect of age was lower than in previous publications, 10 as we also display in the clinical scenarios, and this may be related to our adjustment for multivariate effects and penalization, as well as the dichotomization of age in previous studies. Overall, our study underscores that the unadjusted effect sizes of some prior studies should be interpreted with caution.
4.3. Clinical implications
A key clinical challenge in childhood ITP is the early identification of a patient's disease course to counsel families; inform treatment decisions; and guide additional diagnostics, eg, screening for systemic autoimmune diseases, immunodeficiencies, or genetic thrombocytopenia. Clinical parameters for our model are readily available and there is no cost involved in obtaining them. We suggest that the Childhood ITP Recovery Score allows the targeting of intensive monitoring or additional diagnostic efforts to children with low probability of recovery. On the other hand, in children with high probability of recovery, the rate for persistent and chronic ITP is low and they could be monitored “hands‐off,” if this is clinically judged to be possible. In addition, in these children with a high probability of recovery, emergency treatment with IVIg, or treatment aiming to resolve thrombocytopenia, is likely to be effective. The clinical utility of the score may be assessed in a clinical impact study. The score may also be used for stratification of additional laboratory testing, eg 1 or 3 months after the diagnosis, which may give further information for the ITP prognosis.
There remains a need for research to investigate the integration of additional clinical data as well as molecular information (eg, anti‐platelet antibodies, T and B cell markers; related to pathogenesis of ITP) to further improve the prediction of disease courses and response to therapy in newly diagnosed ITP.
5. CONCLUSIONS
The Childhood ITP Recovery Score could be clinically useful to differentiate transient versus persistent disease courses in newly diagnosed childhood ITP, using clinical variables that are readily available at diagnosis.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
D. E. Schmidt conceived the study, analyzed and interpreted data, and wrote the manuscript. S. Rosthøj and P. Wendtland Edslev provided data from the Nordic NOPHO ITP Study and discussed the findings. K. M. J. Heitink‐Pollé and M. C. A. Bruin coordinated the TIKI trial, and discussed and interpreted data. R. Kapur, G. Vidarsson and C. E. van der Schoot discussed and interpreted data. B. Mertens supervised statistical analyses. M. de Haas, J. van der Bom, and S. Rosthøj supervised the study. All co‐authors revised and approved the manuscript.
Supporting information
App S1
ACKNOWLEDGMENTS
This work was supported by a research grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR) and a doctoral stipend to DES by the Studienstiftung des Deutschen Volkes.
Schmidt DE, Wendtland Edslev P, Heitink‐Pollé KMJ, et al. A clinical prediction score for transient versus persistent childhood immune thrombocytopenia. J Thromb Haemost. 2021;19:121–130. 10.1111/jth.15125
Manuscript handled by: Katsue Suzuki‐Inoue
Final decision: Katsue Suzuki‐Inoue, 07 October 2020
Contributor Information
David E. Schmidt, @schmidtdav.
Masja de Haas, Email: m.dehaas@sanquin.nl.
REFERENCES
- 1. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. [Google Scholar]
- 2. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780‐3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Am Soc Hematol. 2009;113(26):6511‐6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 5. Rosthøj S, Hedlund‐Treutiger I, Rajantie J, et al. Duration and morbidity of newly diagnosed idiopathic thrombocytopenic purpura in children: a prospective Nordic study of an unselected cohort. J Pediatr. 2003;143(3):302‐307. [DOI] [PubMed] [Google Scholar]
- 6. Imbach P, Kuhne T, Müller D, et al. Childhood ITP: 12 months follow‐up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer. 2006;46(3):351‐356. [DOI] [PubMed] [Google Scholar]
- 7. Heitink‐Pollé KMJ, Uiterwaal CSPM, Porcelijn L, et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood. 2018;132(9):883‐891. [DOI] [PubMed] [Google Scholar]
- 8. Flores A, Klaassen RJ, Buchanan GR, Neunert CE. Patterns and influences in health‐related quality of life in children with immune thrombocytopenia: a study from the Dallas ITP Cohort. Pediatr Blood Cancer. John Wiley & Sons, Ltd. 2017;64(8):e26405. [DOI] [PubMed] [Google Scholar]
- 9. Heitink‐Polle KMJ, Haverman L, Annink KV, Schep SJ, de Haas M, Bruin MCA. Health‐related quality of life in children with newly diagnosed immune thrombocytopenia. Haematologica. 2014;99(9):1525‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heitink‐Pollé KMJ, Nijsten J, Boonacker CWB, de Haas M, Bruin MCA. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta‐analysis. Blood. 2014;124(22):3295‐3307. [DOI] [PubMed] [Google Scholar]
- 11. Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer. 2017;65(1):e26736‐e26737. [DOI] [PubMed] [Google Scholar]
- 12. Lord SJ, Staub LP, Bossuyt PMM, Irwig LM. Target practice: choosing target conditions for test accuracy studies that are relevant to clinical practice. BMJ. 2011;343:d4684. [DOI] [PubMed] [Google Scholar]
- 13. Bossuyt PMM, Reitsma JB, Linnet K, Moons KGM. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58(12):1636‐1643. [DOI] [PubMed] [Google Scholar]
- 14. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edslev PW, Rosthøj S, Treutiger I, et al. A clinical score predicting a brief and uneventful course of newly diagnosed idiopathic thrombocytopenic Purpura in children. Br J Haematol. Blackwell Publishing Ltd. 2007;138(4):513‐516. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donato H, Picón A, Martinez M, et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: a multicentered study from Argentina. Pediatr Blood Cancer. Wiley Subscription Services Inc, A Wiley Company. 2009;52(4):491‐496. [DOI] [PubMed] [Google Scholar]
- 18. Revel‐Vilk S, Yacobovich J, Frank S, et al. Age and duration of bleeding symptoms at diagnosis best predict resolution of childhood immune thrombocytopenia at 3, 6, and 12 months. J Pediatr. 2013;163(5):1335.e1‐2‐1339.e1‐2. [DOI] [PubMed] [Google Scholar]
- 19. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 20. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Am Soc Hematol. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 21. Bennett CM, Rogers ZR, Kinnamon DD, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107(7):2639‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoerl A, Kennard R. Ridge regression: biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55. [Google Scholar]
- 23. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1‐22. [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ. Review and evaluation of penalised regression methods for risk prediction in low‐dimensional data with few events. Heinze G, Michiels S, Posch M, editors. Stat Med. 2016;35(7):1159‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning. New York: Springer Science & Business Media; 2013;1:1. [Google Scholar]
- 26. Lever J, Krzywinski M, Altman N. Model selection and overfitting. Nature Methods. 2016;13(9):703‐704. [Google Scholar]
- 27. Lever J, Krzywinski M, Altman N. Regularization. Nature Methods. 2016;13(10):803‐804. [Google Scholar]
- 28. Goeman J, Meijer R, Chaturvedi N. L1 and L2 Penalized Models [Internet]. 0 ed. CRAN; 2018. https://cran.r‐project.org/web/packages/penalized/vignettes/penalized.pdf. Accessed July 1, 2020.
- 29. Austin PC, Brunner LJ. Inflation of the type I error rate when a continuous confounding variable is categorized in logistic regression analyses. Stat Med. 2004;23(7):1159‐1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


