Abstract
Individuals with fetal alcohol spectrum disorder (FASD) experience remarkably high rates of mental health and substance use challenges, beginning early in life and extending throughout adulthood. Proactive intervention can help to mitigate some of these negative experiences. Although the literature on FASD intervention is growing, there is currently a lack of consolidated evidence on interventions that may improve mental health and substance use outcomes in this population. Informed by a life course perspective, we undertook a systematic review of the literature to identify interventions that improve mental wellness through all developmental stages for people with prenatal alcohol exposure (PAE) and FASD. A total of 33 articles were identified, most of which were focused on building skills or strategies that underlie the well‐being of children with PAE and FASD and their families. Other interventions were geared toward supporting child and family wellness and responding to risk or reducing harm. There was a notable lack of interventions that directly targeted mental health and substance use challenges, and a major gap was also noted in terms of interventions for adolescents and adults. Combined, these studies provide preliminary and emerging evidence for a range of intervention approaches that may support positive outcomes for individuals with FASD across the life course.
Keywords: Fetal Alcohol Spectrum Disorder, Prenatal Alcohol Exposure, Mental Health, Substance Use, Intervention, Life Course
People with prenatal alcohol exposure and Fetal Alcohol Spectrum Disorder (FASD) experience high rates of mental health and substance use challenges. Our systematic review identified 33 studies of interventions for improving mental wellness for this population. Most studies targeted children, and were focused on building skills that underlie well‐being in children and their families. There was a significant lack of interventions for directly reducing mental health and substance use challenges, and a major gap in interventions for adolescents and adults.

1.
Fetal alcohol spectrum disorder (FASD) is a lifelong brain‐ and body‐based disability resulting from prenatal alcohol exposure (PAE), affecting up to 5% of people in North America (Popova et al., 2019; Roozen et al., 2016). Individuals with FASD experience a range of impairments across physical, cognitive, social–emotional, and behavioral functioning and, without adequate or appropriate supports, are at risk of experiencing an array of adverse outcomes throughout the life course (McLachlan et al., 2020; Streissguth et al., 1996, 2004). FASD is a heterogeneous disability and no 2 individuals present with the same pattern of needs, though most will require ongoing support with daily living (Doyle et al., 2019).
1.1. Mental Health and Substance Use in FASD
Mental health problems are one of the most prevalent adverse outcomes associated with FASD (Streissguth et al., 1996), with an estimated 90% of this population experiencing mental health challenges over their lifetime (Pei et al., 2011). These problems are evident even among people with low levels of PAE (Ichikawa et al., 2018) and range from emotional adjustment and attachment issues in infancy to complex psychiatric comorbidities in adulthood (O’Connor & Paley, 2009). Elevated rates of both internalizing and externalizing problems have been reported among people with FASD (Khoury et al., 2018), and common comorbid diagnoses include attention‐deficit/hyperactivity disorder, conduct disorder, oppositional defiant disorder, and psychotic disorders (Fryer et al., 2007; Lange et al., 2018; Pagnin et al., 2019; Patel et al., 2020; Popova et al., 2016; Rasmussen et al., 2010; Weyrauch et al., 2017). Individuals with FASD also experience high rates of suicidality (Landgren et al., 2019; O’Connor et al 2019; Streissguth et al., 1996), which has been linked to comorbid mental health and substance use problems, impaired emotion regulation, history of trauma, financial stress, and a lack of stable social support (Dirks et al., 2019; Huggins et al., 2008; Temple et al., 2019). PAE is also associated with the development and high rates of substance use problems later in life (Dodge et al., 2019; Goldschmidt et al., 2019; McLachlan et al., 2020; Streissguth et al., 1996, 2004; Yates et al., 1998).
The etiological mechanisms that seem to underlie mental health and substance use problems among individuals with FASD are complex and likely involve both biological and environmental factors (O’Connor, 2014). In fact, mental health and substance use disorders often co‐occur in this population (Grant et al., 2004a). Beginning in the earliest stages of life, PAE has been shown to disrupt the developing brain’s stress–response system (Hellemans et al., 2010; McLachlan et al., 2016; Weinberg et al., 2008). This disruption can lead to a biological vulnerability that is believed to influence mental health (Hellemans et al., 2010) and a propensity toward substance use (Chotro et al., 2006). Environmental influences may further compound this biological vulnerability, such as the lifelong adversity experienced among people with FASD (Henry et al. 2007; McLachlan et al., 2020; Price et al., 2017; Streissguth et al., 1996, 2004). Experiences of adversity may contribute to problems with attachment, socio‐emotional functioning, mental health, and substance use (Fagerlund et al., 2011; Koponen et al., 2009; Streissguth et al., 2004).
The way in which mental health is conceptualized has evolved over time, and currently, the World Health Organization (WHO) defines the construct as “more than the absence of mental disorders” (WHO, 2020). Broadly, mental health encompasses biological, psychological, social, environmental (Galderisi et al., 2015), and spiritual factors (Michaelson et al., 2019). Given the multifaceted nature of mental health, there are many potential targets for intervention to support positive outcomes among individuals with FASD. Critically, because of the complex needs and ongoing challenges associated with FASD, interventions to promote mental wellness should be delivered as early and proactively as possible.
1.2. A Life Course Perspective on FASD and Well‐Being
It is important to recognize how trajectories and transitions impact one’s life (Clausen, 1986; Grenier, 2012; Hareven, 1994; Hutchison, 2016). Individual’s lives can be understood through structured pathways and trajectories that shift over time to impact identities and behaviors (Elder and Shanahan, 2007). In the context of FASD, PAE can have detrimental effects on well‐being across the life course (Connor and Streissguth, 1996), beginning with biological and genetic vulnerabilities, and exacerbated by behavioral, social, and other environmental factors that vary across the life course (O’Connor, 2014). On the other hand, fostering stability and nurturing skills and abilities that contribute to mental well‐being through all developmental stages may improve long‐term outcomes for people with FASD. Importantly, individuals with FASD possess strengths and abilities (Flannigan et al., 2018) which can be built upon in various ways throughout the life course to promote healthy outcomes (Pei et al., 2019).
1.3. Current Study
Although it is recognized that responses for individuals with FASD should be coordinated, long‐term, and informed by the neurocognitive vulnerabilities associated with PAE (Mela et al., 2019), there is currently a lack of consolidated information about responses intended to support positive mental health and substance use outcomes in this population. Several review studies have been published in the last decade on FASD interventions more broadly (e.g., Paley & O’Connor, 2011; Peadon et al., 2009; Pei et al., 2016; Petrenko & Alto, 2017; Reid et al., 2015). However, none of these have been focused specifically on mental health or substance use outcomes for individuals with FASD, despite there being a notable gap in this area (Pei et al., 2016). To fill this gap, we conducted a systematic literature review to collate the findings of all existing research related to interventions that improve mental health and substance use outcomes for individuals with PAE/FASD across the life course. The goal was to identify meaningful mechanisms of change for this population and to inform FASD best practice and policy. The study was guided by the following research questions:
What interventions exist that improve mental health or substance use in the PAE/FASD population?
What desired outcomes have been identified for these interventions?
How effective have these interventions been at improving mental health or substance use outcomes for individuals with PAE/FASD?
2. Materials and Methods
To conduct this review, we followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, as delineated by Liberati and colleagues (2009). The study was registered with PROSPERO (CRD42018103951).
2.1. Inclusion and Exclusion Criteria
Studies were considered for review if they were original and peer‐reviewed, contributed empirical data (quantitative, qualitative, or mixed), and included the following: (i) interventions for individuals of any age with PAE or FASD, (ii) with quantitatively or qualitatively reported outcomes related to mental health and/or substance use, and (iii) published in English, from the year 2000 onward. Studies were not excluded based on design or setting but were required to involve some type of investigation of an intervention to be considered. Case studies and case series with outcome measures were included, as were feasibility and pilot studies. Review articles, commentaries, book chapters and reviews, dissertations, unpublished studies, and all other anecdotal or nonscientific writing were excluded.
For the purpose of this review, mental health was conceptualized based on the current WHO definition, and informed by a comprehensive wellness perspective, including emotional, psychological, spiritual, behavioral, and social well‐being. In order to be reviewed, intervention studies were required to report on at least one outcome related to this broad definition of mental health. Specifically, we included studies measuring change in symptoms associated with traditional mental illness or formal psychiatric diagnoses (e.g., externalizing and internalizing behaviors, emotional functioning), as well as other, more tangential indicators of mental health (e.g., attachment, self‐regulation, risk behavior related to substance use).
Studies were excluded if they involved animal subjects or focused on dietary or pharmacological interventions. Studies that were focused on improving cognitive skills, language and communication, academics and literacy, and physical or motor outcomes were only included if they also measured a mental health or substance use outcome. Adaptive skills intervention studies were included only if they involved some social or emotional component (e.g., interventions for social skills were included; safety skills and activities of daily living were not). For studies reporting on attention‐related outcomes, only those with a behavioral component were included (e.g., behavioral measures of distractibility were included; cognitive aspects of attention were not).
2.2. Search Strategy and Study Selection
In August 2018, studies were collected through searching the electronic databases MEDLINE (1946‐Present), PsychINFO (1827‐Present), Web of Science (1975‐Present), and the Cochrane Database of Systematic Reviews. Duplicates were removed within the databases when the option was available. This search strategy was developed in collaboration with a librarian who completed each database search. An identical search was conducted in April 2020 to capture any additional articles published since 2018. We also reviewed the reference lists of included publications for other relevant articles that were not captured by our search strategy. Search terms included keywords related to PAE/FASD; mental health treatment, intervention, and services; and substance‐related disorders and addictions treatment. A full list of subject headings and keywords used are outlined in Appendix 1.
Two authors independently screened and reviewed titles and abstracts of potentially relevant articles (KCH, TA). A third author reviewed the articles deemed relevant by the first 2 reviewers and resolved discrepancies (KF). Full texts were reviewed by 2 of these authors (KCH, KF). Any further discrepancies were resolved through discussions with 2 additional authors (MM, JP).
2.3. Quality Assessment
In order to assess the methodological quality of quantitative studies included in this review, we used the Effective Public Health Practice Project (EPHPP) tool (Thomas et al., 2004). This tool was designed to evaluate research on the effectiveness of public health interventions, with items derived from key components identified in the literature. The EPHPP tool encompasses a variety of research designs, not limited to randomized control trials (RCTs), which was important for the current study because of the relative scarcity of research that exists in the FASD intervention literature. The EPHPP tool has also been used in previous reviews of the FASD intervention literature (e.g., Reid et al., 2015; Symons et al., 2018).
Using the EPHPP tool, researchers are guided through multiple domains of quality assessment, yielding 6 component ratings: selection bias; study design; confounders; blinding; data collection methods; and withdrawals and dropouts. These component ratings are combined to form an overall global rating, categorized as “weak,” “moderate,” or “strong.” Intervention integrity and data analysis are also considered, though not included in the global rating. For the 1 qualitative study included in the current review, we followed the Critical Appraisal Skills Program (CASP) Qualitative Checklist (CASP, 2018), which consists of 3 sections to assess the validity, results, and value of the research.
Two authors (KF and KCH) independently assessed the quality of each study and resolved any disagreements through discussion. The individual component scores for each quantitative study included in the current review are presented in Table 1.
Table 1.
Quality Assessment Ratings for Quantitative Studies
| Study | Selection Bias | Design | Confounders | Blinding | Data Collection | Withdrawals/ Dropouts | Global Rating |
|---|---|---|---|---|---|---|---|
| Early childhood (birth to 5 years) | |||||||
| Zarnegar and colleagues (2016) | Moderate | Moderate | N/A | Weak | Strong | Moderate | Moderate |
| Connolly and colleagues (2016) | Weak | Weak | Weak | Moderate | Strong | N/A | Weak |
| Kartin and colleagues (2002) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Wiskow and colleagues (2018) | Weak | Weak | N/A | Moderate | Strong | N/A | Weak |
| Hanlon‐Dearman and colleagues (2017) | Moderate | Weak | Weak | Moderate | Strong | Weak | Weak |
| Hajal and colleagues (2019) | Weak | Weak | N/A | Moderate | Moderate | N/A | Weak |
| Gurwitch and colleagues (in Bertrand, 2009) | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Middle childhood (6 to 12 years) | |||||||
| Kable and colleagues (2007) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Coles and colleagues (2009) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Kable and colleagues (2012) | Moderate | Strong | Strong | Moderate | Moderate | Strong | Strong |
| Petrenko and colleagues (2017) | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Petrenko and colleagues (2019) | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Coles and colleagues (2015) | Moderate | Strong | Strong | Moderate | Moderate | Moderate | Strong |
| Coles and colleagues (2018) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Kable and colleagues (2016) | Moderate | Strong | Strong | Moderate | Moderate | Strong | Strong |
| Olson and colleagues (in Bertrand, 2009) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Clark and colleagues (2014) | Weak | Strong | Weak | Moderate | Strong | Moderate | Weak |
| Adnams and colleagues (in Riley et al., 2003) | Weak | Strong | Weak | Moderate | Strong | Weak | Weak |
| Wells and colleagues (2012) | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Timler and colleagues (2005) | Weak | Weak | N/A | Weak | Strong | N/A | Weak |
| O'Connor and colleagues (2006) | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Keil and colleagues (2010) | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| O’Connor and colleagues (2012) | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| Soh and colleagues (2015) | Weak | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Nash and colleagues (2015) | Weak | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| Griffin and Copeland (2018) | Weak | Weak | N/A | Weak | Strong | N/A | Weak |
| Reid and colleagues (2020) | Moderate | Weak | N/A | Weak | Strong | Moderate | Weak |
| Middle childhood to adolescence (13 to 18 years) | |||||||
| Katz and colleagues (2020) | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| O’Connor and colleagues (2016) | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Emerging adulthood and beyond (19+ years) | |||||||
| Grant and colleagues (2004) | Moderate | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Denys and colleagues (2011) | Moderate | Weak | Weak | Moderate | Moderate | N/A | Weak |
| Brintnell and colleagues (2019) | Weak | Weak | Weak | Moderate | Weak | Strong | Weak |
2.4. Data Extraction and Synthesis
Data were extracted systematically for each study, using a standardized form which included the location, study design, sample size and characteristics, and FASD diagnostic approach (see Table 2). Intervention details were also compiled, including a description of each intervention’s broad aim, target(s)/mechanism(s) of change, delivery format, outcome measure(s), and key findings (see Table 3).
Table 2.
Study Characteristics
| Author(s) and date | Country | Study design | Sample | Mean age (range) | Diagnostic approach |
|---|---|---|---|---|---|
| Early childhood (birth to 5 years) | |||||
| Zarnegar and colleagues (2016) | United States | Cohort (before–after) | 10 children with FASD experiencing maltreatment or loss, and their adoptive parents (n = 20) | 3 years (1 to 4) | Unspecified |
| Connolly and colleagues (2016) | United States | Case study with data collection mid‐ and postintervention | Female with pFAS and PDD‐NOS | 3 years | Unspecified |
| Kartin and colleagues (2002) | United States | CCT | 78 postpartum mothers within 1 month of birth, 78 of their children with PAE; 53 mother–child dyads in treatment group (23 recruited from hospital, 30 from community) and 25 in control group | Child age = 3 years; client mean age = 28 years (ranges not specified) | None |
| Wiskow and colleagues (2018) | United States | Case study with reversal design | Male with FAS and sensory processing disorder | 4 years | Unspecified |
| Hanlon‐Dearman and colleagues (2017) | Canada | Implementation study with 3‐month follow‐up | 12 children with PAE and their caregivers; 8 in intervention group, 4 in treatment‐as‐usual control group | 4 years (2 to 5) | Canadian guidelines |
| Hajal and colleagues (2019) | United States | Case series | 3 children with known or strongly suspected PAE and their caregivers | 3, 4, and 5 years | 4‐Digit Code |
| Gurwitch and colleagues (in Bertrand, 2009) | United States | RCT | 46 children with FASD and their caregivers; 23 in treatment group; and 23 in caregiver‐only support and management comparison group | 5 years (3 to 7) | Modified IOM criteria |
| Middle childhood (6 to 12 years) | |||||
| Kable and colleagues (2007) | United States | RCT | 56 children with FASD or alcohol‐related facial features, and their caregivers; 29 children in math group, 27 in psychoeducational contrast group | 6 years (3 to 10) | IOM criteria |
| Coles and colleagues (2009) | United States | RCT; 6‐month follow‐up to Kable and colleagues (2007) | 54 children with FASD or alcohol‐related facial features, and their caregivers and teachers; 28 children in math group, 26 in psychoeducational contrast group | 6 years (3 to 10) | IOM criteria |
| Kable and colleagues (2012) | United States | RCT | 59 caregivers of children with FASD or alcohol‐related facial features; 23 in workshop group, 18 in web‐based group, 18 in community comparison group | Differed across groups (6 to 7) | IOM criteria |
| Petrenko and colleagues (2017) | United States | Pilot RCT | 27 children with PAE or FASD, and their primary caregivers; 15 in treatment group, 12 in comparison group who received neuropsychological assessments and community referral | 7 years (4 to 8) | Revised IOM guidelines |
| Petrenko and colleagues (2019) | United States | Pilot RCT; 6‐month follow‐up to Petrenko and colleagues (2017) | 24 children with FASD or PAE, and their primary caregivers; 14 in treatment group, 10 in comparison group who received neuropsychological assessments and community referral | 7 years (4 to 8) | Revised IOM guidelines |
| Coles and colleagues (2015) | United States | Pilot RCT | 30 children with FASD or alcohol‐related facial features; 2 treatment groups—GoFAR program (n = 10) and FACELAND contrast (n = 10)—and control group (n = 10) | 7 years (5 to 10) | IOM criteria |
| Coles and colleagues (2018) | United States | Pilot RCT; additional data from Coles and colleagues (2015) | 30 children with FASD or alcohol‐related facial features; 2 treatment groups—GoFAR program (n = 10) and FACELAND contrast (n = 10)—and control group (n = 10) | 7 years (5 to 10) | IOM criteria |
| Kable and colleagues (2016) | United States | Pilot RCT; additional data from Coles and colleagues (2015) | 28 children with FASD or alcohol‐related facial features; 2 treatment groups—GoFAR program (n = 9) and FACELAND contrast (n = 10)—and control group (n = 9) | 7 years (5 to 10) | IOM criteria |
| Olson and colleagues (in Bertrand, 2009) | United States | RCT | 52 children with FASD and their caregivers; 26 in treatment, 26 in community standard of care group | 8 years (5 to 11) | 4‐Digit Code |
| Clark and colleagues (2014) | Canada | CCT | 12 teachers and 13 students with FASD; 6 teachers and 7 students in intervention group, 6 teachers and 6 students in comparison group | 8 years (6 to 12 years) | Gestalt guidelines |
| Adnams and colleagues (in Riley et al., 2003) | South Africa | Cohort (before–after) | 10 students with FAS; 5 in treatment group, 5 in control group | 8 years (range unspecified) | Unspecified |
| Wells and colleagues (2012) | United States | RCT | 78 children with FASD in out‐of‐home placements; 40 in treatment group, 38 in control group | 8 years in treatment group, 9 years in control (6 to 11) | 4‐Digit Code |
| Timler and colleagues (2005) | United States | Feasibility with case study | Female with FASD | 9 years | 4‐Digit Code |
| O’Connor and colleagues (2006) | United States | CCT with 3‐month follow‐up | 96 children with FASD and measurable social skills deficits; 49 in CFT group, 47 in delayed treatment group | 9 years (6 to 12) | 4‐Digit Code |
| Keil and colleagues (2010) | United States | CCT; additional data from O’Connor and colleagues (2006) | 96 children with FASD and measurable social skills deficits; 49 in CFT group, 47 in delayed treatment group | 9 years (6 to 12) | 4‐Digit Code |
| O’Connor and colleagues (2012) | United States | CCT | 67 children, with and without PAE, seeking community mental health support; 32 in CFT group, 35 in standard of care group | 9 years (6 to 12) | 4‐Digit Code |
| Soh and colleagues (2015) | Canada | CCT | 48 children with and without FASD; 29 with FASD (13 treatment, 16 delayed treatment control) and 19 in typically developing control group | 10 years (8 to 12) | Canadian guidelines |
| Nash and colleagues (2015) | Canada | CCT with 6‐month follow‐up (subset of Soh et al., 2015) | 25 children with FASD; 12 in treatment group, 13 in delayed treatment control group | 10 years (8 to 12) | Canadian guidelines |
| Griffin and Copeland (2018) | United States | Case study with reversal design | Male with ARND, ADHD, ODD, RAD, and LD | 11 years | Unspecified |
| Keightley and colleagues (2018) | Canada | Feasibility with case series | 3 children with FASD and their caregivers (n = 3) and program facilitators (n = 4) | 9, 10, and 14 years | Canadian guidelines |
| Reid and colleagues (2017) | Australia | Feasibility with single‐case experimental design | Families of 2 children with FASD | 9 and 12 years | 4‐Digit Code |
| Middle childhood to adolescence (13 to 18 years) | |||||
| Katz and colleagues (2020) | Canada | Cluster RCT | 113 students with neurodevelopmental disabilities (60 with FASD, 31 with ASD, 22 with ID); 61 in treatment group, 52 in control group | Grade 7 (3 through 12; age not specified) | Unspecified |
| O’Connor and colleagues (2016) | United States | CCT with 3‐month follow‐up | 54 adolescents with FASD; 26 in treatment group, 28 in control group | 17 years (13 to 18) | Revised IOM criteria |
| Emerging adulthood and beyond (19 + years) | |||||
| Grant and colleagues (2004b) | United States | Cohort (before–after) nested pilot study | 39 females with diagnosed (n = 11) or suspected (n = 8) FASD | 22 years (14 to 36) | Unspecified |
| Denys and colleagues (2011) | Canada | Retrospective file review | 24 females with diagnosed (n = 12) or suspected (n = 12) FASD | 30 years (19 to 47) | Unspecified |
| Brintnell and colleagues (2019) | Canada | Exploratory study with 3‐ and 6‐month follow‐up | 49 incarcerated males with suspected FASD | 30 years (19 to 50) | Canadian guidelines |
Table 3.
Intervention Details and Key Findings
| Author(s) | Broad aim | Target/mechanism of change | Format | Outcome measures | Key findings |
|---|---|---|---|---|---|
| Early childhood (birth to 5 years) | |||||
| Zarnegar and colleagues (2016) | Improve child developmental outcomes |
Early psychosocial intervention based on the Neurosequential Model of Therapeutics model (NMT), integrating biological, psychological, social, learning, and problem‐solving development, and involving:
|
6 months; twice‐weekly CPP (joint child‐caregiver) and weekly MPE (caregiver only) sessions (length unspecified) |
Child
|
|
| Connolly and colleagues (2016) | Improve adaptive behavior and functional communication skills | Verbal Behavior intervention, a one‐on‐one applied‐behavior analysis‐based day treatment involving an assessment of strengths and challenges, and targeted at improving functional communication and verbal behavior skills | 23 months; 15 hours/week in therapy setting and natural settings (frequency unspecified) |
Child
|
|
| Kartin and colleagues (2002) | Substance use recovery and reduce associated challenges; improve child outcomes | Seattle Birth to 3 program (now known as PCAP), a paraprofessional home visitation advocacy and mentorship intervention for postpartum women, based on relational theory and focused on connecting women with services to reduce challenges associated with substance use | 3 years; weekly visits for 6 weeks, followed by minimum twice monthly visits (length unspecified) |
Child
|
|
| Wiskow and colleagues (2018) | Improve behavior | Good Behavior Game, a summer group program involving therapist‐delivered praise, corrective feedback, and positive reinforcement, with rewards for rule following and appropriate behavior | 3 weeks; 2.5 hours/day, game sessions administered 1‐4 times per day |
Child
|
|
| Hanlon‐Dearman and colleagues (2017) | Promote child‐caregiver attachment | Circle of Security, a community home‐based intervention targeted at improving caregiver emotion regulation through increasing observation skills, sensitivity and responsiveness, and self‐reflection | 9 months; 36 sessions (joint child‐caregiver; frequency and length unspecified) |
Child
|
|
| Hajal and colleagues (2019) | Promote school readiness |
Strategies for Enhancing Early Developmental Success (SEEDS), a manualized preschool program involving:
|
14 weeks; twice‐weekly 3‐hour sessions (combination of child‐only, caregiver only, and joint activities) |
Child
|
|
| Gurwitch and colleagues (in Bertrand, 2009) | Reduce child behavior problems and caregiver stress | Parent‐Child Interaction Therapy, involving caregiver training on behavioral parenting skills to enhance child–caregiver relationship, increase social skills, decrease negative behavior, and implement positive discipline | 14 weeks; weekly 90‐minute sessions (joint child–caregiver) |
Child
|
|
| Middle childhood (6 to 12 years) | |||||
| Kable and colleagues (2007) | Improve math skills and behavioral functioning |
Math Interactive Learning Environment, a socio‐cognitive habilitation intervention involving:
|
Two 2‐hour caregiver workshops, followed by 6 weeks of tutoring services (child‐only) with concurrent weekly caregiver instruction (session length unspecified) |
Child
|
|
| Coles and colleagues (2009) | Six‐month follow‐up to Kable and colleagues (2007) | See Kable and colleagues (2007) | See Kable and colleagues (2007) |
Child
|
|
| Kable and colleagues (2012) | Improve behavior | Caregiver training focused on increasing knowledge about the neurodevelopmental consequences of PAE, strategies to provide behavioral supports, and improving capacity and advocacy skills | Two 2‐hour in‐person caregiver workshops, or web‐based translation of the same material (length and frequency unspecified) |
Child
|
|
| Petrenko and colleagues (2017) | Improve family adaptation and reduce risk for adverse outcomes |
Families on Track , a preventative in‐home intervention for reducing risk factors and promoting protective factors, and involving:
|
30 weeks; weekly 90‐minute child groups, biweekly 90‐minute caregiver visits |
Child
Caregiver
|
|
| Petrenko and colleagues (2019) | Six‐month follow‐up to Petrenko and colleagues (2017) | See Petrenko and colleagues (2017) | See Petrenko and colleagues (2017) |
Child
|
|
| Coles and colleagues (2015) | Improve self‐regulation and adaptive living skills |
GoFAR self‐regulation intervention involving:
|
5 weeks; weekly 1‐hour concurrent but separate child and caregiver sessions, followed by 5 weeks of weekly BAT (joint child‐caregiver) |
Child
|
|
| Coles and colleagues (2018) | Additional data from Coles and colleagues (2015) | See Coles and colleagues (2015) | See Coles and colleagues (2015) |
Child
Caregiver
|
|
| Kable and colleagues (2016) | Additional data from Coles and colleagues (2015) | See Coles and colleagues (2015) | See Coles and colleagues (2015) |
Child
Caregiver
|
|
| Olson and colleagues (in Bertrand, 2009) | Improve caregiver efficacy; meet family needs; reduce problematic child behaviors | Families Moving Forward, a behavioral consultation intervention based on social learning theory and intended to modify caregiver attitudes and responses, emphasizing positive behavior support and increasing caregiver capacity to advocate for their child | 9‐11 months; 16 biweekly 90‐minute sessions |
Child
Caregiver
|
|
| Clark and colleagues (2014) | Improve student behavior and opportunities for learning | Professional development program for teachers, including an overview of FASD, and training on how to use and organize information from student records to identify accommodations based on student strengths | One school year; 2 full‐day and 4 half‐day workshops, along with weekly meetings between teacher and mentor |
Child
Teacher
|
|
| Adnams and colleagues (in Riley et al., 2003) | Improve self‐observation and self‐regulation | Cognitive Control Therapy, a classroom group intervention targeted at rehabilitation of metacognitive control, focused attention, resisting distraction, information processing, and improving body and self‐awareness | 10 months; weekly 1‐hour sessions |
Child
|
|
| Wells and colleagues (2012) | Improve self‐regulation |
Neurocognitive habilitation intervention, an adaptation of the Alert Program, involving:
|
12 weeks; weekly 75‐minute sessions (first separate child and caregiver, then joint) |
Child
|
|
| Timler and colleagues (2005) | Improve social communication | Social communication intervention targeted at improving mental state verb use and social cognitive skills | 6 weeks; 2 weeks of individual sessions (1 hour, 2X/week), followed by 4 weeks of group sessions (2 hours, 3X/week) |
Child
|
|
| O’Connor and colleagues (2006) | Improve social skills |
Children’s Friendship Training (CFT), a parent‐assisted intervention based on social learning theory and involving:
Intervention was modified to account for neurocognitive difficulties |
12 weeks; weekly 90‐minute concurrent but separate child and caregiver sessions |
Child
|
|
| Keil and colleagues (2010) | Improve social skills | Additional data from O’Connor and colleagues (2006) | See O’Connor and colleagues (2006) |
Child
|
|
| O’Connor and colleagues (2012) | Improve social skills | Community mental health‐based implementation of Children’s Friendship Training (see O’Connor et al., 2006) | 12 weeks; weekly 90‐minute concurrent but separate child and caregiver sessions |
Child
Caregiver and therapist
|
|
| Soh and colleagues(2015) | Improve self‐regulation | Alert Program, an intervention focused on teaching children how to identify and manage their level of arousal through mind–body awareness, metacognitive strategies, and sensory coping strategies; no caregiver component in this study | 12 weeks; weekly 1‐hour sessions (child‐only) |
Child
|
|
| Nash and colleagues (2015) | Subset of Soh and colleagues(2015) | See Soh and colleagues (2015) | See Soh and colleagues (2015) |
Child
|
|
| Griffin and Copeland (2018) | Improve behavior | In‐home behavioral intervention informed by a functional behavioral assessment, and involving explicit teaching of self‐monitoring strategies and use of contingent reinforcement | 20 sessions (frequency and length unspecified) |
Child
|
|
| Keightley and colleagues (2018) | Facilitate social communication and engagement | Intensive theater‐based skills training group intervention emphasizing collaboration, awareness of self and others, relaxation, listening, and curiosity and imagination | 4 weeks; 4‐hour daily sessions 5 days/week |
Youth
Youth, caregivers, and facilitators
|
|
| Reid and colleagues (2017) | Improve the child–caregiver relationship | Parents Under Pressure, a home‐based individualized program focused on improving the child–caregiver relationship and teaching mindfulness and self‐regulation strategies within the ecological context of the family | 27 weeks; weekly or biweekly 1‐ to 2‐hour sessions (child‐only, caregiver‐only activities in each session) |
Child
Caregiver
|
|
| Middle childhood to adolescence (13 to 18 years) | |||||
| Katz and colleagues (2020) | Increase protective factors related to resilience |
School‐based mental health program delivered by teachers, including:
|
13 lessons throughout the school year (length unspecified) |
Child
|
|
| O’Connor and colleagues (2016) | Prevent/reduce alcohol‐related harms |
Project Step Up intervention to reduce alcohol use, involving:
Intervention was modified to account for neurocognitive difficulties |
6 weeks; weekly 60‐minute concurrent but separate youth and caregiver sessions |
Youth
Youth and caregiver
|
|
| Emerging adulthood and beyond (19 + years) | |||||
| Grant and colleagues. (2004b) | Reduce risk for substance use and alcohol‐exposed pregnancies |
Community‐based mentorship intervention (based on PCAP), focused on addressing environment challenges, facilitating client connection with community resources, and supporting health and safety through relationship‐based case management and mentorship, and education and collaboration with service providers Intervention was modified for clients with FASD |
12 months of visits (frequency and length unspecified) |
Client
|
|
| Denys and colleagues (2011) | Stabilize mothers and strengthen connection to community |
Step by Step mentorship program (based on PCAP), a strengths‐based and individualized goal‐oriented intervention focused on connecting clients with their communities and accessing resources to ensure the health, safety, and overall well‐being of women and their children Intervention was modified for clients with FASD |
3 years of visits (frequency and length unspecified) |
Client
|
|
| Brintnell and colleagues (2019) | Promote overall health and well‐being | Mind, Body, and Spirit group therapy program, focused on improving communication, interpersonal skills, personal strengths, and physical health (i.e., hygiene and sexuality); exploring cultural manifestations of spirituality; and incorporating a regular exercise program; concurrent individual meetings with Transitional Advocates | 10 weeks; 4 days/week (length unspecified) |
Client
|
|
ADHD, attention‐deficit/hyperactivity disorder; ARND, alcohol‐related neurodevelopmental disorder; ASD, autism spectrum disorder; ASI‐5, Addictions Severity Index—5th Ed.; AUDIT, Alcohol Use Disorders Identification Test; BASC‐2, Behavior Assessment Scale for Children—2nd Ed.; BDI‐2, Battelle Developmental Inventory—2nd Ed.; BPI, Berkeley Puppet Interview; BRIEF, Behavior Rating Inventory of Executive Functioning; BSID‐II, Bayley Scales of Infant Development—2nd Ed.; CANTAB, Cambridge Neuropsychological Test Automated Battery; CBCL, Child Behavior Checklist; CBM, Curriculum‐Based Measure; CBQ, Child Behavior Questionnaire; CCT, Controlled Clinical Trial; CRAFFT, 6‐item tool to rate alcohol use; CSQ, Client Satisfaction Questionnaire; DAS‐2, Differential Abilities Scale—2nd Ed.; DASS, Depression Anxiety Stress Scale; DBRF, Disruptive Behavior Record Form; DPICS‐II, Dyadic Parent‐Child Interaction Coding System‐II; ECBI, Eyberg Child Behavior Inventory; ERC, Emotion Regulation Checklist; GPSMHY, Global Portrait of Social and Moral Health for Youth; HOMES, Hull Outcome Monitoring Evaluation System; ID, Intellectual Disability; IRS, Impairment Rating Scale; K&A, Knowledge and Advocacy Scale; LD, learning disability; MRI, magnetic resonance imaging; NMT, Neurosequential Model of Therapeutics; ODD, oppositional defiant disorder; PCAP, Parent‐Child Assistance Program; PDD‐NOS, Pervasive Developmental Disorder—Not Otherwise Specified; PEI‐FOT, Parent Evaluation Inventory—FOT; pFAS, partial fetal alcohol syndrome; PPI, Parenting Practices Interview; PSI‐3, Parenting Stress Index—3rd Ed.; PSI‐4‐SF, Parenting Stress Index—4th Ed. Short Form PSI‐SF, Parenting Stress Index—Short Form; PSOC, Parenting Sense of Competence; PSSS, Perceived Social Support Scale; RAD, reactive attachment disorder; RAPI, Rutgers Alcohol Problem Index; RATC, Roberts Apperception Test for Children; RCT, randomized control trial; RI, Resilience Inventory; SBSH, Secure Based‐Safe Haven; SCC, Sense of Classroom as a Community Scale; SDQ, Strengths and Difficulties Questionnaire; SDQ‐GS, Self‐Description Questionnaire—General Subscale; SOS, Student Observation System; SSIS, Social Skills Improvement System; SSRS, Social Skills Rating System; TEA‐Ch, Test of Everyday Attention for Children; TOVA, Test of Variables of Attention; TRF, Teacher Report Form; TRS, Teacher Rating Scale; TSC, Test of Social Cognition; TSSK, Test of Social Skills Knowledge; VABS‐2, Vineland Adaptive Behavior Scale—2nd Ed.
Because of the exploratory nature of this review and the heterogeneity of studies identified, our data synthesis was largely descriptive and narrative. Interventions were first organized developmentally (see Table 3) and then further synthesized into broad categories based on the intervention’s mechanism(s) of change (see Table 4). In cases where an intervention comprised multiple components, we relied on the overarching aim of the intervention to determine categorization. Categories were as follows: (i) supporting attachment and family wellness; (ii) building skills and strategies; and (iii) responding to risk and reducing harm. Generally, interventions with a caregiver component were considered in the attachment and family category, as the primary mechanism of change was to improve family wellness. However, in some interventions, caregiver components were a secondary focus intended to support skill‐building with the child, which was the primary mechanism of change, and these interventions were therefore considered in the skills and strategies category. Although some interventions in the risk and harm category also included elements of skill‐building and family support, the primary aims of these interventions were to stabilize individuals and reduce risk or harm.
Table 4.
Intervention Categories and Mechanisms of Change Across the Life Course
| Category | Early childhood (birth to 5 years) | Middle childhood (6 to 12 years) | Middle Childhood to Adolescence (13 to 18 years) | Emerging Adulthood and Beyond (19 + years) |
|---|---|---|---|---|
| Supporting attachment and family wellness |
|
|
No studies identified | No studies identified |
| Building skills and strategies |
Behavior |
Self‐regulation
Behavior
Social skills
|
Mental health literacy
|
No studies identified |
| Responding to risk and reducing harm | No studies identified | No studies identified |
|
|
3. Results
3.1. Study Selection and Characteristics
Our searches yielded a total of 10,129 articles (8,877 in 2018 and an additional 1,252 in 2020), 1,882 of which were duplicates and 641 of which were deemed relevant through preliminary screening. Of these screened titles, 206 abstracts and 59 full‐text articles were reviewed. The final number of articles included in this study was 33. See Figure 1 for a PRISMA flowchart of study selection.
Figure 1.
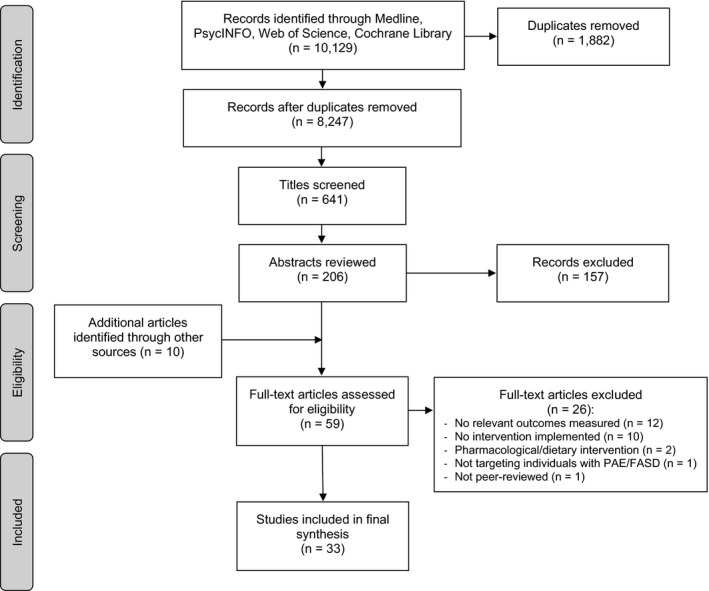
PRISMA flowchart of study selection.
Most studies were RCTs (n = 12) and controlled clinical trials (CCTs; n = 8); 4 were case studies, 3 were case series, 3 were cohort (before and after) studies, one was a file review, one was an implementation study, and one was an exploratory study. The most common age group of participants was middle childhood, aged 6 to 12 years (n = 21). Seven studies included participants with a mean age in early childhood (birth to 5 years), 2 studies included participants in middle childhood to adolescence (13 to 18 years), and 3 studies included participants in emerging adulthood and beyond (19 + years).
Twenty‐three studies were conducted in the United States, 8 were from Canada, one was from South Africa, and one was from Australia. The FASD diagnostic approach used in studies was most often the Institute of Medicine (IOM) guidelines (n = 10), followed by the University of Washington’s 4‐Digit Code system (n = 8), the 2005 Canadian guidelines (n = 5), and the Gestalt method (n = 1). Diagnostic approach was unspecified or absent in 9 studies. See Table 2 for a complete description of study characteristics.
3.2. Intervention Characteristics
Within the early childhood age group, most interventions involved supporting attachment and family wellness as the mechanisms of change (n = 9), and all involved caregiver participation as a critical component of the intervention. For middle childhood and early adolescence, interventions primarily involved building skills and strategies as a mechanism of change to support behavioral, social, or emotional functioning. In this category, the most common targets for change were self‐regulation (n = 7), social skills (n = 6), and behavioral skills (n = 5). Only one intervention was designed to teach mental health literacy skills more holistically. For later adolescence, emerging adulthood, and adulthood, there were 5 studies identified, categorized as responding to risk and reducing harm, most often related to substance use (n = 4) and one intervention administered in a correctional setting. Interestingly, across studies, very few mechanisms of change were specifically targeted at improving mental health per se. Rather, more commonly, the measured outcomes of the interventions were operationalized in terms of mental health functioning. See Tables 3 and 4 for more intervention details.
3.3. Quality Assessment
All but one study identified in this review were primarily quantitative, and their overall global EPHPP quality assessment ratings were roughly evenly distributed, with 12 studies assessed as having weak methodological rigor, 10 as moderate, and 10 as strong (see Table 1). The 1 qualitative study, assessed using the CASP Qualitative Checklist, was determined to have adequate validity, results, and research value.
3.4. Summary and Synthesis
3.4.1. Supporting Attachment and Family Wellness
We identified 9 interventions that were targeted toward supporting early child development, attachment, and positive child–caregiver interactions within the family context. All of these studies included caregiver components (with 1 study including only caregivers), many were based in the home, and the target children were all in early or middle childhood. Zarnegar and colleagues (2016) tested a psychosocial intervention program for infants and young children with PAE and their caregivers, focused on attachment and caregiver mindfulness. They reported notable positive impacts of the program on child developmental and regulatory skills, as well as a reduction in caregiver stress, when measured pre‐ to posttreatment. Parents Under Pressure was another intervention integrating mindfulness as a means to improve the child–caregiver relationship (Reid et al., 2017). Findings from this study were mixed for the 2 families completing the intervention. However, pre‐ to posttreatment data indicated that this training led to some significant improvements in child distress and behavior. Caregivers perceived the program to increase their understanding of themselves and their children, enhance feelings of support, and improve overall family functioning.
Hanlon‐Dearman and colleagues (2017) examined the Circle of Security intervention program with preschool‐aged children with PAE and their caregivers. They found clinically significant pre‐ to posttreatment improvements in attachment quality for the majority of children receiving the intervention, as well as reductions in caregiver stress that were maintained at 3‐month follow‐up. In another attachment‐based intervention, Hajal and colleagues (2019) implemented the Strategies for Enhancing Early Developmental Success (SEEDS) preschool program for children with PAE and their caregivers, rooted in attachment and positive child–caregiver interactions. Descriptive findings for 3 cases were mixed, with positive and clinically significant pre‐ to posttreatment impacts for 2 children, but inconsistent impacts for the third. Gurwitch and colleagues (as cited in Bertrand, 2009) implemented another intervention focused on supporting the child–caregiver relationship, Parent‐Child Interaction Therapy, and reported significant improvements throughout the course of treatment in terms of both child behavior and caregiver stress. However, these improvements were not significantly greater than those found in a control group.
We identified a number of interventions that involved in‐home consultation for caregivers and their children with PAE/FASD. Two studies were published on the Families on Track program, which is focused on family interactions and experiences and includes group treatment to support the child’s social competence (Petrenko et al., 2017, 2019). This intervention was received with high satisfaction and led to significant pre‐ to posttreatment gains in caregiver knowledge of FASD and advocacy, family needs being better met, and improvements in caregiver‐reported child emotion regulation in the treatment group compared to families who did not receive the intervention (Petrenko et al., 2017). In both groups, children showed significant pre‐ to posttreatment improvements in disruptive behavior and negative affect (no group differences). Gains in caregiver knowledge were maintained at 6‐month follow‐up in both groups (Petrenko et al., 2019). Those who completed the intervention also had a greater sense of efficacy and family needs were better met at follow‐up than those in the comparison group, although both groups reported an overall decline in needs met (Petrenko et al., 2019). Interestingly, caregivers in the comparison group reported significant improvements in parenting satisfaction at follow‐up, but those in the treatment group did not. Although all children showed continued improvements in disruptive behavior and negative affect at follow‐up (no group differences), they also all showed significantly worsening self‐esteem across time points, and the treatment group experienced diminishing gains in emotion regulation whereas the comparison group showed significant increases (Petrenko et al., 2019).
In the Families Moving Forward program, the in‐home consultation intervention was intended to modify caregiver attitudes and responses, and increase child advocacy (Olson et al., as cited in Bertrand, 2009). Significant pre‐ to posttreatment improvements were reported in the treatment group but not the control group for child behavior problems as well as caregiver self‐care, sense of competence, family needs met, and caregiver satisfaction, though caregiver stress was comparable across groups.
To test whether the delivery format of caregiver training influences caregiver knowledge or child behavior outcomes, Kable and colleagues (2012) compared the impacts of in‐person workshop versus web delivery. Caregivers in both the workshop and web‐based groups made significant pre‐ to posttraining gains in advocacy skills and knowledge of behavior regulation, whereas the community comparison group showed significant knowledge gains in behavior regulation only. Caregiver satisfaction was highest in the workshop group, and only caregivers in the workshop group reported significant behavioral change in their children, indicating that the effectiveness of caregiver training may be specific to the context in which it is implemented.
3.4.2. Building Skills and Strategies
Our search yielded 19 studies focused on developing skills or strategies related to emotional, social, and behavioral functioning as a mechanism to improve mental health outcomes for individuals with PAE/FASD. Almost all of the participants with PAE/FASD in these studies were in their middle childhood years, though 2 case studies were reported on younger children, and 2 studies also included adolescents. Nearly half (n = 9) of these interventions involved caregiver or teacher training to complement the direct instruction provided to children and adolescents. Most of the studies were based on standardized interventions established as effective in other populations, implemented with adaptations to account for the neurocognitive deficits associated with FASD.
3.4.2.1. Self‐regulation
In one of the first attempts to examine the impacts of a self‐regulation intervention for children with FASD, Adnams and colleagues (in Riley et al., 2003) delivered Cognitive Control Therapy in a classroom setting to a small group of children, designed to teach metacognitive strategies and ultimately improve self‐observation and self‐regulation. The researchers found that after 10 months of weekly sessions, children in the treatment group but not the control group showed significant pre‐ to posttreatment improvements in classroom behavior, though no group differences in neuropsychological outcomes were reported.
The GoFAR program is another self‐regulation intervention designed to build metacognitive strategies in children through computer game learning and caregiver training. In the 3 studies reporting data from this intervention, researchers compared outcomes of a small group of children who completed the GoFAR program with a contrast group who received a modified treatment (differing only in terms of the computer game content, but similar caregiver training components) and a nontreatment control group. Results indicated that both the GoFAR and contrast treatment groups showed significant pre‐ to posttreatment reductions in disruptive behavior (Coles et al., 2015), improvements in adaptive skills, and decreased negative affect (Coles et al., 2018) compared to the control group. Compared to the contrast and control groups, children in the GoFAR group showed significantly greater pre‐ to posttreatment improvements in attention regulation (Coles et al., 2018), sustained mental effort, and reductions in frustration levels (Kable et al., 2016). With respect to the GoFAR caregiver component, caregivers across studies who received the training were highly satisfied (Coles et al., 2018; Kable et al., 2016), and caregiver engagement was significantly related to improvements in child regulatory abilities (Kable et al., 2016).
Three additional self‐regulation intervention studies were identified in which researchers examined the effectiveness of the Alert Program (Williams and Shellenberger, 1996) for children with PAE/FASD, which is designed to improve self‐regulatory skills through “neurocognitive habilitation.” In the first study, researchers implemented an adaptation of the Alert Program with children with FASD and reported significant pre‐ to posttreatment improvements in both caregiver‐reported executive functioning and emotional problem solving compared to a control group (Wells et al., 2012). Similar positive outcomes were reported by Nash and colleagues (2015), who noted significant pre‐ to posttreatment improvements in inhibition as well as affect and behavior regulation among children with FASD who received the intervention compared to a control group. Soh and colleagues (2015) provided additional evidence for the positive impacts of the Alert Program on emotion regulation and inhibition, as well as data suggesting gray matter changes in brain areas critical to self‐regulation in children who received the intervention, but not in those who had not.
3.4.2.2. Behavioral Skills
Five studies were identified where children with PAE/FASD were taught skills and strategies to improve behavioral functioning. Three of these were case studies (Connolly et al., 2016; Griffin and Copeland, 2018; Wiskow et al., 2018). in which behavioral interventions were delivered in various settings to children with FASD (ranging in age from 3 to 11 years), and findings were predominantly positive. In Connolly and colleagues’ (2016) study, a long‐term, intensive, and multidisciplinary applied‐behavior analysis‐based treatment led to posttreatment improvements in functional communication, adaptive behavior, and emotional–behavioral functioning for a young girl with FASD. Griffin and Copeland (2018) reported on an in‐home behavior therapy delivered to an 11‐year‐oldboy with FASD and his caregiver, which resulted in increased task completion and reduced argumentativeness over the course of treatment. Lastly, a summer program involving a game‐like behavioral treatment in a small‐group setting led to substantial reductions in disruptive behavior from baseline to completion for a preschool‐aged boy with fetal alcohol syndrome (Wiskow et al., 2018).
Two, more rigorous, RCT studies provided outcome data from the socio‐cognitive Math Interactive Learning Environment (MILE) intervention. In the first study, Kable and colleagues (2007) reported on immediate outcomes of the intervention, noting that children in the intervention group showed gains in math skills that were significantly greater than those seen in a psychoeducational contrast group. Caregivers in both groups experienced high levels of treatment satisfaction, and significant pre‐ to posttreatment improvements in their knowledge of caregiving advocacy and behavioral regulation principles. Caregivers also reported significant pre‐ to posttreatment reductions in child problem behaviors, though these were not significantly greater in the MILE group than those in the contrast group. The lack of group differences in caregiver knowledge and child behavioral gains suggests that the math tutoring itself may not have been as influential on behavioral outcomes as was the caregiver training workshops, delivered to both groups. Coles and colleagues (2009) conducted a follow‐up study of the same sample of families and reported that treatment impacts were maintained at 6 months postintervention. For all children, reductions in problem behavior extended to the classroom, with significant teacher‐reported behavior improvements from pretest to 6 months posttreatment. Again, there were significantly greater gains in the intervention group than the contrast group for math skills, but not for behavioral outcomes or caregiver knowledge.
Adding to the evidence that external support may play a key role in improving outcomes for children with PAE/FASD, researchers in 1 small study reported that a teacher professional development intervention that was designed to improve student behavior and learning had positive impacts on teaching practice, and led to significant gains in student adaptive behavior and reductions in classroom problem behaviors, none of which was apparent in a comparison group (Clark et al., 2014).
3.4.2.3. Social Skills
Five studies were identified that focused on building social skills to improve well‐being among children with PAE/FASD. In 3 of these studies, researchers examined the impacts of the Children’s Friendship Training (CFT) intervention, with relatively large samples of children with FASD. These studies provided evidence that CFT, modified to account for neurocognitive deficits among school‐aged children with FASD, can significantly improve social skills knowledge, as well as reduce hostile attributions (Keil et al., 2010) and caregiver‐reported problem behaviors (O’Connor et al., 2006), compared to a control group. However, the CFT intervention was not shown to impact teacher perceptions of social skills (O’Connor et al., 2006), suggesting that learned skills may not always transfer across settings. In a translation of the CFT intervention to a community mental health setting, O’Connor and colleagues (2012) tested its effectiveness among children both with and without PAE and reported significantly improved social skills knowledge, self‐concept, and some caregiver‐reported social skills for all children who completed the intervention compared to those who did not. Children with PAE benefited from the intervention as much as children without PAE, suggesting that some children with PAE may be successfully integrated into widely offered community treatment programs that take into consideration their unique neurocognitive challenges. Positive feedback from program facilitators indicated the acceptability of this intervention in community settings (O’Connor et al., 2012).
Two additional social skills interventions were identified, both more limited in size and scope. Timler and colleagues (2005) presented a feasibility case study from a social communication intervention with a 9‐year‐old girl with FASD and reported improvements in her ability to generate social strategies, but no change in her ability to take the perspectives of others. More recently, Keightley and colleagues (2018) piloted an innovative theater‐based intervention with a small group of children and youth with FASD, their caregivers, and intervention facilitators. Qualitative data captured in this study on feasibility, usability, acceptability, and perceived outcomes revealed that the intervention was perceived to improve self‐confidence, social interaction and communication, and emotional awareness among participants. Although these 2 studies represent a small number of people with FASD, they offer preliminary evidence on potential targets for intervention to promote skills important for social well‐being.
3.4.2.4. Mental Health Literacy
In the final intervention in the skill‐building category, researchers tested a comprehensive school‐based holistic mental health program to promote resiliency among students through building mental health literacy and teaching dialectical behavior therapy (DBT) strategies (Katz et al., 2020). Participants included a large group of students with various neurodevelopmental disabilities. The researchers did not single out FASD in their analysis of outcomes, but the intervention led to significant improvements in self‐concept, coping skills, and social support for all students receiving the intervention compared to those in the control group, and these gains were maintained throughout the school year.
3.4.3. Responding to Risk and Reducing Harm
In the third category of studies (n = 5), interventions were targeted at responding supportively to high‐risk behaviors and reducing related harms as a means to improving mental wellness. Three of these studies were related to substance use and related challenges within a larger context of mentorship, advocacy, and access to resources. One study was an intervention to reduce alcohol use and related risks among youth with FASD. The last study was a holistic mental health intervention for adults with FASD who were incarcerated.
3.4.3.1. Substance Use
Three of the interventions in this category were based on the Parent‐Child Assistance Program (PCAP) model, a home visitation mentorship and advocacy program for women, designed to reduce the risk of alcohol‐exposed pregnancies (Ernst et al., 1999; Grant et al., 1999). The first of these studies was conducted with women with substance use challenges who recently gave birth to alcohol‐exposed infants (Kartin et al., 2002). The intervention was focused on connecting clients with services to reduce substance use and associated challenges, and after 3 years, the researchers measured child developmental outcomes. No significant differences were found between children of mothers who completed the program and those who did not.
In a community pilot study, Grant and colleagues (2004b) implemented PCAP for the first time with modifications for clients with FASD, and reported a range of positive impacts. These impacts included increases in mental and physical health needs being met, use of reliable contraception, and attainment of adequate housing, as well as a decrease in substance use. Additional evidence for the positive impacts of supportive mentorship for women with FASD was provided by Denys and colleagues (2011), who conducted a retrospective record review of clients who had completed the Step by Step program, another modified version of PCAP for clients with FASD. Clients who partook in this program experienced a significant pre‐ to postprogram reduction in self‐reported needs ranging from experiences of abuse and problems with substance use to community access and housing. This decrease in needs was significantly greater for those who had a formal diagnosis rather than those with suspected FASD. Moreover, clients experienced a significant increase in their achievement of personal goals. Although these interventions were wide‐ranging in scope, they had a specific positive impact on issues related to substance use for women with PAE/FASD.
Only 1 study specifically targeted substance use and associated harms in the FASD population, and it was limited to adolescents, most of whom (67%) were abstinent or consumed alcohol infrequently (O’Connor et al., 2016). The Project Step Up intervention (modified to account for neurocognitive deficits associated with FASD) was implemented to youth, along with concurrent, but separate, caregiver sessions. The intervention was reported to have no impact on abstinent/infrequent drinkers. However, in light/moderate drinkers, treatment was associated with significantly lower levels of self‐reported alcohol‐related risk behaviors posttreatment compared to those in the comparison group, with some of these gains maintained at 3‐month follow‐up.
3.4.3.2. Justice Involvement
As part of a larger study aimed at developing services and supports for individuals with FASD involved with the justice system, Brintnell and colleagues (2019) delivered the holistic Mind, Body, Spirit (MBS) program to incarcerated adult males with FASD. Although no analyses were conducted to examine the independent impacts of the MBS intervention, participants perceived that the overall program improved their self‐esteem and insight, emotional functioning, coping skills, relationships, and appreciation for the benefits of exercise.
4. Discussion
The goal of the current review was to consolidate the available evidence on interventions aimed at improving mental health and substance use outcomes for individuals with PAE/FASD. Mental health was conceptualized broadly in this review and comprised emotional, psychological, spiritual, behavioral, and social well‐being. Much of the current FASD intervention literature is focused on improving functioning, and our goal was to explore this broad work to tease apart specific outcomes associated with mental health. Additional objectives were to identify gaps and inform best practice and policy change in order to support lifelong well‐being for individuals with PAE/FASD. This study offers a valuable contribution to the literature by identifying promising areas for promoting mental wellness among individuals with PAE/FASD, particularly in terms of the mechanisms that may underlie and impact mental wellness, and how these mechanisms may evolve across the life course. We found favorable and emerging evidence for interventions to support attachment and family wellness, build skills and strategies, and respond to risk and reduce harm. Combined, these approaches may reflect the components critical to integrated and interdependent care planning for individuals with PAE/FASD across the life course.
Broadly, the findings from this review revealed that interventions to support mental wellness for people with PAE/FASD may be beneficial in different ways across the life course, through both preventative and responsive approaches. Preliminary work that explored attachment and family‐based interventions was original and specifically designed for the PAE/FASD child–caregiver dyad and may be particularly (although not necessarily exclusively) impactful in early childhood. These interventions had positive impacts on attachment and child adjustment, including improved relationships, enhanced caregiving experiences, and increased family functioning. The positive results of these studies support a preventative model in which better bonding may aid the developmental process in the child and diminish the risk of adversity. Beyond early childhood, intervention efforts were responsive to the changing functional needs of individuals with PAE/FASD and their families. Acquiring skills and strategies to support optimal functioning took precedence in middle childhood. In later adolescence, emerging adulthood, and adulthood, as needs may become more complex, interventions shifted to a more responsive approach to mitigate risk and reduce harm.
Adopting a developmental approach to intervention allows for flexibility at each life stage for individuals with FASD, where there are multiple opportunities to tailor intervention efforts to strengthen the foundational experiences, skills, and support systems that underlie mental wellness. By such a mechanism, mental health challenges and the associated negative outcomes may be mitigated as in the non‐FASD population (Rokita et al., 2018). Complex clinical presentations and comorbid conditions complicate treatment delivery for people with FASD, with case management often necessitating support for mental health and substance use needs, sexuality, legal issues, and medical issues (Paley & O’Connor, 2009). By considering mental health needs early and integrating FASD screening and diagnosis with assessment to identify any comorbid mental health concerns, clinicians are in a better position to cater to the unique needs of individual with PAE/FASD so that clinical recommendations are as comprehensive, holistic, and impactful as possible (Patel et al., 2020). It is important that people with FASD have access to these holistic assessments to ensure that their unique functional needs, abilities, and circumstances are understood and adequately addressed. This holistic approach may not only inform treatment planning, but also allow for a more comprehensive understanding of mental health trajectories among people with FASD, and help to guide future research about the multi‐faceted needs of people with FASD and their families. Lifelong coordinated, multidisciplinary, accessible, and community‐based services and supports, that are aimed to help individuals with FASD live and function independently, will be necessary to support the well‐being of individuals with FASD as they age (Paley & O’Connor, 2009).
Interestingly, very few studies in this review were focused on mental health or substance use as primary targets of intervention. Rather, interventions most often targeted functional or adaptive skills, and mental health factors were considered to be significant potential outcomes. Notably, we found that intervention efforts can support individuals with FASD to adopt and apply skills and strategies to improve their ability to function at their best. Nineteen articles, reporting on 7 evidence‐based interventions indicate this potential impact. Self‐regulation and social skills strategies have the strongest evidence for use in children with PAE/FASD, and there is also promising evidence for interventions to support the development of positive behavioral skills and strategies. Skill‐building was not exclusive to the individual with PAE/FASD; in many cases, interventions also incorporated external support through facilitators, caregivers, teachers, or mentors. Importantly, these interventions led to improved indicators of mental health, suggesting that the acquisition of skills and strategies is one viable mechanism for individuals with FASD (and in many cases, their families), to cope, interact, and feel better.
Although preliminary evidence suggests that individuals with FASD can acquire skills and strategies through more direct psychotherapeutic approaches, such as building mindfulness, distress tolerance, and coping skills via holistic mental health programs (Brintnell et al., 2019; Katz et al., 2020), current interventions are largely focused on improving underlying skill sets that indirectly influence mental health through regulation of behavior or problem‐solving skills. For individuals with FASD, the current approach for managing mental health seems to be focused more on supporting adaptive or functional capacity rather than providing psychotherapy in the traditional sense. Considering the outcome measures reported in the reviewed studies (i.e., behavioral, emotional, social functioning), and their association with mental health function, one may infer that these interventions have the potential to make a positive impact. In many ways, current FASD interventions are more preventative in nature, attempting to either prevent the onset of mental health problems, or prevent additional harm. There is currently no evidence on effective mental health treatment approaches or best practices for individuals with FASD who are diagnosed with comorbid mental health problems, such as anxiety or depressive disorders, ADHD, or other psychiatric disorders, which are very common in this population. Moreover, there are no established best practices for individuals with FASD in the midst of a mental health crisis. Although not directly incorporating the traditional therapeutic approach, the skill‐building interventions reviewed here are promising, considering that some of the negative outcomes associated with FASD (e.g., high‐risk behavior, suicidality) may be rooted in the neurocognitive deficits associated with PAE (e.g., poor emotion regulation, impaired decision making, impulsivity; Rasmussen and Wyper, 2007; Temple et al., 2019). Again, targeted approaches to mitigating the functional consequences of these impairments are important and may help to prevent or minimize the later development of mental health or substance use problems.
Finally, our findings indicate that investing efforts to engage and train supporters may lead to environmental shifts that are conducive to optimized fit and positive outcomes for individuals with PAE/FASD. A recurring theme apparent from our review relates to the importance of supporters who understand FASD; engage in advocacy for their children, students, or clients; and practice skills to support everyday success. A common element of studies targeting child skill‐ and strategy‐building was improved caregiver knowledge and skills to complement the child’s learning. Caregiver confidence, support, and skills appear to be some of the key ingredients for developing effective interventions for children with FASD. Accordingly, a comprehensive treatment approach requires the service provider to include specific recommendations geared toward knowledge and skills enhancement among those who support and care for people with FASD. Interactions between caregivers and service providers, virtually or in‐person, may also provide additional resources for acquiring relevant knowledge and support. It is important to consider that intervention studies involving caregivers required active and intensive participation, and given the high rates of caregiver instability in this population (Olson et al., 2009), these positive outcomes may be more difficult to achieve for some children with FASD. As well, follow‐up data from several studies indicated that not all of the gains made during intervention delivery were maintained, highlighting the necessity of ongoing supports across the life course.
The neurocognitive and learning deficits associated with PAE may impact a person’s receptiveness to treatment and subsequent completion rates (Grant et al., 2013). Modification of existing programs to account for PAE‐related impairments was common (e.g., the Alert Program, CTF, PCAP, Project Step Up). Knowing that specific adaptations may be warranted for individuals with FASD, it is critical that screening for FASD and identification of functional needs and impairments is conducted within mental health and substance use treatment settings to inform program planning (Grant et al., 2013). For individuals with FASD who have had difficulty completing mental health or substance use treatment in the past, modifying the program rather than framing the difficulties as individual shortcomings can make the difference between treatment failure and success (Grant et al., 2013).
4.1. Limitations
Many of the studies identified in this review had methodological limitations, particularly in terms of selection bias and blinding, where no studies were rated strongly. Weak ratings in selection bias reflected the limited representativeness of the study populations, which was primarily because participants were identified through clinic databases or self‐referral, or the authors did not describe the proportion of selected individuals who decided to participate. No studies were rated strongly with respect to blinding, because in all studies, either the outcome assessor was aware of the intervention status of the participants, the participants were aware of the research question, or blinding was not explicitly described. Additionally, most studies relied on small sample sizes, and numerous interventions were with single cases, accounting for the weak ratings in study design. On the other hand, numerous studies had strong ratings in terms of study design and data collection because many were RCTs or CCTs, and data collection measures were reliable and valid. For most outcomes, data were primarily collected through informant report (i.e., self, caregiver, and/or teacher ratings) rather than via direct measures of functioning. Although this informant data reflects important perspectives with strong ecological validity, future research using a wider range of data collection tools would provide complementary information.
Notably, dropouts were reported as minimal in most studies, suggesting that as interventions currently stand, these approaches have appeal for individuals with FASD and their families. This high rate of participation may reflect a combination of a strong desire for supports and intervention on the part of families (Pepper et al., 2018). This desire may be combined with a belief that the concerns caregivers hold can be addressed to some extent and that their child has the capacity to learn and grow in response to these interventions.
Several studies included follow‐up data, but this was limited to 6 months, at most, which is not sufficient to ascertain any long‐term effects of the intervention. Almost all studies were conducted in North America—predominantly in the United States—which also limits generalizability to other geographical regions where there are high rates of FASD. Despite these limitations, the studies identified in this review contribute preliminary evidence to build upon in our efforts to establish empirically supported interventions for people with FASD across the life course.
4.2. Gaps and Future Research Considerations
One of the most noteworthy findings of the current review is the stark absence of traditional psychotherapeutic interventions to improve mental health or substance use outcomes for individuals with PAE/FASD. Although elements of this approach are integrated into some of the interventions identified, holistic mental health responses were implemented in only 2 studies (Brintnell et al., 2019; Katz et al., 2020), neither of which were strongly generalizable, and one of which was not specific to FASD. Psychotherapy is one of the most common recommendations for children with FASD and comorbid psychiatric diagnoses (Patel et al., 2020), and anecdotal evidence suggests that these approaches are being used in front‐line FASD practice. However, research is critically needed to examine the use of evidence‐based psychotherapeutic approaches, such as DBT, cognitive behavior therapy, acceptance and commitment therapy, eye movement desensitizing and reprocessing therapy, and others, within this population. Studies are needed to explore the effectiveness of these specific interventions in order to generate an understanding of what modifications may be required to best account for the brain‐based difficulties experienced by individuals with PAE/FASD. In the future, researchers should explore the use of these therapies to address common psychiatric comorbidities in FASD, as well as examine the effectiveness or required adaptations for individuals with added layers of risk and complexity such as trauma, out‐of‐home care, self‐harm and suicidal behaviors, and justice involvement. As more evidence is developed, clinicians should be relatively confident in the existing findings supported by the ease of initiation and completion of interventions. Specific suggestions regarding ways to leverage the diverse strengths and abilities of individuals with FASD will be critical, which should be incorporated into planning geared toward achieving and promoting healthy outcomes.
Another significant gap identified in this review is the lack of substance use interventions for individuals with PAE/FASD. Although we noted several studies where substance use outcomes were measured, most of these were set within a larger context of strengthening connection with community resources and reducing risk through mentorship and advocacy for mothers. In the single study where researchers directly targeted substance use and related risk behaviors, participants were adolescents, and two‐thirds were abstinent or infrequent drinkers (O’Connor et al., 2016). This research offers important data on the feasibility and promise of substance use treatment for youth with FASD, especially as a means to intervene early and prevent the onset or worsening of substance use and related harms. However, research is needed with youth and adults with FASD who present with more severe challenges and risks. It has been suggested that the heightened risk of substance use in FASD results from a combination of complex factors, such as neurophysiological vulnerability, unstable early home environments, and concurrent mental disorders (Grant et al., 2013). Therefore, scientists and clinicians working in this area should take into consideration these biopsychosocial components as they relate to treatment and future research directions.
Finally, there are significant gaps in our knowledge about interventions for adolescents, emerging adults, and adults with FASD. In the current review, only 6 studies included participants over the age of 12 years, and only 3 were with individuals over 18 years. The lack of evidence‐based services for adolescents and adults with FASD is of critical concern. It is during the adolescent period that mental health and substance use challenges often emerge, and then as individuals with FASD transition to adulthood, they may become increasingly disconnected from the very supports and services that are most essential. Indeed, the transition to adulthood seems to be a particularly vulnerable time for individuals with FASD (Burnside and Fuchs, 2013; Coons‐Harding et al., 2019), with mental health and substance use problems especially relevant for this age group (Lynch et al., 2017). There is a critical and timely need for increased research and targeted service delivery during this life stage to provide wraparound supports for individuals with FASD who may otherwise lack resources and supports to promote healthy outcomes (McLachlan et al., 2020; Pei et al., 2019).
5. Conclusion
Recognizing the intersectoral nature of a life course approach, investing in early and ongoing intervention, supporting the acquisition of functional skills and strategies, engaging in preventative and harm‐reduction responses, and promoting a supportive environment across the life course are all essential components for fostering well‐being among individuals with PAE and FASD. Although early experiences are important, risk and protective factors are influential across the life course and trajectories can be altered by interventions at varying times. A developmental focus helps to elucidate the enduring nature of challenges related to FASD while also enabling our understanding of the implementation of effective interventions at various stages of life. The existing evidence illustrates a broad range of mental wellness interventions for people with FASD, the goals of which should be situated in symptom improvement, functional benefits, and ultimately supporting quality of life.
Acknowledgments
The authors would like to acknowledge Sophie Regalado, Research and Scholarly Communications Librarian at the Northern Ontario School of Medicine, for peer‐reviewing the search strategy. We have no conflicts of interest to declare.
Appendix 1. Search Histories
Ovid MEDLINE(R)
| 1 | Fetal Alcohol Spectrum Disorders/ |
| 2 | (alcohol adj related adj birth adj defect*).mp. |
| 3 | (alcohol adj related adj neurodevelopmental adj disorder*).mp. |
| 4 | (alcohol‐related adj birth adj defect*).mp. |
| 5 | FAES.mp. |
| 6 | FAE.mp. |
| 7 | FASD*.mp. |
| 8 | (fetal adj alcohol adj syndrome*).mp. |
| 9 | (growth adj2 retard* adj2 facial abnormalit* adj2 central adj2 nervous adj2 system adj2 dysfunction*).mp. |
| 10 | (fetal adj alcohol adj spectrum adj disorder*).mp. |
| 11 | FAS.mp. |
| 12 | (prenatal adj alcohol adj expos*).mp. |
| 13 | PAE.mp. |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 |
| 15 | exp Mental Disorders/ |
| 16 | (behavio* adj2 disorder*).mp. |
| 17 | (diagnos* adj2 psychiatric).mp. |
| 18 | (mental* adj3 disorder*).mp. |
| 19 | (psychiatric* adj2 disorder*).mp. |
| 20 | Mental Health/ |
| 21 | (mental* adj3 health*).mp. |
| 22 | (mental* adj3 hygien*).mp. |
| 23 | exp Self‐Injurious Behavior/ |
| 24 | suicid*.mp. |
| 25 | (self adj2 injur*).mp. |
| 26 | (self adj2 destruct*).mp. |
| 27 | (self adj2 harm*).mp. |
| 28 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 |
| 29 | exp Substance‐Related Disorders/ |
| 30 | addict*.mp. |
| 31 | alcohol*.mp. |
| 32 | drug*.mp. |
| 33 | substance*.mp. |
| 34 | exp Alcohol Drinking/ |
| 35 | exp Drug‐Seeking Behavior/ |
| 36 | (drug adj2 seek*).mp. |
| 37 | Alcoholics/ |
| 38 | Drug Users/ |
| 39 | exp Street Drugs/ |
| 40 | Heroin/ |
| 41 | heroin*.mp. |
| 42 | exp Analgesics, Opioid/ |
| 43 | opioid*.mp. |
| 44 | Cannabis/ |
| 45 | cannabis*.mp. |
| 46 | exp Cannabinoids/ |
| 47 | cannabinoid*.mp. |
| 48 | dronabinol*.mp. |
| 49 | tetrahydrocannabinol*.mp. |
| 50 | THC.mp. |
| 51 | cannabidiol*.mp. |
| 52 | marijuana*.mp. |
| 53 | marijuana smoking/ |
| 54 | (hemp* or marihuana* or hashish or ganja* or cannabi* or bhang*).mp. |
| 55 | exp Cocaine/ |
| 56 | cocaine*.mp. |
| 57 | crack.mp. |
| 58 | cocaine smoking/ |
| 59 | exp Amphetamines/ |
| 60 | amphetamine*.mp. |
| 61 | methamphetamine*.mp. |
| 62 | exp Hallucinogens/ |
| 63 | hallucinogen*.mp. |
| 64 | 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 |
| 65 | 28 or 64 |
| 66 | exp Health Services Accessibility/ |
| 67 | exp Rehabilitation/ |
| 68 | substance abuse treatment centers/ or exp community mental health centers/ |
| 69 | Hospitals, Psychiatric/ |
| 70 | Psychiatric Department, Hospital/ |
| 71 | exp Health Services/ |
| 72 | exp Psychotherapy/ |
| 73 | exp "Behavioral Disciplines and Activities"/ |
| 74 | exp Psychological Techniques/ |
| 75 | Case Management/ |
| 76 | (case adj2 manage*).mp. |
| 77 | exp housing/ |
| 78 | (hous* or lodg*).mp. |
| 79 | exp Methadone/ |
| 80 | methadone*.mp. |
| 81 | exp Self‐Help Groups/ |
| 82 | (peer adj2 support*).mp. |
| 83 | (group* adj2 support*).mp. |
| 84 | (treat* or therap* or manag* or servic* or rehab*).mp. |
| 85 | 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 |
| 86 | 14 and 65 and 85 |
| 87 | animals/ |
| 88 | 86 not 87 |
| 89 | limit 88 to (english language and yr="2000 ‐Current") |
| 90 | limit 89 to (editorial or letter) |
| 91 | 89 not 90 |
| 92 | remove duplicates from 91 |
PsycINFO
| 1 | exp fetal alcohol syndrome/ |
| 2 | (alcohol adj related adj birth adj defect*).mp. |
| 3 | (alcohol adj related adj neurodevelopmental adj disorder*).mp. |
| 4 | (alcohol‐related adj birth adj defect*).mp. |
| 5 | FAES.mp. |
| 6 | FAE.mp. |
| 7 | FASD*.mp. |
| 8 | (fetal adj alcohol adj syndrome*).mp. |
| 9 | (growth adj2 retard* adj2 facial abnormalit* adj2 central adj2 nervous adj2 system adj2 ysfunction*).mp. |
| 10 | (fetal adj alcohol adj spectrum adj disorder*).mp. |
| 11 | FAS.mp. |
| 12 | (prenatal adj alcohol adj expos*).mp. |
| 13 | PAE.mp. |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 |
| 15 | exp Mental Disorders/ |
| 16 | (behavio* adj2 disorder*).mp. |
| 17 | (diagnos* adj2 psychiatric).mp. |
| 18 | (mental* adj3 disorder*).mp. |
| 19 | (psychiatric* adj2 disorder*).mp. |
| 20 | Mental Health/ (59834) |
| 21 | (mental* adj3 health*).mp. |
| 22 | (mental* adj3 hygien*).mp. |
| 23 | exp Self‐Injurious Behavior/ |
| 24 | suicid*.mp. |
| 25 | (self adj2 injur*).mp. |
| 26 | (self adj2 destruct*).mp. |
| 27 | (self adj2 harm*).mp. |
| 28 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 |
| 29 | exp Drug Abuse/ |
| 30 | addict*.mp. |
| 31 | alcohol*.mp. |
| 32 | drug*.mp. |
| 33 | substance*.mp. |
| 34 | exp Alcohol Drinking Patterns/ |
| 35 | exp Drug Seeking/ |
| 36 | (drug adj2 seek*).mp. |
| 37 | alcoholics/ |
| 38 | Heroin/ |
| 39 | heroin*.mp. |
| 40 | exp Opiates/ |
| 41 | opioid*.mp. |
| 42 | Cannabis/ |
| 43 | cannabis*.mp. |
| 44 | exp Cannabinoids/ |
| 45 | cannabinoid*.mp. |
| 46 | dronabinol*.mp. |
| 47 | tetrahydrocannabinol*.mp. |
| 48 | THC.mp. |
| 49 | cannabidiol*.mp. |
| 50 | marijuana*.mp. |
| 51 | marijuana usage/ |
| 52 | (hemp* or marihuana* or hashish or ganja* or cannabi* or bhang*).mp. |
| 53 | exp Cocaine/ |
| 54 | cocaine*.mp. |
| 55 | crack.mp. |
| 56 | amphetamine/ |
| 57 | amphetamine*.mp. |
| 58 | methamphetamine*.mp. |
| 59 | exp Hallucinogenic Drugs/ |
| 60 | halluncinogen*.mp. |
| 61 | 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 |
| 62 | exp Rehabilitation/ |
| 63 | exp Treatment Facilities/ |
| 64 | Psychiatric Units/ |
| 65 | exp Health Care Services/ |
| 66 | exp Treatment/ |
| 67 | exp Diagnosis/ |
| 68 | exp case management/ |
| 69 | (case adj2 manage*).mp. |
| 70 | exp housing/ |
| 71 | (hous* or lodg*).mp. |
| 72 | exp Methadone/ |
| 73 | methadone*.mp. |
| 74 | exp support groups/ |
| 75 | (group* adj2 support*).mp. |
| 76 | (peer* adj2 support*).mp. |
| 77 | (treat* or therap* or manag* or servic* or rehab*).mp. |
| 78 | 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 |
| 79 | 28 or 61 |
| 80 | 14 and 78 and 79 |
| 81 | animals/ |
| 82 | 80 not 81 |
| 83 | limit 82 to yr="2000 ‐Current" |
| 84 | limit 83 to english language |
| 85 | limit 84 to (editorial or letter) |
| 86 | 84 not 85 |
| 87 | remove duplicates from 86 |
Web of Science
| 1 | TS=(alcohol NEAR related NEAR birth NEAR defect*) OR TS=(alcohol NEAR related NEAR neurodevelopmental NEAR disorder*) OR TS=(alcohol‐related NEAR birth NEAR defect*) OR TS=(FAES OR FAE OR FAS OR FASD OR PAE) OR TS=(fetal NEAR alcohol NEAR syndrome) OR TS=(growth NEAR/2 retard* NEAR/2 facial abnormalit* NEAR/2 central NEAR/2 nervous NEAR/2 system NEAR/2 dysfunction*) OR TS=(fetal NEAR alcohol NEAR spectrum NEAR disorder) OR TS=(prenatal NEAR alcohol NEAR expos*) |
| 2 | TS=(behavio* NEAR/2 disorder*) OR TS=(diagnos* NEAR/2 psychiatric) OR TS=(mental* NEAR/3 disorder*) OR TS=(psychiatric* NEAR/2 disorder*) OR TS=(mental* NEAR/3 health*) OR TS=(mental* NEAR/3 hygien*) OR TS=(suicid*) OR TS=(self NEAR/2 injur*) OR TS=(self NEAR/2 destruct*) OR TS=(self NEAR/2 harm*) |
| 3 | TS=(addict*) OR TS=(alcohol*) OR TS=(drug*) OR TS=(substance*) OR TS=(drug adj2 seek*) OR TS=(heroin*) OR TS=(opioid*) OR TS=(cannabis*) OR TS=(cannabinoid*) OR TS=(dronabinol*) OR TS=(tetrahydrocannabinol*) OR TS=(THC) OR TS=(cannabidiol*) OR TS=(marijuana*) OR TS=(hemp* OR marihuana* OR hashish OR ganja* OR cannabi* OR bhang*) OR TS=(cocaine*) OR TS=(crack) OR TS=(amphetamine*) OR TS=(methamphetamine*) OR TS=(hallucinogen*) |
| 4 | TS=(case adj2 manage*) OR TS=(hous* or lodg*) OR TS=(methadone*) OR TS=(group* adj2 support*) OR TS=(peer adj2 support*) OR TS=(treat* or therap* or manag* or servic* or rehab*) |
| 5 | #3 OR #2 |
| 6 | #5 AND #4 AND #1 |
| 7 | #5 AND #4 AND #1Refined by: [excluding]: PUBLICATION YEARS: (1992 OR 1999 OR 1991 OR 1998 OR 1990 OR 1997 OR 1989 OR 1996 OR 1988 OR 1995 OR 1987 OR 1994 OR 1984 OR 1993 OR 1982) |
| 8 |
#5 AND #4 AND #1 Refined by: [excluding]: PUBLICATION YEARS: (1992 OR 1999 OR 1991 OR 1998 OR 1990 OR 1997 OR 1989 OR 1996 OR 1988 OR 1995 OR 1987 OR 1994 OR 1984 OR 1993 OR 1982) AND LANGUAGES: (ENGLISH) |
| 9 | #8 NOT TS = animal* |
Cochrane Database of Systematic Reviews
| 1 | (alcohol adj related adj birth adj defect*) OR (alcohol adj related adj neurodevelopmental adj disorder*) OR (alcohol‐related adj birth adj defect*) OR (FAES OR FAE OR FAS OR FASD OR PAE) OR (fetal adj alcohol adj syndrome) OR (growth adj2 retard* adj2 facial abnormalit* adj2 central adj2 nervous adj2 system adj2 dysfunction*) OR (fetal adj alcohol adj spectrum adj disorder) OR (prenatal adj alcohol adj expos*) |
| 2 | (behavio* adj2 disorder*) OR (diagnos* adj2 psychiatric) OR (mental* adj3 disorder*) OR (psychiatric* adj2 disorder*) OR (mental* adj3 health*) OR (mental* adj3 hygien*) OR (suicid*) OR (self adj2 injur*) OR (self adj2 destruct*) OR (self adj2 harm*) |
| 3 | (addict*) OR (alcohol*) OR (drug*) OR (substance*) OR (drug adj2 seek*) OR (heroin*) OR (opioid*) OR (cannabis*) OR (cannabinoid*) OR (dronabinol*) OR (tetrahydrocannabinol*) OR (THC) OR (cannabidiol*) OR (marijuana*) OR (hemp* OR marihuana* OR hashish OR ganja* OR cannabi* OR bhang*) OR (cocaine*) OR (crack) OR (amphetamine*) OR (methamphetamine*) OR (hallucinogen*) |
| 4 | 2 or 3 |
| 5 | (case adj2 manage*) OR (hous* or lodg*) OR (methadone*) OR (group* adj2 support*) OR (peer adj2 support*) OR (treat* or therap* or manag* or servic* or rehab*) |
| 6 | 1 and 4 and 5 |
| 7 | 6 not animals.mp. |
| 8 | limit 7 to last 18 years |
References
- Bertrand J (2009) Interventions Children Fetal A. Interventions for children with fetal alcohol spectrum disorders (FASDs): Overview offindings for five innovative research projects. Research in Developmental Disabilities 30(5):986–1006. 10.1016/j.ridd.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Brintnell ES, Sawhney AS, Bailey PG, Nelson M, Pike AD, Wieldandt P (2019) Corrections and connection to the community: a diagnostic and service program for incarcerated adult men with FASD. Int J Law Psychiatry 64:8–17. [DOI] [PubMed] [Google Scholar]
- Brown J, Harr D (2019) Perceptions of Fetal Alcohol Spectrum Disorder (FASD) at a mental health outpatient treatment provider in Minnesota. Int J Environ Res Public Health 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside L, Fuchs D (2013) Bound by the clock: the experiences of youth with FASD transitioning to adulthood from child welfare care. First Peoples Child Family Rev 8: 40–61. [Google Scholar]
- Chotro MG, Arias C, Laviola G (2007) Increased ethanol intake after prenatal ethanol exposure: Studies with animals. Neurosci Biobehav Rev 31(2):181–91. 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Clark E, George MA, Hardy C, Hall WA, MacMillan PD, Wakabayashi S, Hughes K (2014) Exploratory study of the effectiveness of a professional development program on the academic achievement and classroom behavior of students with Fetal Alcohol Spectrum Disorder in British Columbia, Canada. Int J Alcohol Drug Res 3:25–34. [Google Scholar]
- Clausen JA (1986) The Life Course: A Sociological Perspective, 1st edn Prentice Hall, New York. [Google Scholar]
- Coles CD, Kable JA, Taddeo E (2009) Math performance and behavior problems in children affected by prenatal alcohol exposure: intervention and follow‐up. J Dev Behav Pediatr 30:7–15. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E, Strickland DC (2015) A metacognitive strategy for reducing disruptive behavior in children with Fetal Alcohol Spectrum Disorders: GoFAR pilot. Alcohol Clin Exp Res 39:2224–2233. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E, Strickland D (2018) GoFAR: improving attention, behavior and adaptive functioning in children with Fetal Alcohol Spectrum Disorders: brief report. Dev Neurorehabil 2:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SC, Millians M, Peterman R, Shillingsburg MA (2016) The clinical application of applied behavior analysis in a child with partial Fetal Alcohol Syndrome: a case study. Clin Case Stud 15:225–242. [Google Scholar]
- Connor PD, Streissguth AP (1996) Effects of prenatal exposure to alcohol across the life span. Alcohol Health Res World 20:170–174. [PMC free article] [PubMed] [Google Scholar]
- Coons‐Harding KD, Azulai A, McFarlane A (2019) State‐of‐the‐art review of transition planning tools for youth with fetal alcohol spectrum disorder in Canada. J Dev Disabil 24:81–98. [Google Scholar]
- Critical Appraisal Skills Program (2018) CASP Checklist: 10 questions to help you make sense of a qualitative research. Available at: https://casp‐uk.net/wp‐content/uploads/2018/01/CASP‐Qualitative‐Checklist‐2018.pdf. Accessed June 26, 2020.
- Denys K, Rasmussen C, Henneveld D (2011) The effectiveness of a community‐based intervention for parents with FASD. Community Ment Health J 47:209–219. [DOI] [PubMed] [Google Scholar]
- Dirks H, Francke L, Würz V, Kretschmann C, Dehghan‐Sanji S, Scherbaum N (2019) Substance use, comorbid psychiatric disorders and suicide attempts in adult FASD patients. Adv Dual Diagn 12:6–13. [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW (2019) Effects of fetal substance exposure on offspring substance use. Pediatr Clin North Am 66:1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LR, Glass L, Wozniak JR, Kable JA, Riley EP, Coles CD, Sowell ER, Jones KL, Mattson SN (2019) Relation between oppositional/conduct behaviors and executive function among youth with histories of heavy prenatal alcohol exposure. Alcohol Clin Exp Res 43:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH Jr, Shanahan MJ (2007) The life course and human development, in Handbook of Child Psychology (Damon W, Lerner RM, Lerner RM, eds), pp. 665–715. John Wiley & Sons, New Jersey. [Google Scholar]
- Ernst CC, Grant TM, Streissguth AP, Sampson PD (1999) Intervention with high‐risk alcohol and drug‐abusing mothers: II. three‐year findings from the Seattle model of paraprofessional advocacy. J Community Psychol 27:19–38. [Google Scholar]
- Fagerlund A, Autti‐Ramo I, Hoyme H, Mattson SN, Korkman M (2011) Risk factors for behavioural problems in Foetal Alcohol Spectrum Disorders. Acta Paediatr 100:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan K, Coons K, Reid D,Family Advisory Committee (2018) Strengths among individuals with FASD. Available at: https://canfasd.ca/wp‐content/uploads/publications/Strengths‐Among‐Individuals‐with‐FASD.pdf. Accessed June 26, 2020.
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119:e733–e741. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Heinz A, Kastrup M, Beezhold J, Sartorius N (2015) Toward a new definition of mental health. World Psychiatry 14:231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, De Genna NM, Cornelius MD, Day NL (2019) Prenatal alcohol exposure and offspring alcohol use and misuse at 22 years of age: a prospective longitudinal study. Neurotoxicol Teratol 71:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K (2004a) Prevalence and co‐occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61:807–816. [DOI] [PubMed] [Google Scholar]
- Grant TM, Brown NN, Dubovsky D, Sparrow J, Ries R (2013) The impact of prenatal alcohol exposure on addiction treatment. J Addict Med 7:87–95. [DOI] [PubMed] [Google Scholar]
- Grant T, Ernst C, Streissguth A (1999) Intervention with high‐risk alcohol and drug‐abusing mothers: I. administrative strategies of the Seattle model of paraprofessional advocacy. J Community Psychol 27:1–18. [Google Scholar]
- Grant T, Huggins J, Connor P, Pedersen JY, Whitney N, Streissguth A (2004b) A pilot community intervention for young women with Fetal Alcohol Spectrum Disorders. Community Ment Health J 40:499–511. [DOI] [PubMed] [Google Scholar]
- Grenier A (2012) Transitions and the Life Course: Challenging the Constructions of 'Growing Old', 1st edn The Policy Press, Chicago. [Google Scholar]
- Griffin MM, Copeland SR (2018) Effects of a self‐management intervention to improve behaviors of a child with Fetal Alcohol Spectrum Disorder. Educ Train Autism Dev Disabil 53:405–414. [Google Scholar]
- Hajal NJ, Paley B, Delja JR, Gorospe CM, Mogil C (2019) Promoting family school‐readiness for child‐welfare involved preschoolers and their caregivers: case examples. Child Youth Serv Rev 96:181–193. [Google Scholar]
- Hanlon‐Dearman A, Malik S, Wellwood J, Johnston K, Gammon H, Andrew KN, Maxwell B, Longstaffe S (2017) A descriptive study of a community‐based home‐visiting program with preschool children prenatally exposed to alcohol. J Popul Ther Clin Pharmocol 24:e61–e71. [DOI] [PubMed] [Google Scholar]
- Hareven TK (1994) Aging and generational relations: a historical and life course perspective. Annu Rev Sociol 20:437–461. [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J (2010) Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev 34:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Sloane M, Black‐Pond C (2007) Neurobiology and neurodevelopmental impact on childhood traumatic stress and prenatal alcohol exposure. Lang Speech Hear Serv Sch 38:99–108. [DOI] [PubMed] [Google Scholar]
- Huggins JE, Grant T, O'Malley K, Streissguth AP (2008) Suicide attempts among adults with Fetal Alcohol Spectrum Disorders: clinical considerations. Ment Health Aspects Dev Disabil 1:33–41. [Google Scholar]
- Hutchison ED (2016) Essentials of Human Behavior: Integrating Person, Environment, and the Life Course, 2nd edn SAGE Publications, Thousand Oaks. [Google Scholar]
- Ichikawa K, Fujiwara T, Kawachi I (2018) Prenatal alcohol exposure and child psychosocial behavior: a sibling fixed‐effects analysis. Front Psychiatry 9:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Strickland D, Taddeo E (2012) Comparing the effectiveness of on‐line versus in‐person caregiver education and training for behavioral regulation in families of children with FASD. Int J Ment Health AD 10:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Taddeo E (2007) Socio‐cognitive habilitation using the math interactive learning experience program for alcohol‐affected children. Alcohol Clin Exp Res 31:1425–1434. [DOI] [PubMed] [Google Scholar]
- Kable JA, Taddeo E, Strickland D, Coles CD (2016) Improving FASD children’s self‐regulation: piloting phase 1 of the GoFAR intervention. Child Fam Behav Ther 38:124–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartin D, Grant TM, Streissguth AP, Sampson PD, Ernst CC (2002) Three‐year developmental outcomes in children with prenatal alcohol and drug exposure. Pediatr Phys Ther. Fall; 14(3):145–153. 10.1097/00001577-200214030-00004. [DOI] [PubMed] [Google Scholar]
- Katz J, Knight V, Mercer SH, Skinner SY. (2020) Effects of a universal school‐based mental health program on the self‐concept, coping skills, and perceptions of social support of students with developmental disabilities. J Autism Dev Dis 50(11):4069–4084. [DOI] [PubMed] [Google Scholar]
- Keightley M, Agnihotri S, Subramaniapillai S, Gray J, Keresztesi J, Colantonio A, Polatajko HJ, Cameron D, Wiseman‐Hakes C (2018) Investigating a theatre‐based intervention for Indigenous youth with Fetal Alcohol Spectrum Disorder. Can J Occup Ther 85:128–136. [DOI] [PubMed] [Google Scholar]
- Keil V, Paley B, Frankel F, O’Connor MJ (2010) Impact of a social skills intervention on the hostile attributions of children with prenatal alcohol exposure. Alcohol Clin Exp Res 34:231–241. [DOI] [PubMed] [Google Scholar]
- Khoury JE, Jamieson B, Milligan K (2018) Risk for childhood internalizing and externalizing behavior problems in the context of prenatal alcohol exposure: a meta‐analysis and comprehensive examination of moderators. Alcohol Clin Exp Res 42:1358–1377. [DOI] [PubMed] [Google Scholar]
- Koponen AM, Kalland M, Autti‐Ramo I (2009) Caregiving environment and socio‐emotional development of foster‐placed FASD‐children. Child Youth Serv Rev 31:1049–1056. [Google Scholar]
- Landgren V, Svensson L, Gyllencreutz E, Aring E, Grönlund MA, Landgren M (2019) Fetal Alcohol Spectrum Disorders from childhood to adulthood: a Swedish population‐based naturalistic cohort study of adoptees from Eastern Europe. BMJ Open 9:e032407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Rehm J, Anagnostou E, Popova S (2018) Prevalence of externalizing disorders and Autism Spectrum Disorders among children with Fetal Alcohol Spectrum Disorder: systematic review and meta‐analysis. Biochem Cell Biol 96:241–251. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidid JPA, Clarke M, Devereau PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Kable JA, Coles CD (2017) Effects of prenatal alcohol exposure in a prospective sample of young adults: mental health, substance use, and difficulties with the legal system. Neurotoxicol Teratol 64:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan K, Flannigan K, Temple V, Unsworth K, Cook JL. (2020) Difficulties in daily living experienced by adolescents, transition‐aged youth, and adults with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 44(8):1609–1624. [DOI] [PubMed] [Google Scholar]
- McLachlan K, Rasmussen C, Oberlander TF, Loock C, Pei J, Andrew G, Reynolds J, Weinberg J (2016) Dysregulation of the cortisol diurnal rhythm following prenatal alcohol exposure and early life adversity. Alcohol 53:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela M, Coons‐Harding KD, Anderson T (2019) Recent advances in Fetal Alcohol Spectrum Disorder for mental health professionals. Curr Opin Psychiatry 32:328–335. [DOI] [PubMed] [Google Scholar]
- Michaelson V, King N, Inchley J, Currie D, Brooks F, Pickett W (2019) Domains of spirituality and their associations with positive mental health: a study of adolescents in Canada, England and Scotland. Prev Med 125:12–18. [DOI] [PubMed] [Google Scholar]
- Nash K, Stevens S, Greenbaum R, Weiner J, Koren G, Rovet J (2015) Improving executive functioning in children with Fetal Alcohol Spectrum Disorders. Child Neuropsychol 21:191–209. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, Marquardt R (2006) A controlled social skills training for children with Fetal Alcohol Spectrum Disorders. J Consult Clin Psychol 74:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, Laugeson EA, Mogil C, Lowe E, Welch‐Torres K, Keil V, Paley B (2012) Translation of an evidence‐based social skills intervention for children with prenatal alcohol exposure in a community mental health setting. Alcohol Clin Exp Res 36:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, Paley B (2009) Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev 15:225–234. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ (2014) Mental Health Outcomes Associated with Prenatal Alcohol Exposure: Genetic and Environmental Factors. Curr Dev Disord Rep 1:181–188. 10.1007/s40474-014-0021-7. [DOI] [Google Scholar]
- O'Connor MJ, Portnoff LC, Lebsack‐Coleman M, Dipple KM (2019) Suicide risk in adolescents with Fetal Alcohol Spectrum Disorders. Birth Defects Res 111:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ, Quattlebaum J, Castañeda M, Dipple KM (2016) Alcohol intervention for adolescents with Fetal Alcohol Spectrum Disorders: Project Step Up, a treatment development study. Alcohol Clin Exp Res 40:1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Oti R, Gelo J, Beck S (2009) "Family matters:" Fetal Alcohol Spectrum Disorder and the family. Dev Disabil Res Rev 15:235–249. [DOI] [PubMed] [Google Scholar]
- Pagnin D, Grecco MLZ, Furtado EF (2019) Prenatal alcohol use as a risk for Attention‐Deficit/Hyperactivity Disorder. Eur Arch Psy Clin N 269:681–687. [DOI] [PubMed] [Google Scholar]
- Paley B, O'Connor MJ (2009) Intervention for individuals with Fetal Alcohol Spectrum Disorders: treatment approaches and case management. Dev Disabil Res Rev 15:258–267. [DOI] [PubMed] [Google Scholar]
- Paley B, O'Connor MJ (2011) Behavioral interventions for children and adolescents with fetal alcohol spectrum disorders. Alcohol Res Health. 34(1):64–75. [PMC free article] [PubMed] [Google Scholar]
- Patel M, Agnihotri S, Hawkins C, Levin L, Goodman D, Simpson A (2020) Identifying Fetal Alcohol Spectrum Disorder and psychiatric comorbidity for children and youth in care: A community approach to diagnosis and treatment. Children Youth Serv Rev 108:104606 [Google Scholar]
- Peadon E, Rhys‐Jones B, Bower C, Elliott EJ (2009) Systematic review of interventions for children with Fetal Alcohol Spectrum Disorders. BMC Pediatr.9:35 10.1186/1471-2431-9-35.Published May 25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C (2011) Mental health issues in Fetal Alcohol Spectrum Disorder. J Ment Health 20:473–483. [DOI] [PubMed] [Google Scholar]
- Pei J, Kapasi A, Kennedy KE, Joly V (2019). Towards Healthy Outcomes for Individuals with Fetal Alcohol Spectrum Disorder. The Canada FASD Research Network in collaboration with the University of Alberta, Vancouver, BC: Available at: https://canfasd.ca/wp‐content/uploads/2019/11/Final‐Towards‐Healthy‐Outcomes‐Document‐with‐links.pdf. Accessed June 26, 2020. [Google Scholar]
- Pei J, Flannigan K, Walls L, Rasmussen C (2016) Interventions for Fetal Alcohol Spectrum Disorder: Meeting Needs Across the Lifespan. Int J Neurorehabilitation 3:192 10.4172/2376-0281.1000192. [DOI] [Google Scholar]
- Pepper J, Coons KD, Bibr C, Watson SL (2018) Waving a magic wand: supports for families raising school‐aged children with Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder in Ontario. J Fetal Alcohol Spec Risk Prev 1:e2–e16. [Google Scholar]
- Petrenko CLM, Demeusy EM, Alto ME (2019) Six‐month follow‐up of the Families on Track Intervention pilot trial for children with Fetal Alcohol Spectrum Disorders and their families. Alcohol Clin Exp Res 43:2242–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko CLM, Pandolfino ME, Robinson LK (2017) Findings from the Families on Track Intervention pilot trial for children with Fetal Alcohol Spectrum Disorders and their families. Alcohol Clin Exp Res 41:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko CL, Alto ME (2017) Interventions in fetal alcohol spectrum disorders: An international perspective. Eur J Med Genet. 60(1):79–91. 10.1016/j.ejmg.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lange S, Shield K, Burd L, Rehm J (2019) Prevalence of Fetal Alcohol Spectrum Disorder among special subpopulations: a systematic review and meta‐analysis. Addiction 114:1150–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RAS, Bekmuradov D, Rehm J (2016) Comorbidity of Fetal Alcohol Spectrum Disorder: a systematic review and meta‐analysis. Lancet 387:978–987. [DOI] [PubMed] [Google Scholar]
- Price A, Cook PA, Norgate S, Mukherjee R (2017) Prenatal alcohol exposure and traumatic childhood experiences: a systematic review. Neurosci Biobehav Rev 80:89–98. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele‐Webster L, Alton C, Lord L (2010) The impact of an ADHD co‐morbidity on the diagnosis of FASD. Can J Clin Pharmacol 17:e165–e176. [PubMed] [Google Scholar]
- Rasmussen C, Wyper K (2007) Decision making, executive functioning and risky behaviors in adolescents with prenatal alcohol exposure. Int J Disabil Hum Dev 6:405–416. [Google Scholar]
- Reid N, Dawe S, Shelton D, Harnett P, Warner J, Armstrong E, LeGros K, O’Callaghan F (2015) Systematic review of Fetal Alcohol Spectrum Disorder interventions across the life span. Alcohol Clin Exp Res 39:2283–2295. [DOI] [PubMed] [Google Scholar]
- Reid N, Dawe S, Harnett P, Shelton D, Hutton L, O’Callaghan F (2017) Feasibility study of a family‐focused intervention to improve outcomes for children with FASD. Research in Developmental Disabilities 67:34–46. 10.1016/j.ridd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Rokita KI, Dauvermann MR, Donohoe G (2018) Early life experiences and social cognition in major psychiatric disorders: A systematic review. European Psychiatry. 53:123–133. 10.1016/j.eurpsy.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L (2016) Worldwide prevalence of Fetal Alcohol Spectrum Disorders: a systematic literature review including meta‐analysis. Alcohol Clin Exp Res 40:18–32. [DOI] [PubMed] [Google Scholar]
- Soh DW, Skocic J, Nash K, Stevens S, Turner GR, Rovet J (2015) Self‐regulation therapy increases frontal gray matter in children with Fetal Alcohol Spectrum Disorder: evaluation by voxel‐based morphometry. Front Hum Neurosci 9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL (1996) Understanding the Occurrence of Secondary Disabilities in Clients with Fetal Alcohol Syndrome (FAS), and Fetal Alcohol Effects (FAE). University of Washington School of Medicine and Department of Psychiatry and Behavioral Sciences, Washington. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK (2004) Risk factors for adverse life outcomes in Fetal Alcohol Syndrome and Fetal Alcohol Effects. J Dev Behav Pediatr 25:228–238. [DOI] [PubMed] [Google Scholar]
- Symons M, Pedruzzi RA, Bruce K, Milne E (2018) A systematic review of prevention interventions to reduce prenatal alcohol exposure and Fetal Alcohol Spectrum Disorder in Indigenous communities. BMC Public Health 18:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple VK, Cook JL, Unsworth K, Rajani H, Mela M (2019) Mental health and affect regulation impairment in Fetal Alcohol Spectrum Disorder (FASD): Results from the Canadian National FASD Database. Alcohol Alcohol 54:545–550. [DOI] [PubMed] [Google Scholar]
- Thomas BH, Ciliska D, Dobbins M, Micucci S (2004) A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 1:176–184. [DOI] [PubMed] [Google Scholar]
- Timler GR, Olswang LB, Coggins TE (2005) “Do I know what I need to do?” a social communication intervention for children with complex clinical profiles. Lang Speech Hear Serv Sch 36:73–85. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KGC (2008) Prenatal alcohol exposure: foetal programming, the hypothalamic‐pituitary‐adrenal axis and sex differences in outcome. J Neuroendocrinol 20:470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Chasnoff IJ, Schmidt CA, Telford E, Schwartz LD (2012) Neurocognitive habilitation therapy for children with fetal alcohol spectrum disorders: An adaptation of the Alert Program®. Am J Occup Ther 66(1):24–34. 10.5014/ajot.2012.002691. [DOI] [PubMed] [Google Scholar]
- Weyrauch D, Schwartz M, Hart B, Klug MG, Burd L (2017) Comorbid mental disorders in Fetal Alcohol Spectrum Disorders: a systematic review. J Dev Behav Pediatr 38:283–291. [DOI] [PubMed] [Google Scholar]
- Williams MS, Shellenberger S (1996) “How Does Your Engine Run?” A Leader’s Guide to the Alert Program for Self‐Regulation. Therapy Works, Albuquerque. [Google Scholar]
- Wiskow KM, Ruiz‐Olivares R, Matter AL, Donaldson JM (2018) Evaluation of the good behavior game with a child with Fetal Alcohol Syndrome in a small‐group context. Behav Interv 33:150–159. [Google Scholar]
- World Health Organization (2020) Mental health: Strengthening our response. Available at: https://www.who.int/news‐room/fact‐sheets/detail/mental‐health‐strengthening‐our‐response. Accessed June 26, 2020.
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS (1998) Effect of Fetal Alcohol Exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res 22:914–920. [PubMed] [Google Scholar]
- Zarnegar Z, Hambrick EP, Perry BD, Azen SP, Peterson C (2016) Clinical improvements in adopted children with Fetal Alcohol Spectrum Disorders through neurodevelopmentally informed clinical intervention: a pilot study. Clin Child Psychol Psychiatry 21:551–567. [DOI] [PubMed] [Google Scholar]


