Abstract
Early onset of type 2 diabetes and a high prevalence of co‐morbidities predispose the Asian population to a high risk for, and rapid progression of, diabetic kidney disease (DKD). Apart from renin‐angiotensin system inhibitors, sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors have been shown to delay renal disease progression in patients with DKD. In this review article, we consolidate the existing literature on SGLT‐2 inhibitor use in Asian patients with DKD to establish contemporary guidance for clinicians. We extensively reviewed recommendations from international and regional guidelines, data from studies on Asian patients with DKD, global trials (DAPA‐CKD, CREDENCE and DELIGHT) and cardiovascular outcomes trials. In patients with DKD, SGLT‐2 inhibitor therapy significantly reduced albuminuria and the risk of hard renal outcomes (defined as the onset of end‐stage kidney disease, substantial decline in renal function from baseline and renal death), cardiovascular outcomes and hospitalization for heart failure. In all the cardiovascular and renal outcomes trials, there was an initial decline in the estimated glomerular filtration rate (eGFR), which was followed by a slowing in the decline of renal function compared with that seen with placebo. Despite an attenuation in glucose‐lowering efficacy in patients with low eGFR, there were sustained reductions in body weight and blood pressure, and an increase in haematocrit. Based on the available evidence, we conclude that SGLT‐2 inhibitors represent an evidence‐based therapeutic option for delaying the progression of renal disease in Asian patients with DKD and preserving renal function in patients at high risk of kidney disease.
Keywords: diabetes, diabetic kidney disease, diabetic nephropathy, gliflozins, renal disease, SGLT, type 2 diabetes
1. INTRODUCTION
A large proportion of patients with type 2 diabetes (T2D) develop diabetic kidney disease (DKD), which is characterized by glomerular hyperfiltration, microalbuminuria, structural changes and eventual decline in renal function. 1 , 2 The silent nature of DKD calls for close monitoring of renal function, especially in patients with hypertension and diabetes, who have more rapid decline in renal function than those with one condition alone. 3 , 4 Patients with T2D have considerable variations in the trajectories of renal function decline, with the annual rate of eGFR decline ranging from 0.7% to 14.3%. 5 Microalbuminuria and diabetic retinopathy are the strongest predictors of accelerated decline in renal function. DKD is also the most common cause of end‐stage kidney disease (ESKD). 2 , 6 , 7
In the UK Prospective Diabetes Study (UKPDS), 40% of patients with T2D developed albuminuria and 30% developed renal impairment (eGFR <60 mL/min/1.73 m2) within 15 years of diagnosis. 8 , 9 From 2005 to 2015, the global prevalence of DKD increased by 27%, with a higher increase observed in developing countries. 10 , 11 Asian patients with T2D have a higher prevalence of DKD and a faster deterioration of renal function than their Caucasian counterparts. 12 , 13 , 14 This may be attributed to early onset of diabetes and frequent co‐existence of metabolic syndrome and other risk factors, such as genetic propensity and chronic hepatitis B virus infection. 15 , 16 , 17 , 18 , 19
Optimal control of hypertension, hyperglycaemia and dyslipidaemia, 20 , 21 , 22 as well as the early use of renin‐angiotensin system inhibitors (angiotensin‐converting enzyme inhibitors [ACEis] or angiotensin II receptor blockers [ARBs]), are the main strategies for disease control in patients with DKD. 23 , 24 , 25 In addition, several randomized controlloed trials (RCTs) have confirmed the benefits of intensive glycaemic control in delaying the progression of renal functional decline in T2D. 26 , 27 Apart from these strategies and, to a lesser extent, use of mineralocorticoid inhibitors (e.g. spironolactone), 28 , 29 , 30 no novel therapy has been shown to alter the natural course of DKD in the last 2 decades. The renoprotective effects of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors as a new class of oral glucose‐lowering drugs (GLDs) have provided a novel therapeutic option for the management of DKD. In the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, canagliflozin reduced the risk of ESKD by 32% in patients with DKD who were on optimal treatment, including ACEis or ARBs. 31 In the recently completed Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA‐CKD) trial, treatment with dapagliflozin was associated with significant reductions in the risk of renal outcomes in patients with CKD with or without T2D. 32 The renoprotective effects of SGLT‐2 inhibitors now add to the cardiovascular (CV) benefits confirmed in multiple cardiovascular outcomes trials (CVOTs). 33 , 34 , 35 These results have motivated the conduct of several ongoing trials aiming to confirm the efficacy and safety of SGLT‐2 inhibitors in patients with a broad spectrum of kidney disease, with or without T2D (Figure 1). Given the potential impact of the results from CVOTs and the CREDENCE and DAPA‐CKD trials on the management of DKD, we have summarized and consolidated current literature on the renoprotective effects of SGLT‐2 inhibitors in Asian patients with T2D to produce clinical guidelines for physicians.
FIGURE 1.
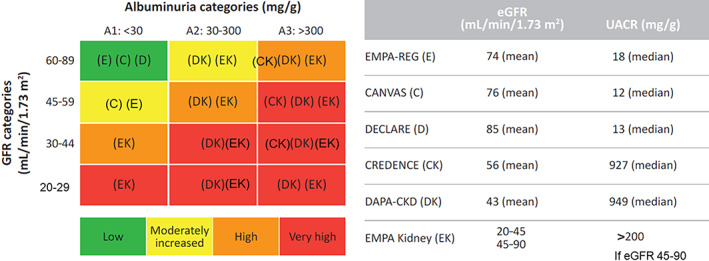
SGLT‐2 inhibitors: clinical evidence across the renal disease continuum. C, CANVAS Program; CK, CREDENCE; D, DECLARE‐TIMI56; DK, DAPA‐CKD; E, EMPA‐REG OUTCOME; eGFR, estimated glomerular filtration rate; EK, EMPA‐Kidney; GFR, glomerular filtration rate
2. METHODOLOGY
An expert panel comprising 13 endocrinology experts from Australia, China, Hong Kong, India, Indonesia, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam met five times (Bangkok, November 2017; Shanghai, March 2018; Orlando, June 2018; Kuala Lumpur, November 2018; and Taipei, May 2019) to develop expert recommendations on the use of SGLT‐2 inhibitors in Asian patients with T2D. A literature search was conducted in the MEDLINE database for articles published up to 30 June 2019 using search terms (‘canagliflozin’ OR ‘dapagliflozin’ OR ‘empagliflozin’ OR ‘ipragliflozin’ OR ‘luseogliflozin’ OR ‘tofogliflozin’ OR ‘ertugliflozin’ OR ‘remogliflozin’) AND ‘type 2 diabetes’. The search results were screened for efficacy and safety studies of SGLT‐2 inhibitors conducted in Asian patients. In addition, the panel critically analysed the recommendations from international guidelines, as well as results from renal outcomes trials and CVOTs. Following discussion, the panel reached consensus on a series of recommendations based on scientific evidence and expertsʼ opinions.
3. EPIDEMIOLOGY AND ASSOCIATED COMPLICATIONS OF DKD IN ASIA
Diabetes is the most common cause of ESKD; the risk of ESKD is 10‐fold higher among patients with diabetes compared with those without. In a pooled analysis of data from 54 countries, up to 80% of ESKD was caused by diabetes, hypertension or a combination of these conditions. Diabetes alone accounted for 12%‐55% of all ESKD cases. 36
In Asia, population and cohort surveys indicate that as many as 13% of adults have renal impairment. 37 , 38 , 39 , 40 The Asia‐Pacific region is also an epicentre of the diabetes epidemic, accounting for more than 57% (240 of 425 million patients) of the global burden. A further 156 million adults in the region have impaired glucose tolerance. 36 The high prevalence of diabetes portends a growing burden of DKD in Asia. 13 , 14 , 36 Furthermore, in a meta‐analysis of studies of genetic associations with DKD, Mooyaart et al. found that the genetic polymorphisms of ELMO1 and CCR5 (susceptible genes for DKD) were found only in Asian people. 41
In the Developing Education on Microalbuminuria for Awareness of renal and cardiovascular risk in Diabetes (DEMAND) study, which evaluated the prevalence and risk factors of albuminuria in patients with T2D (N = 24 151; 9111 patients from Asia, 38%) from 33 countries, 55% of Asian patients (mean age, 59.9 years; duration of diabetes, 7.1 years) had albuminuria (microalbuminuria, 43%; macroalbuminuria, 12%) compared with 40.6% (microalbuminuria, 33%; macroalbuminuria, 7.6%) of Caucasians (mean age, 63.2 years; duration of diabetes, 7.8 years), with an odds ratio (OR) of 1.77 (95% CI, 1.59‐1.97; P < .0001). 13 In the Pathways study, which assessed racial/ethnic differences in the prevalence of albuminuria among patients with T2D in a primary care setting, the prevalence of albuminuria was similar among Asians and Caucasians. However, in patients without hypertension, Asians were two times more probable to have microalbuminuria (OR, 2.01 [95% CI, 1.14‐3.53]) and three times more probable to have macroalbuminuria (OR, 3.17 [95% CI, 1.09‐9.26]) compared with Caucasians, which was mainly attributed to a body mass index of 30 kg/m2 or higher. 42 Furthermore, many patients with DKD may have completely normal albuminuria levels. In the DEMAND study, 17% of T2D patients with advanced renal impairment (stage ≥3) had normoalbuminuria. 43
Patients with DKD have a greater risk of developing microvascular and macrovascular complications than those without DKD. Both renal function and albuminuria are independent predictors of CV outcomes in patients with T2D. 44 Microalbuminuria and macroalbuminuria are associated with a 1.76‐fold and 2.96‐fold greater risk of CV mortality compared with normoalbuminuria, respectively. 7 The combination of albuminuria and an eGFR of less than 60 mL/min/1.73 m2 is associated with a 4.2‐fold greater risk of CV mortality compared with patients with neither of these risk factors. 7 In a cross‐sectional study of patients with T2D, the proportion of patients with macrovascular complications was higher in those with DKD than those without (36.5% vs. 14.2%; P < .001). 45 This included lower limb arterial disease (8.1% vs. 2.8%; P < .001), coronary heart disease (17.1% vs. 6.5%; P < .001) and stroke (3.0% vs. 0.8%; P = .003). The incidence of retinopathy was 42.5% in patients with DKD compared with 20.4% in those without DKD (P < .001). The corresponding figures for neuropathy were 20.1% versus 8.7% (P < .001). 45 Furthermore, an analysis of predictors of long‐term outcomes showed that low eGFR, but not retinopathy, was an independent predictor of all‐cause mortality and CV mortality in patients with T2D and albuminuria. 46
4. MANAGEMENT OF PATIENTS WITH DKD
Early detection of DKD is important for the prevention of ESKD. International and regional guidelines recommend regular monitoring of albuminuria and eGFR at least annually in patients with diabetes, and more often among patients with DKD. A comprehensive and multifaceted treatment approach, including diet restrictions and optimal control of hyperglycaemia, hypertension and dyslipidaemia, has been shown to delay progression of kidney dysfunction in patients with DKD (Table S1). 47 , 48 Blood pressure (BP) of less than 140/90 mmHg is recommended to slow the decline of renal function and to reduce the risk of CV disease. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend a lower BP target of less than 130/80 mmHg in patients with albuminuria of less than 30 mg/24 hours. Blockade of the renin‐angiotensin system using ACEis or ARBs is the recommended first‐line therapy for BP control in patients with DKD. However, combination therapy with ACEis and ARBs is not recommended because of an increased risk of hyperkalaemia and/or acute kidney injury (AKI).
The National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend the use of renin‐angiotensin system inhibitors (ACEis or ARBs) in normotensive T2D patients with albuminuria to delay the progression of DKD. 49 Similarly, the Malaysian clinical practice guidelines and the Chinese Diabetes Society guidelines recommend the use of ACEis or ARBs in T2D patients with albuminuria regardless of the presence of hypertension. 50 , 51 Although ACEis or ARBs are often prescribed in normotensive patients with albuminuria, there is no evidence that they slow GFR decline in these patients. Hence, the American Diabetes Association (ADA) recommends the use of renin‐angiotensin system inhibitors only in hypertensive T2D patients with albuminuria. 20 In the absence of albuminuria or kidney disease or hypertension, there is no benefit of ACEi or ARB therapy for the prevention of kidney disease in patients with T2D; hence, both the ADA and NKF KDOQI guidelines do not recommend the use of renin‐angiotensin system inhibitors for the primary prevention of kidney disease in normotensive normoalbuminuric patients with T2D. 20 , 49
In several RCTs, intensive glycaemic control delayed the onset and progression of DKD. However, patients with DKD have a high risk for hypoglycaemia, necessitating the individualization of glycaemic targets. Dosage adjustment for GLDs or use of drugs with a low risk of hypoglycaemia is recommended. 52 Table S2 summarizes the dose modifications required for each GLD in patients with DKD. Most of the oral GLDs are not recommended for patients with stage 4 and 5 chronic kidney disease (CKD; eGFR <30 mL/min/1.73 m2). Metformin is not recommended in patients with an eGFR of less than 30 mL/min/1.73 m2. In patients with an eGFR of less than 45 mL/min/1.73 m2, metformin treatment should be initiated with caution, while in those already on treatment, metformin can be continued with reduced dosage following an assessment of the benefits and risks. 20
The ADA/European Association for the Study of Diabetes 2019 algorithm has incorporated current evidence into its clinical decision‐making pathway, with a recommendation to choose GLDs based on the presence of established CV disease or CKD. 20 In patients with predominant heart failure or CKD, SGLT‐2 inhibitors are recommended as the preferred add‐on therapy to metformin. While renal outcomes were evaluated as secondary endpoints in CVOTs, results from the CREDENCE and DAPA‐CKD trials, which were designed to evaluate the renoprotective effects of SGLT‐2 inhibitors, provided definitive evidence of their benefits in patients with DKD.
5. SGLT‐2 INHIBITORS
The glucose‐lowering effects of SGLT‐2 inhibitors are independent of β‐cell function and insulin resistance. 53 , 54 As the glucose‐lowering effect of SGLT‐2 inhibitors is dependent on renal function, their efficacy is attenuated in patients with reduced eGFR. The recommended lower cut‐off for eGFR varies among different SGLT‐2 inhibitors, ranging from 30 to 60 mL/min/1.73 m2.
In general, SGLT‐2 inhibitors are well‐tolerated with a very low risk of hypoglycaemia. Mycotic genital infections are the most common adverse events (AEs) reported with SGLT‐2 inhibitors; these can be managed with standard treatment and good personal hygiene. In addition, urinary tract infections and volume depletion‐related AEs have also been reported.
In the early postmarketing surveillance data, there were sporadic case reports of AKI with SGLT‐2 inhibitors, especially in those with hypovolaemia, renal insufficiency, congestive heart failure and concomitant medications such as diuretics, ACEis, ARBs and non‐steroidal anti‐inflammatory drugs. 55 However, with better understanding of their pharmacodynamic effects and careful patient selection, there was no significant increase in the risk of AKI with SGLT‐2 inhibitors in the subsequent CVOTs. In a meta‐analysis of three CVOTs, there was a significant reduction in the risk of AKI among patients randomized to SGLT‐2 inhibitors (HR 0.66; 95% CI, 0.54‐0.80) compared with placebo. 56 In addition, the real‐world evidence did not show an increased risk of AKI among T2D patients initiated on SGLT‐2 inhibitor therapy. 57 , 58 Nevertheless, caution should be exercised in patients with multiple stressors of volume status, such as the elderly, patients with low body weight, and those receiving diuretic therapy.
6. ROLE OF SGLT‐2 INHIBITORS IN THE MANAGEMENT OF PATIENTS WITH DKD
The continuum of renal disease includes several well‐defined stages: microalbuminuria, macroalbuminuria, CKD and ESKD. 59 Patients with DKD have an accelerated loss of renal function, calling for optimal control of glycaemia and hypertension. Most of the available GLDs have limited utility in the management of DKD because of the risk of hypoglycaemia and weight gain. To date, SGLT‐2 inhibitors are the only class of GLD with proven renoprotective effects in high‐risk patients with DKD, and those are probably independent of their glucose‐lowering effects. In the following sections we discuss the renal and metabolic effects of SGLT‐2 inhibitors in Asian patients with DKD, as well as data from global trials.
6.1. Effect of SGLT‐2 inhibitors on kidney disease progression in patients with DKD
Overall, we found seven studies evaluating the effects of SGLT‐2 inhibitors in Asian patients with DKD (sample size range: 20‐1030 patients; duration of treatment range: 12‐104 weeks) and three global studies including Asian patients with DKD. The effects of SGLT‐2 inhibitors on renal outcomes in Asian patients with DKD and data from global studies are summarized in Table 1. 31 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 A pooled analysis of four 52‐week, phase III trials evaluated the effects of luseogliflozin in Japanese patients with T2D and varying degrees of renal function: normal (eGFR ≥90 mL/min/1.73 m2; n = 275), mild (eGFR ≥60 to <90 mL/min/1.73 m2; n = 598) and moderate renal impairment (eGFR ≥30 to <60 mL/min/1.73 m2; n = 157). 60 At 52 weeks, the urinary albumin‐to‐creatinine ratio (UACR) was modestly increased in the mild renal impairment group (+3.5 mg/g), whereas it was reduced by −27.4 mg/g in patients with moderate renal impairment. In both of these groups, eGFR was reduced during the first 2 weeks of treatment, returning to baseline levels after week 36. At 52 weeks, eGFR remained stable in the mild and moderate renal impairment groups, but was decreased in patients with normal renal function. This reduction in eGFR in individuals with normal renal function could be explained by a decrease in glomerular hyperfiltration as a subgroup analysis in patients with normal eGFR showed that a significant decrease from baseline was only observed in those with an eGFR of 100 to less than 110 mL/min/1.73 m2 (P = .030) and 120 mL/min/1.73 m2 or higher (P = .001). 60 This effect on glomerular hyperfiltration was also shown in previous studies with renin angiotensin system inhibitors, where the initial acute decline in eGFR was associated with a slower decline in long‐term renal function. 68
TABLE 1.
Effects of SGLT‐2 inhibitors on eGFR and UACR in patients with DKD
| Study | Design and population | Intervention | Effect on eGFR | Effect on UACR |
|---|---|---|---|---|
|
DAPA‐CKD Heerspink et al. 2020 32 |
Phase III, randomized, international trial in patients with CKD with or without T2D (N = 4304) Number (%) of Asians: 1467 (34.0%) |
Dapagliflozin (10 mg); placebo |
Week 2 Dapa: −3.97 ± 0.15 mL/min/1.73 m2 PBO: −0.82 ± 0.15 mL/min/1.73 m2 Annual changes in eGFR from week 2 to month 30 Dapa: −1.67 ± 0.11 mL/min/1.73 m2 per year PBO: −3.59 ± 0.11 mL/min/1.73 m2 per year |
‐ |
|
DELIGHT Pollock et al. 2019 67 |
Phase II/III, 24‐wk, randomized, international study in patients with DKD (N = 448) Number (%) of Asians: 177 (39.5%) |
Dapagliflozin‐saxagliptin (10/2.5 mg), Dapagliflozin (10 mg), placebo |
Week 1 Dapa: −4.8 (−6.3; −3.3) mL/min per 1.73 m2 Dapa/Saxa: −4.6 (−6.0; −3.1) mL/min/1.73 m2 Week 24 Dapa: −2.4 (−4.2; −0.5) mL/min/1.73 m2 Dapa/Saxa: −2.4 (−4.2; −0.7) mL/min/1.73 m2 |
Week 4 Dapa: −28.3% (−36.8; −18.7) Dapa/Saxa: −34.5% (−42.1; −25.9) Week 24 Dapa: −21.0% (−34.1; −5.2) Dapa/Saxa: −38.0% (−48.2; −25.8) |
|
CREDENCE Perkovic et al. 2019 31 |
Phase III, randomized, international study, in patients with DKD (N = 4401) Number (%) of Asians: 877 (19.9%) |
Canagliflozin (100 mg); placebo |
Week 3 Cana: −3.72 ± 0.25 mL/min/1.73 m2 PBO: −0.55 ± 0.25 mL/min/1.73 m2 Annual changes in eGFR from week 3 to end of study Cana: −1.85 ± 0.13 mL/min/1.73 m2 per year PBO: −4.59 ± 0.14 mL/min/1.73 m2 per year |
Mean change during follow‐up Cana: −31% (26 to 35%) |
| Haneda et al. 2016 60 |
Pooled analysis of four Phase III, 52‐wk studies in Japanese T2D patients with mild or moderate renal impairment (N = 1030) Number (%) of Asians: 1030 (100%); from Japan |
Luseogliflozin (2.5, 5 mg/d) |
Week 52 Normal eGFR (≥90 mL/min/1.73 m2) ≥90 to <100: −0.56 ≥100 to <110: −2.46 ≥110 to <120: −3.15 ≥120: −6.92 Mild renal impairment (≥60 to <90 mL/min/1.73 m2) +0.23 (−0.40 to 0.85) Moderate renal impairment (≥30 to <60 mL/min/1.73 m2) −0.13(−1.05 to 0.78) |
Week 52 Normal eGFR −3.2 mg/g (−8.5 to 2.1) Mild renal impairment +3.5 mg/g (−11.9 to 19.0) Moderate renal impairment −27.4 mg/g (−88.2 to 33.4) |
| Ito et al. 2018 61 |
Open‐label, 104‐wk study in Japanese with DKD (N = 50) Number (%) of Asians: 50 (100%); from Japan |
Ipragliflozin (50 mg) |
Baseline 82.1 ± 19.8 mL/min/1.73 m2 Week 24 78.5 ± 17.4 mL/min/1.73 m2 Week 52 82.2 ± 20.7 mL/min/1.73 m2 Week104 81.0 ± 20.2 mL/min/1.73 m2 |
Baseline 15.5 (8.0‐95.7) mg/g Week 24 12.9 (7.4‐36.3) mg/g Week 52 15.2 (8.0‐39.0) mg/g Week 104 14.0 (7.3‐34.6) mg/g |
| Jian et al. 2018 62 |
12‐mo study in patients with DKD (N = 126) Number (%) of Asians: 126 (100%); from China |
Dapagliflozin (0.05 g/d) or placebo for 6 mo |
Baseline Dapa: 45.3 ± 12.1 mL/min/1.73 m2 PBO: 42.3 ± 10.3 mL/min/1.73 m2 3 months Dapa: 52.3 ± 12.2 mL/min/1.73 m2 PBO: 51.2 ± 9.8 mL/min/1.73 m2 6 months Dapa: 55.3 ± 18.5 mL/min/1.73 m2 PBO: 45.3 ± 9.4 mL/min/1.73 m2 12 months Dapa: 64.4 ± 12.5 mL/min/1.73 m2 PBO: 54.3 ± 10.7 mL/min/1.73 m2 |
|
| Kashiwagi et al. 2015 63 |
24‐wk, randomized, double‐blind study followed by open‐label 52‐wk extension in Japanese patients with DKD (N = 165) Number (%) of Asians: 165 (100%); from Japan |
Ipragliflozin (50 mg) |
All patients Week 2 Ipra: −2.8 ± 4.78 mL/min/1.73 m2 PBO: +0.1 ± 4.34 mL/min/1.73 m2 Week 4 Ipra: −2.0 ± 5.77 mL/min/1.73 m2 PBO: +1.4 ± 5.35 mL/min/1.73 m2 Week 12 Ipra: −1.4 ± 5.85 mL/min/1.73 m2 PBO: +0.5 ± 5.60 mL/min/1.73 m2 Week 24 Ipra: +0.2 ± 6.56 mL/min/1.73 m2 PBO: +1.5 ± 6.00 mL/min/1.73 m2 Mild renal impairment Week 2 Ipra: −3.3 ± 5.07 mL/min/1.73 m2 PBO: −0.1 ± 4.92 mL/min/1.73 m2 Week 4 Ipra: −1.4 ± 5.99 mL/min/1.73 m2 PBO: +2.4 ± 6.19 mL/min/1.73 m2 Week 12 Ipra: −1.7 ± 6.39 mL/min/1.73 m2 PBO: −0.8 ± 6.80 mL/min/1.73 m2 Week 24 Ipra: +0.9 ± 7.57 mL/min/1.73 m2 PBO: +1.4 ± 6.84 mL/min/1.73 m2 Moderate renal impairment Week 2 Ipra: −2.4 ± 4.47 mL/min/1.73 m2 PBO: +0.3 ± 3.76 mL/min/1.73 m2 Week 4 Ipra: −2.5 ± 5.53 mL/min/1.73 m2 PBO: +0.3 ± 4.12 mL/min/1.73 m2 Week 12 Ipra: −1.2 ± 5.30 mL/min/1.73 m2 PBO: +1.9 ± 3.65 mL/min/1.73 m2 Week 24 Ipra: −0.4 ± 5.29 mL/min/1.73 m2 PBO: +1.6 ± 5.18 mL/min/1.73 m2 Week 52 +0.2 ± 6.56 mL/min/1.73 m2 |
All patients Week 2 Ipra: −35.64 ± 170.28 mg/g PBO: −2.03 ± 108.58 mg/g Week 24 Ipra: −23.72 ± 229.10 mg/g PBO: +4.28 ± 62.64 mg/g Mild renal impairment Week 2 Ipra: −19.83 ± 68.48 mg/g PBO: +3.21 ± 52.28 mg/g Week 24 Ipra: −10.79 ± 168.70 mg/g PBO: −9.51 ± 29.51 mg/g Moderate renal impairment Week 2 Ipra: −51.99 ± 232.66 mg/g PBO: −7.28 ± 146.02 mg/g Week 24 Ipra: −37.10 ± 279.14 mg/g PBO: +18.08 ± 82.19 mg/g Week 52 −23.72 ± 229.10 mg/g |
| Osonoi et al. 2018 64 |
Open‐label, 12‐wk study in Japanese T2D with microalbuminuria (N = 20) Number (%) of Asians: 20 (100%); from Japan |
Canagliflozin (100 mg) |
Week 12 −8.9 ± 7.5 (−12.4 to −5.3) mL/min/1.73 m2 |
Week 12 −19 mg/g % change from baseline (95% CI): 22% (−41.7 to 4.1) |
| Sugiyama et al. 2019 65 |
1‐y study of Japanese with DKD (N = 42) Number (%) of Asians: 42 (100%); from Japan |
SGLT‐2 inhibitor therapy |
1 year +0.3 (−0.9 to 2.7) mL/min/1.73 m2 Annual changes in eGFR (mL/min/1.73 m2 per year) Before SGLT‐2 inhibitor therapy: −3.8 (−6.0 to −1.7) After SGLT‐2 inhibitor therapy: +0.1 (−0.8 to +1.5) |
1 year −0.13 (−0.72 to −0.10) g/g |
| Takashima et al. 2018 66 |
52‐wk, randomized, open‐label study in patients with DKD (N = 42) Number (%) of Asians: 42 (100%); from Japan |
Canagliflozin (100 mg) |
Week 52 Cana: +0.7 ± 6.4 mL/min/1.73 m2 PBO: −3.4 ± 4.5 mL/min/1.73 m2 |
Week 52 Cana: −83 (−266 to −31) mg/g PBO: +27 (−11 to +131) mg/g |
Abbreviations: Cana, canagliflozin; Dapa, dapagliflozin; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; Empa, empagliflozin; Ipra, ipragliflozin; Saxa, saxagliptin; SGLT‐2, sodium‐glucose co‐transporter‐2; PBO, placebo; T2D, type 2 diabetes.
Another study evaluated the effects of ipragliflozin (50 mg) in Japanese T2D patients (N = 164) with mild (≥60 to <90 mL/min/1.73 m2) or moderate renal impairment (≥30 to <60 mL/min/1.73 m2). 63 The eGFR initially decreased during the first 4 weeks, but increased continuously until 24 weeks in patients with mild (difference vs. placebo: −3.8 mL/min/1.73 m2 at 4 weeks [P < .05] to +0.5 mL/min/1.73 m2 at 24 weeks [NS]) and moderate (−2.8 mL/min/1.73 m2 at 4 weeks to −2.0 mL/min/1.73 m2 at 24 weeks [P < .05 at both time points]) renal impairment. 63 The initial reduction in eGFR was considered to be a decrease in hyperfiltration, followed by stabilization of eGFR over time. In addition, UACR (mg/g Cr) was reduced by −10.79 and −37.10 mg/g in the mild and moderate renal impairment groups, respectively, at 24 weeks.
The randomized, double‐blind, multinational DELIGHT study assessed the albuminuria‐lowering effect (primary endpoint) of dapagliflozin alone (n = 145) or in combination with saxagliptin (n = 155) compared with placebo (n = 148) in patients with moderate‐to‐severe DKD (eGFR 25‐75 mL/min/1.73 m2) who were on stable optimal therapy. 67 The study enrolled patients from nine countries, including three Asian countries (Japan, South Korea and Taiwan; 177 patients from Asia, 39.5%). After 4 weeks of treatment, there was a significant reduction from baseline in UACR in the dapagliflozin (difference vs. placebo: −28.3%; 95% CI, −36.8% to −18.7%; P < .0001) and dapagliflozin‐saxagliptin groups compared with placebo (−34.5%; −42.1% to −25.9%; P < .0001). These reductions in UACR were sustained at week 24 (primary endpoint), with corresponding changes of −21.0% (−34.1% to −5.2%; P = .011) and −38.0% (−48.2% to −25.8%; P < .0001), respectively. The effect on UACR was significant even after adjustment for concomitant changes in HbA1c, systolic blood pressure (SBP), eGFR and uric acid. There was an initial decrease in eGFR at week 1 in both treatment groups (−4.8 and −4.6 mL/min/1.73 m2, respectively), followed by an increase and stabilization thereafter. At 24 weeks, the difference from baseline versus placebo was −2.4 mL/min/1.73 m2 in both treatment groups. 67
The CREDENCE trial confirmed the beneficial effects of SGLT‐2 inhibitors on renal outcomes. This trial was the first randomized, double‐blind study to evaluate the efficacy and safety of SGLT‐2 inhibitors on renal outcomes in patients with DKD across a wide range of renal function. 31 The trial randomized 4401 patients (including 877 [19.9%] Asians) with T2D and albuminuric kidney disease (eGFR 30‐90 mL/min/1.73 m2 and UACR >300‐5000) to receive canagliflozin (100 mg) or placebo added to renin‐angiotensin system inhibitors and GLDs. The primary outcome was a composite of ESKD, doubling of serum creatinine level from baseline for at least 30 days (central laboratory), or death from renal disease or CV disease. The trial was stopped early after a median follow‐up of 2.62 years because of an overwhelming benefit observed in the patients allocated to SGLT‐2 inhibitor treatment. There was a significant 30% risk reduction in the primary outcome in the canagliflozin group compared with placebo (HR 0.70; 95% CI, 0.59‐0.82) (Table 2). This effect was consistent across all ranges of baseline eGFR and UACR levels, in patients with or without CV disease, 69 as well as between Asian and Caucasian subgroups (interaction P‐value: NS). When analysed by individual endpoint, the risk of ESKD was reduced by 32% (HR 0.68, 95% CI, 0.54‐0.86; P = .002) and the risk of doubling of serum creatinine was reduced by 40% (HR 0.60, 95% CI, 0.48‐0.76; P < .001) in favour of canagliflozin. The risk of the composite of ESKD, doubling of the serum creatinine level, or renal death was lowered by 34% in the canagliflozin group compared with placebo (HR 0.66, 95% CI, 0.53‐0.81; P < .001). At 3 weeks, there was a greater decline in eGFR in the canagliflozin group compared with placebo (−3.72 ± 0.25 vs. −0.55 ± 0.25 mL/min/1.73 m2; between‐group difference: −3.17 mL/min/1.73 m2; 95% CI, −3.87 to −2.47). Thereafter, the decline in eGFR was slower in the canagliflozin than in the placebo group (−1.85 ± 0.13 vs. −4.59 ± 0.14 mL/min/1.73 m2; between‐group difference of 2.74 mL/min/1.73 m2 per year; 95% CI, 2.37‐3.11). 31
TABLE 2.
Effects on cardiorenal outcomes in patients with DKD
| No. of events | HR (95% CI) | P‐value | ||
|---|---|---|---|---|
|
CREDENCE (canagliflozin vs. placebo) 31 T2D patients with established kidney disease, N = 4401 Number (%) of Asians: 877 (19.9%) |
Cana (N = 2202) n |
Placebo (N = 2199) n |
||
| Renal outcomes | ||||
| ESKD, doubling of the serum creatinine level from baseline, or death from renal or CV disease | 245 | 340 | 0.70 (0.59‐0.82) | .00001 |
| ESKD, doubling of the serum creatinine level from baseline, or death from renal disease | 153 | 224 | 0.66 (0.53‐0.81) | <.001 |
| Doubling of the serum creatinine | 118 | 188 | 0.60 (0.48‐0.76) | <.001 |
| ESKD | 116 | 165 | 0.68 (0.54‐0.86) | .002 |
| eGFR <15 mL/min/1.73 m2 | 78 | 125 | 0.60 (0.45‐0.80) | ‐ |
| Dialysis initiated or kidney transplantation | 76 | 100 | 0.74 (0.55‐1.00) | ‐ |
| Dialysis, kidney transplantation or renal death | 78 | 105 | 0.72 (0.54‐0.97) | ‐ |
| CV outcomes | ||||
| MACE | 217 | 269 | 0.80 (0.67‐0.95) | .01 |
| CV death or HHF | 179 | 253 | 0.69 (0.57‐0.83) | <.001 |
| CV death | 110 | 140 | 0.78 (0.61‐1.00) | .05 |
| HHF | 89 | 141 | 0.61 (0.47‐0.80) | .001 |
|
DAPA‐CKD (dapagliflozin vs. placebo) 32 CKD patients with or without T2D, N = 4304 (T2D, n = 2906; no T2D, n = 1398) Number (%) of Asians: 1467 (34.0%) |
Dapa (N = 2152) n |
Placebo (N = 2152) n |
||
| Renal outcomes | ||||
| ≥50% sustained decline in eGFR, ESKD or death from renal or CV disease | 197 | 312 | 0.61 (0.51‐0.72) | <.001 |
| ≥50% sustained decline in eGFR, ESKD or death from renal disease | 142 | 243 | 0.56 (0.45‐0.68) | <.001 |
| ≥50% sustained decline in eGFR | 112 | 201 | 0.53 (0.42‐0.67) | ‐ |
| ESKD | 109 | 161 | 0.64 (0.50‐0.82) | ‐ |
| eGFR <15 mL/min/1.73 m2 | 84 | 120 | 0.67 (0.51‐0.88) | ‐ |
| Long‐term dialysis | 68 | 99 | 0.66 (0.48‐0.90) | ‐ |
| Kidney transplantation | 3 | 8 | ‐ | ‐ |
| Death from renal causes | 2 | 6 | ‐ | ‐ |
| CV outcomes | ||||
| CV death | 65 | 80 | 0.81 (0.58‐1.12) | ‐ |
| CV death or HHF | 100 | 138 | 0.71 (0.55‐0.92) | .009 |
Abbreviations: CV, cardiovascular; cana, canagliflozin; ESKD, end‐stage kidney disease; HHF, heart failure hospitalizations; MACE, major adverse cardiac events; T2D, type 2 diabetes.
The recently completed DAPA‐CKD trial showed that the beneficial renal effects of SGLT‐2 inhibitors extend to patients with CKD regardless of diabetes. The randomized, double‐blind, placebo‐controlled DAPA‐CKD trial evaluated the efficacy and safety of dapagliflozin on renal outcomes in patients with CKD with or without T2D. 32 A total of 4304 participants (including 1467 Asians [34.0%]; T2D, n = 2906; no T2D, n = 1398) with an eGFR of 25‐75 mL/min/1.73 m2 and a UACR of 200‐5000 mg/g who were on stable therapy with ACEi/ARB, were randomized to receive dapagliflozin (10 mg once daily) or placebo. The primary composite endpoint was worsening of kidney function (≥50% sustained decline in eGFR or onset of ESKD) or death because of kidney or CV disease. The trial was stopped early after a median follow‐up of 2.4 years because of an overwhelming benefit observed in the patients allocated to dapagliflozin. There was a significant 39% risk reduction in the primary composite endpoint in the dapagliflozin compared with the placebo group (HR 0.61; 95% CI, 0.51‐0.72; P < .001) (Table 2). This effect of dapagliflozin on primary outcome was consistent in patients with diabetes (HR 0.64; 95% CI, 0.52‐0.79) and in those without (HR 0.50; 95% CI, 0.35‐0.72). When analysed by the individual component of primary endpoint, the risk of ESKD was reduced by 36% (HR 0.64, 95% CI, 0.50‐0.82) and the risk of 50% or more sustained decline in eGFR was reduced by 47% (HR 0.53, 95% CI, 0.42‐0.67) in favour of dapagliflozin. The risk of kidney composite endpoint (≥50% sustained decline in eGFR, ESKD or death from renal causes), a secondary endpoint, was reduced by 46% in the dapagliflozin compared with the placebo group (HR 0.56, 95% CI, 0.45‐0.68; P < .001). Overall, the least‐square mean (SE) annual change in eGFR from baseline to 30 months was −2.86 ± 0.11 mL/min/1.73 m2 in the dapagliflozin versus −3.79 ± 0.11 mL/min/1.73 m2 in the placebo group (between‐group difference: +0.93 mL/min/1.73 m2; 95% CI, +0.61 to +1.25). During the first 2 weeks, there was a greater reduction in eGFR in the dapagliflozin than in the placebo group (−3.97 ± 0.15 vs. −0.82 ± 0.15 mL/min/1.73 m2). Thereafter, the annual decline in the mean eGFR was smaller in the dapagliflozin than in the placebo group (−1.67 ± 0.11 vs. −3.59 ± 0.11 mL/min/1.73 m2; between‐group difference: +1.92 mL/min/1.73 m2 per year; 95% CI, 1.61‐2.24). 32
6.2. Effect of SGLT‐2 inhibitors on the metabolic variables in patients with DKD
Apart from their glucose‐lowering effects, SGLT‐2 inhibitors also lower BP (range: SBP, −1.2 to −7.9 mmHg; diastolic BP, −0.8 to −6.1 mmHg), body weight (range: −1.29 to −3.9 kg) and serum uric acid (range: −0.2 to −1.0 mg/dL) and increase haematocrit (range: +0.59% to +5.5%), all of which have been shown to prevent or slow the progression of kidney disease. 70 , 71 , 72 , 73 , 74 Reduced levels of HbA1c or haematocrit are independent predictors of ESKD in patients with DKD, 75 , 76 , 77 which may result from the renal damage, and promote the decline of renal function. SGLT‐2 inhibitor therapy has been shown to increase haematocrit through augmentation of erythropoiesis, which may contribute to its beneficial cardiorenal effects. 78
In the pooled analysis of four 52‐week studies in Japanese patients with T2D, treatment with luseogliflozin was associated with reductions in HbA1c across the range of eGFR subgroups: normal (−0.67%), mild (−0.55%) and moderate renal impairment (−0.32%). 60 There was also a reduction in body weight (−2.68, −2.52 and −2.03 kg, respectively) and SBP (−4.7, −4.4 and −5.5 mmHg, respectively) and an increase in haematocrit (+2.02%, +2.31% and +2.32%, respectively) across the renal function subgroups. 60 In another 24‐week study in Japanese T2D patients with mild or moderate renal impairment, treatment with ipragliflozin reduced HbA1c in patients with mild or moderate renal impairment compared with placebo (difference vs. placebo: −0.35% and −0.17%, respectively), albeit only significantly in the mild renal impairment group (P < .001). Both groups also had a decrease in body weight and SBP, and an increase in haematocrit. 63
In the CREDENCE study, HbA1c was reduced from baseline in the canagliflozin group and remained lower than for the placebo group throughout the study, with a between‐group mean difference of −0.25% (95% CI, −0.31% to −0.20%). 31 Similarly, there were reductions in SBP and body weight in the canagliflozin group that were sustained throughout the study, with a between‐group mean difference of −3.30 mmHg (95% CI, −3.87 to −2.73) and −0.80 kg (95% CI, −0.93 to −0.69), respectively, compared with placebo.
In the DELIGHT study involving patients with T2D with moderate‐to‐severe renal impairment, HbA1c was reduced in dapagliflozin alone (difference vs. placebo: −0.2%, 95% CI, −0.4% to 0.1%) and dapagliflozin‐saxagliptin (−0.6%, 95% CI, −0.8% to −0.4%; P < .0001) groups, with a significant difference only for the dapagliflozin‐saxagliptin group. 67 There were reductions in body weight (dapagliflozin: −0.9%; dapagliflozin‐saxagliptin: −0.04%) and SBP (−2.8 and −4.8 mmHg) and an increase in haematocrit (+0.03% and +0.03%) in both the dapagliflozin and dapagliflozin‐saxagliptin groups.
6.3. Effect on CV outcomes in patients with DKD
CV death was evaluated as a part of the primary composite endpoint in the CREDENCE study in patients with DKD, whereas other CV outcomes, such as major adverse cardiovascular events (MACE) and hospitalization for heart failure (HHF), were evaluated as secondary endpoints. At a median follow‐up of 2.62 years, canagliflozin treatment reduced (a) MACE by 20%, (b) CV death or HHF by 31%, and (c) HHF alone by 39%, compared with placebo (Table 2). 31 The relative risk of CV death was reduced by 22% (HR, 0.78; 95% CI, 0.61‐1.00; P = .05). The risk reduction in CV outcomes was consistent in both primary and secondary prevention subgroups. 69 Of note, in the primary prevention cohort (without established CV disease), canagliflozin significantly reduced the risk of MACE by 32% (HR, 0.68; 95% CI, 0.49‐0.94) and HHF by 39% (HR, 0.61; 95% CI, 0.39‐0.96). 69
In the DAPA‐CKD trial, the composite of death from CV causes or HHF was reduced by 29% in the dapagliflozin compared with the placebo group (HR, 0.71; 95% CI, 0.55‐0.92; P = .009). The HR for the risk of CV death in the dapagliflozin versus placebo group was 0.81 (95% CI, 0.58‐1.12). 32
The effects of SGLT‐2 inhibition on CV outcomes stratified by eGFR subgroups in the CVOTs are summarized in Table S3. Overall, the CV benefits observed in these trials were consistent across the eGFR subgroups, including those with renal impairment. 33 , 34 , 35 , 79 However, these trials were not primarily designed to evaluate the effects on CV outcomes in patients with DKD, and the enrolment or randomization was not stratified by the baseline eGFR.
6.4. Safety of SGLT‐2 inhibitors in patients with DKD
In the CREDENCE trial, the rates of AEs and serious AEs were comparable between the canagliflozin and placebo groups. 31 There was no significant difference in the risk of amputation (events/1000 patient‐years: 12.3 vs. 11.2), fractures (11.8 vs. 12.1), hyperkalaemia (29.7 vs. 36.9), AKI (16.9 vs. 20.0) or acute pancreatitis (1.0 vs. 0.4) between the treatment groups. The overall rate of diabetic ketoacidosis was low; however, the incidence was higher in the canagliflozin than in the placebo group (2.2 vs. 0.2). In addition, the incidence of mycotic genital infection was higher in the canagliflozin group (males: 8.4 vs. 0.9; females: 12.6 vs. 6.1). 31
In the DAPA‐CKD trial, the incidences of AEs and serious AEs (proportion of patients: 29.5% vs. 33.9%) were comparable between the dapagliflozin and placebo groups. 32 There was no significant difference in the risk of amputation (1.6% vs. 1.8%), fractures (4.0% vs. 3.2%), renal‐related AEs (7.2% vs. 8.7%), volume depletion (5.9% vs. 4.2%) and major hypoglycaemia (0.7% vs. 1.3%) between the dapagliflozin and placebo groups. There was no incidence of diabetic ketoacidosis in the dapagliflozin group as well as no incidence of severe hypoglycaemia observed in individuals without T2D. 32
In the DELIGHT study, the rates of renal AEs were comparable between the dapagliflozin and placebo groups (proportion of patients: 3% vs. 4%). 67 The incidence of urinary tract infection (3% vs. 3%), mycotic genital infection (3% vs. 0%), volume depletion (3% vs. 3%) and diabetic ketoacidosis (1% vs. 0%) was comparable between the dapagliflozin and placebo groups. 67
7. RENAL EFFECTS OF SGLT‐2 INHIBITORS IN T2D PATIENTS (PREDOMINANTLY WITHOUT KIDNEY DISEASE)
Several clinical studies on Asian T2D patients without kidney disease reported effects of SGLT‐2 inhibitors on eGFR and UACR (Table S4). In placebo‐controlled monotherapy trials in treatment‐naïve patients with T2D (duration up to 24 weeks), the change in eGFR with SGLT‐2 inhibitor therapy ranged from +2.11 to −3.8 mL/min/1.73 m2 (a decrease by −3.8 units was reported in an 8‐week study). As an add‐on to other GLDs, the change in eGFR ranged from +6.0 to −9.8 mL/min/1.73 m2 (study duration up to 52 weeks).
The effects on renal outcomes in patients with T2D were assessed as a secondary endpoint in the CVOTs and are summarized in Table 3. In a meta‐analysis of three CVOTs that included 34 322 patients with T2D (5123 [15%] patients with eGFR <60 mL/min/1.73 m2), SGLT‐2 inhibitors reduced the risk of a composite renal endpoint (decline in eGFR and/or end‐stage renal disease [ESRD] and/or renal death) by 45% compared with placebo (HR 0.55, 95% CI, 0.48‐0.64; P < .0001). 80 The reduction in the composite renal endpoint was consistent across all baseline eGFR levels, with the greatest effect size in those with preserved renal function at baseline. The respective risk reductions were 33%, 44% and 56% in patients with an eGFR of less than 60, 60‐90 and 90 mL/min/1.73 m2 or higher, respectively.
TABLE 3.
SGLT‐2 inhibitor CVOTs: renal outcomes in T2D patients without DKD
| No. of events | HR (95% CI) | P‐value | ||
|---|---|---|---|---|
|
EMPA‐REG OUTCOME (empagliflozin vs. placebo) 35 , 81 T2D with or without CKD (26% of patients with CKD, 40% with albuminuria at baseline), N = 7020 Number (%) of Asians: 1517 (21.6%) |
Empa n/N |
Placebo n/N |
||
| Incident or worsening nephropathy or cardiovascular death | 675/4170 | 497/2102 | 0.61 (0.55‐0.69) | <.001 |
| Incident or worsening nephropathy | 525/4124 | 388/2061 | 0.61 (0.53‐0.70) | <.001 |
| Progression to macroalbuminuria | 459/4091 | 330/2033 | 0.62 (0.54‐0.72) | <.001 |
| Doubling of serum creatinine level accompanied by eGFR of ≤45 mL/min/1.73 m2 | 70/4645 | 60/2323 | 0.56 (0.39‐0.79) | <.001 |
| Initiation of renal replacement therapy | 13/4687 | 14/2333 | 0.45 (0.21‐0.97) | .04 |
| Doubling of serum creatinine level accompanied by eGFR of ≤45 mL/min/1.73 m2, initiation of renal replacement therapy or death from renal disease | 81/4645 | 71/2323 | 0.54 (0.40‐0.75) | <.001 |
| Incident albuminuria in patients with normoalbuminuria at baseline | 1430/2779 | 703/1374 | 0.95 (0.87‐1.04) | .25 |
|
CANVAS (canagliflozin vs. placebo) 33 T2D with or without CKD (20% of patients with CKD, 30% with albuminuria at baseline), N = 10 142 Number (%) of Asians: 1284 (12.7%) |
Cana n/N |
Placebo n/N |
||
| 40% reduction in eGFR, renal‐replacement therapy, or renal death | 124/5795 | 125/4347 | 0.60 (0.47‐0.77) | ‐ |
| Progression of albuminuria | 1341/5196 | 1114/3819 | 0.73 (0.67‐0.79) | ‐ |
| Regression of albuminuria | 885/1679 | 445/1257 | 1.70 (1.51‐1.91) | ‐ |
|
DECLARE‐TIMI 58 (dapagliflozin vs. placebo) 34 , 87 T2D with or without CKD (7.3% of patients with CKD at baseline), N = 17 160 Number (%) of Asians: 2303 (13.4%) |
Dapa (N = 8582) n |
Placebo (N = 8578) n |
||
| ≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD or death from renal or cardiovascular cause | 370 | 480 | 0.76 (0.67‐0.87) | <.0001 |
| ≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD or death from renal cause | 127 | 238 | 0.53 (0.43‐0.66) | <.0001 |
| ≥40% decrease in eGFR to <60 mL/min/1.73 m2 | 120 | 221 | 0·54 (0·43‐0·67) | <.0001 |
| ESRD | 6 | 19 | 0·31 (0·13‐0·79) | .013 |
| Renal death | 6 | 10 | 0·60 (0·22‐1·65) | .32 |
| ESRD or renal death | 11 | 27 | 0·41 (0·20‐0·82) | .012 |
Abbreviations: Cana, canagliflozin; CKD, chronic kidney disease; CVOTs, cardiovascular outcomes trials; dapa, dapagliflozin; DKD, diabetic kidney disease; empa, empagliflozin; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HHF, heart failure hospitalizations; MACE, major adverse cardiac events; SGLT‐2, sodium‐glucose co‐transporter‐2; UACR, urine albumin‐to‐creatinine ratio.
The EMPA‐REG OUTCOME trial included patients (N = 7034) with an eGFR of 30 mL/min/1.73 m2 or higher at screening, and 26% of patients had renal impairment (eGFR <60 mL/min/1.73 m2) at baseline. 35 , 81 Microalbuminuria was reported in 29% and macroalbuminuria in 11% of patients. Following the initiation of treatment with empagliflozin, a transient but significant decline in eGFR was observed at week 4 compared with placebo (mean ± SE change from baseline [mL/min/1.73 m2]: −0.62 ± 0.04 in the 10 mg group, −0.82 ± 0.04 in the 25 mg group and +0.01 ± 0.04 in the placebo group; P < .001 for both comparisons). However, during long‐term treatment from week 4 to end of study, eGFR remained stable in the empagliflozin compared with the placebo group, where there was a steady decline in the eGFR (mean ± SE annual rate of decline [mL/min/1.73 m2]: −0.19 ± 0.11 in both 10 and 25 mg groups vs. −1.67 ± 0.13 in the placebo group; P < .001 for both comparisons). The risk of doubling of serum creatinine was 44% lower in the empagliflozin group than placebo (HR 0.56, 95% CI, 0.39‐0.79; P < .001). 81 The relative risk of incident or worsening nephropathy (defined as progression to macroalbuminuria, doubling of serum creatinine level, initiation of renal‐replacement therapy or death from renal disease) was 39% lower in the empagliflozin compared with the placebo group (HR 0.61, 95% CI, 0.53‐0.70; P < .001). In addition, empagliflozin reduced progression to macroalbuminuria by 38% (HR 0.62, 95% CI, 0.54‐0.72; P < .001) and the risk of renal‐replacement therapy by 55% (HR 0.45, 95% CI, 0.21‐0.75; P < .001). There was no significant difference between the empagliflozin and placebo groups for the risk of incident albuminuria in patients with normal albuminuria. 81
A recent analysis from the EMPA‐REG OUTCOME trial reported renal outcomes in the Asian subgroup (N = 1517). 82 The beneficial effects of empagliflozin on renal outcomes in Asians were consistent with those in the overall study population. Empagliflozin reduced the risk of incident or worsening nephropathy (HR 0.64, 95% CI, 0.49‐0.83), progression to macroalbuminuria (HR 0.64, 95% CI, 0.49‐0.85), and the composite of doubling of serum creatinine, initiation of renal‐replacement therapy, or renal death (HR 0.48, 95% CI, 0.25‐0.92). There was an initial short‐term decline in eGFR, which then stabilized over the rest of the trial (192 weeks). The adjusted mean difference in change from baseline in eGFR between empagliflozin and placebo was +5.0 mL/min/1.73 m2 at follow‐up. 82
Similar renal effects were observed with canagliflozin in the CANVAS trial programme. The trial included patients (N = 10 142) with an eGFR of 30 mL/min/1.73 m2 or higher at screening, and 20.1% of patients had renal impairment (an eGFR of <60 mL/min/1.73 m2) at baseline. Microalbuminuria was reported in 22.6% and macroalbuminuria in 7.6% of patients. 33 , 83 Canagliflozin treatment reduced the risk of renal composite outcome (reduction in eGFR, renal‐replacement therapy or renal death) by 40% compared with placebo (HR 0.60, 95% CI, 0.47‐0.77). 33 The risk of progression of albuminuria was 27% lower in the canagliflozin compared with the placebo group (HR 0.73, 95% CI, 0.67‐0.79), with an increased likelihood of regression of albuminuria in the canagliflozin group (HR 1.70, 95% CI, 1.51‐1.91). 33 In a post hoc analysis, 79 risk reduction in the renal composite outcome was consistent across all the eGFR category subgroups (≥90, 60 to <90, 45 to <60 and <45 mL/min/1.73 m2). Within the first 13 weeks, a decline in eGFR was observed in the canagliflozin group, which was similar across the eGFR subgroups (placebo‐subtracted differences of −1.89, −2.33, −2.85 and −2.75 mL/min/1.73 m2, respectively). From week 13 to the end of follow‐up, the annual rate of decline in eGFR was lower in all canagliflozin subgroups, with placebo‐subtracted mean slope differences of +1.47, +1.09, +1.05 and +1.35 mL/min/1.73 m2 per year for the respective eGFR subgroups. 79
A secondary analysis of the Canagliflozin Treatment and Trial Analysis versus Sulphonylurea (CANTATA‐SU) study evaluated the renal effects of canagliflozin (100 and 300 mg) compared with glimepiride (uptitrated to 6‐8 mg) in T2D patients on metformin (N = 1450). 84 Over the 2 years of follow‐up, eGFR decline was slower in the canagliflozin than in the glimepiride group (−0.5 units per year [100 mg] and −0.9 units per year [300 mg] vs. −3.3 units per year [glimepiride]). In patients with albuminuria at baseline, UACR was reduced by 31.7% and 49.3% with canagliflozin 100 and 300 mg, respectively, compared with glimepiride. 84 By contrast, similar studies with dipeptidyl peptidase‐4 inhibitors (such as linagliptin) failed to show a benefit on either CV or renal outcomes compared with glimepiride or other oral GLDs. 85 , 86
The DECLARE‐TIMI 58 trial included patients (N = 17 160) with an eGFR of 60 mL/min/1.73 m2 or higher at screening, although 7.3% of patients had renal impairment (eGFR <60 mL/min/1.73 m2) at baseline. 34 Treatment with dapagliflozin reduced the risk of renal composite outcome (≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD, or death from renal or CV cause) by 24% compared with placebo (HR 0.76, 95% CI, 0.67‐0.87). Similar effects were observed after excluding CV deaths (≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD, or death from renal cause; HR 0.53, 95% CI, 0.43‐0.66). 34 A prespecified secondary analysis of the DECLARE‐TIMI 58 trial showed a reduction in the risk of renal outcomes with dapagliflozin treatment. 87 The risk of sustained 40% or more reduction in eGFR leading to moderate CKD (eGFR <60 mL/min/1.73 m2) was reduced by 46%, and that of ESRD by 69% in the dapagliflozin group compared with placebo (Table 3). The favourable effect of dapagliflozin on renal composite outcome was consistent in patients with mild renal impairment (HR 0.54, 95% CI, 0.40‐0.73). Other subgroup analyses of the DECLARE‐TIMI 58 trial showed that the effects of dapagliflozin on renal outcomes were consistent in patients with or without prior myocardial infarction, and in those with or without prior heart failure. 88 , 89
The beneficial renal effects of SGLT‐2 inhibitors observed in the CVOTs are corroborated by evidence from real‐world studies. The CVD‐REAL‐3 study evaluated the renal outcomes in T2D patients newly initiated on SGLT‐2 inhibitors versus other GLDs using data from medical claims, primary care/hospital records and national registries in five countries (Israel, Italy, Japan, Taiwan and the UK). 90 After propensity score matching, 35 561 episodes of treatment initiation of either SGLT‐2 inhibitors or other GLDs, from 65 231 patients with T2D, were included. Before initiation of index treatments, the mean (SD) annual rate of eGFR change was −0.73 (7.3) and −0.75 (11.9) mL/min/1.73 m2 in the SGLT‐2 inhibitor and other GLD groups, respectively; the mean follow‐up time was 14.9 months for both groups. After initiation of treatment, the annual rate of eGFR change was +0.46 (95% CI, 0.34 to 0.58) and −1.21 (−1.35 to −1.06) in the SGLT‐2 inhibitor and other GLD groups, respectively (between‐group difference: +1.53 mL/min per 1.73 m2 per year; 95% CI, 1.34 to 1.72; P < .0001). In addition, initiation of SGLT‐2 inhibitor was associated with a 51% reduction in relative risk of renal composite outcome (a 50% eGFR decline or ESKD; HR 0.49, 95% CI, 0.35 to 0.67; P < .0001). These results were similar across HbA1c and eGFR subgroups, and were consistent regardless of the presence or absence of CV disease or concomitant treatment with diuretics or ACEis or ARBs. 90
8. POTENTIAL MECHANISMS MEDIATING THE RENAL BENEFITS OF SGLT‐2 INHIBITORS
The renoprotective effects of SGLT‐2 inhibition appear to be independent of its blood glucose‐lowering effects and can be explained by its renal and systemic effects (Figure 2). Like ACEis or ARBs, SGLT‐2 inhibitors may exert favourable effects on the renal haemodynamics. Single nephron glomerular hyperfiltration is a characteristic feature in DKD caused by dilation of afferent and constriction of efferent arterioles. Under physiological conditions, the SGLT‐2 is responsible for ~5% of sodium reabsorption. Patients with T2D have increased expression of SGLT‐2 receptors, resulting in increased proximal tubular reabsorption and decreased delivery of sodium to the distal tubules, which are in close proximity to the macula densa. This results in decreased tubuloglomerular feedback leading to dilation of the afferent arteriole and increased glomerular perfusion. This is accompanied by high levels of angiotensin II leading to efferent arteriolar constriction, with a net effect of increased intraglomerular pressure and hyperfiltration. Inhibition of SGLT‐2 increases the distal delivery of sodium and activates tubuloglomerular feedback, resulting in constriction of the afferent arterioles, thereby reducing the glomerular hyperfiltration. The proximal natriuretic effect of SGLT‐2 inhibitors is enhanced by functional blockade of the sodium‐hydrogen exchanger 3, which further increases distal delivery of sodium. The effect of SGLT‐2 inhibitors on glomerular hyperfiltration can be accentuated in the presence of ACEis/ARBs, which cause efferent arteriolar vasodilation. 91 , 92
FIGURE 2.
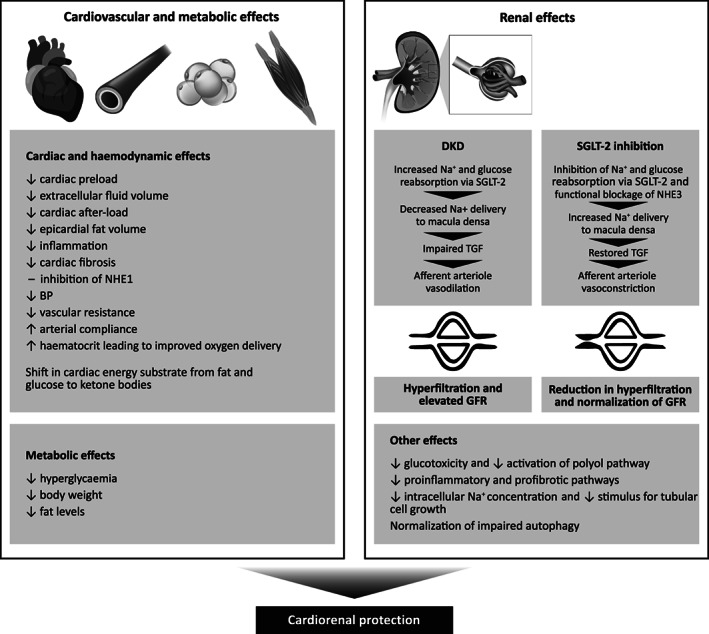
SGLT‐2 inhibitors: mechanism of cardiorenal benefits. Physiological mechanisms implicated in the cardiovascular and renal protection with SGLT‐2 inhibition. BP, blood pressure; DKD, diabetic kidney disease; GFR, glomerular filtration rate; NHE1, sodium‐hydrogen exchanger 1; NHE3, sodium‐hydrogen exchanger 3; SGLT‐2, sodium‐glucose co‐transporter‐2; TGF, tubuloglomerular feedback
Other renal effects of SGLT‐2 inhibition include (a) reduction in oxygen consumption and energy demand in the renal cortex with preserved tubular cell structure integrity and function; (b) reduction of glucose flux through the proximal tubular cells limiting glucotoxicity and activation of the polyol pathway; (c) reduction of intracellular sodium concentration in the proximal tubules with reduced stimulus for tubular cell growth; (d) inhibition of proinflammatory and profibrotic pathways; and (e) normalization of impaired autophagy. Other favourable haemodynamic and metabolic effects that may confer renoprotection include (a) improved cardiac function with maintenance of renal perfusion; (b) BP lowering; (c) shift in energy substrate from fat and glucose metabolism to more efficient ketone body oxidation; (d) decrease in endothelial dysfunction and arterial stiffness; (e) glycaemic control; (f) reduction in body weight and adiposity; and (g) increase in haematocrit leading to improved oxygen delivery. 93 , 94
9. THE FUTURE ROLE OF SGLT‐2 INHIBITORS IN DKD
Until recently, only ACEi or ARB therapy had been shown to improve outcomes in DKD. The CREDENCE study has confirmed the independent renoprotective effects of SGLT‐2 inhibitors in patients with DKD. The DAPA‐CKD trial has further established the beneficial renal effects of SGLT‐2 inhibitors in patients with CKD with or without T2D. Of note, renal outcomes were the primary endpoint in both the CREDENCE and DAPA‐CKD trials, and the beneficial effects of canagliflozin and dapagliflozin were additive to those of ACEis or ARBs. Most SGLT‐2 inhibitors are now approved for use in T2D patients with moderate (stage 3A) renal impairment (eGFR 45‐60 mL/min/1.73 m2). The mechanism of renoprotection of SGLT‐2 inhibitors appears to be independent of its blood glucose‐lowering effects as the same renal benefits are also observed in patients with non‐diabetic kidney disease. The results from the ongoing EMPA‐KIDNEY (NCT03594110) trial will further establish the role of SGLT‐2 inhibitors in delaying the progression of renal disease in patients with both DKD and non‐diabetic kidney disease.
In addition, as with other therapeutic intervention trials, there is an under‐representation of the Asian population in most of the completed and ongoing large randomized controlled trials that evaluate the efficacy and safety of SGLT‐2 inhibitors. Studies in Asian populations have mainly included patients from China, Japan and Korea, thus there is a lack of data from the South Asian region, which accounts for a large proportion of the global diabetes population. Hence, there is a need for more studies on the Asian population with DKD, ideally with a pragmatic or innovative design, considering the limited resources in the region.
10. CONCLUSIONS AND RECOMMENDATIONS
The rising incidence of CKD poses a major public health problem, especially in developing countries. In Asia, the problem is compounded by the growing prevalence of diabetes, obesity and hypertension, as well as limited healthcare resources for renal dialysis and kidney transplantation. In patients with DKD, optimal management includes intensive control of glycaemia and hypertension, and the use of ACEi or ARB therapy. SGLT‐2 inhibitors are GLDs that have unequivocally been shown in multiple clinical trials to improve renal outcomes in patients with DKD. Now, we have the evidence of the efficacy and safety of SGLT‐2 inhibitors with regard to renal outcomes in patients with CKD with or without T2D. Based on currently available evidence on SGLT‐2 inhibitors and clinical experience in patients with DKD, a series of clinical recommendations have been developed for the use of SGLT‐2 inhibitors in this population (Table 4).
TABLE 4.
Clinical recommendations on the use of SGLT‐2 inhibitors for the management of Asian patients with diabetic kidney disease (DKD)
| The burden of DKD in Asia | |
| 1 | Early‐onset diabetes and high prevalence of metabolic risk factors predispose Asian patients with T2D to a higher risk of DKD. |
| 2 | Patients with DKD are at a high risk of cardiovascular disease and progression to ESKD. |
| Management of DKD | |
| 3 | Patients with diabetes may have silent progression of kidney disease before the onset of clinical disease. Therefore, monitoring of renal function (at least annually) and albuminuria is critical for early detection and control of DKD. |
| 4 | In patients with DKD, a multifactorial management including optimal control of hyperglycaemia, blood pressure and dyslipidaemia is essential to delay the progression of renal disease and to reduce adverse cardiorenal outcomes. |
| SGLT‐2 inhibitors for the management of Asian patients with DKD | |
| 5 |
Effect on renal outcomes In patients with DKD, SGLT‐2 inhibitors significantly reduce the risk of renal disease progression defined as onset of ESKD or doubling of creatinine level from baseline or death from renal or CV disease.
|
| 6 | Treatment with SGLT‐2 inhibitors is associated with an initial decline in glomerular filtration rate, which is followed by progressive recovery and slowing in the decline of renal function with follow‐up. |
| 7 | In patients with moderate‐to‐severe DKD, treatment with SGLT‐2 inhibitors is associated with a sustained reduction in albuminuria. |
| 8 |
Effect on CV outcomes In patients with DKD, SGLT‐2 inhibitors significantly reduce the risk of major adverse CV events (defined as CV death or myocardial infarction or stroke) and HF hospitalizations. |
| 9 |
Effect on metabolic variables The blood glucose‐lowering effects of SGLT‐2 inhibitors are attenuated in patients with moderate or severe DKD.
|
| 10 | Treatment with SGLT‐2 inhibitors is associated with reduction in body weight and SBP, and improvements in uric acid and haematocrit in patients with DKD. |
| 11 |
Safety Before initiating SGLT‐2 inhibitor therapy, consider factors that may predispose patients to AKI, including hypovolaemia, dehydration, chronic renal insufficiency, congestive heart failure, peripheral vascular disease and concomitant medications such as diuretics, ACEi, ARBs and non‐steroidal anti‐inflammatory drugs. |
| Renal effects of SGLT‐2 inhibitors in patients with T2D (including those with CKD) | |
| 12 | In T2D patients with established or high risk of CV disease, including those with CKD, SGLT‐2 inhibitor therapy:
|
| Potential mechanism of renal effects | |
| 13 | The beneficial renal effects of SGLT‐2 inhibitors can be attributed to their renal and systemic effects.
|
| Role of SGLT‐2 inhibitors in the management of patients with DKD or those at high risk of DKD | |
| 14 | Considering their beneficial CV and renal effects, SGLT‐2 inhibitors represent a preferred therapy for:
|
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AKI, acute kidney injury; CKD, chronic kidney disease; CV, cardiovascular; DKD, diabetic kidney disease; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HHF, heart failure hospitalizations; MACE, major adverse cardiac events; SBP, systolic blood pressure; SGLT‐2, sodium‐glucose co‐transporter‐2; T2D, type 2 diabetes; UACR, urine albumin‐to‐creatinine ratio.
CONFLICT OF INTEREST
CMK has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. CD has received honoraria as the speaker or advisor or research grant from AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Eli Lilly, Abbott, Novartis, Pfizer, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda. SPC has received honoraria as speaker and advisor for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Servier. BM has received honoraria and CME grants from AstraZeneca, Boehringer Ingelheim, Lilly, Merck, Multicare, MSD, NatraPharm, Novartis, Novo Nordisk, Pfizer, Sanofi, Servier and Torrent Pharma. WHHS has been advisor and/or speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals., Daiichi‐Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi‐Aventis and Takeda Pharmaceutical Company. JC is the Chief Executive Officer (on pro‐bono basis) of Asia Diabetes Foundation, a charitable foundation established under The Chinese University of Hong Kong Foundation for developing the JADE Technology; she has received honoraria and travel support for consultancy or giving lectures, and her affiliated institutions have received research and educational grants from Amgen, Ascencia, AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi‐Sankyo, Eli‐Lilly, GlaxoSmithKline, Medtronic, Merck Serono, Merck Sharp & Dohme, Novo Nordisk, Pfizer and Sanofi. AM has received honoraria as speaker and advisor from AstraZeneca, Abbott, Boehringer Ingelheim, Cipla, Dr Reddyʼs, Eli Lilly, Glenmark, GlaxoSmithKline, Ipca, Janssen, Lupin, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, Serdia Servier, Sun Pharma, Torrent, Wockhardt and Zydus Nutrition. AL is a member of advisory boards for AstraZeneca, Boehringer Ingelheim, Sanofi and Amgen; she has received research grants from Boehringer Ingelheim, MSD, Sanofi and Amgen, and travel grants from AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Novo Nordisk and Sanofi. KS has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Sanofi, Merck and Servier. KHY has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda; and research support from AstraZeneca and Takeda. LJ is a member of the DISCOVER Scientific Committee and has received support from AstraZeneca to attend DISCOVER planning and update meetings; he has also received honoraria from Eli Lilly, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Bayer, Merck Sharp & Dohme, Takeda, Sanofi, Roche, Boehringer Ingelheim and AstraZeneca; and research support from Roche, Sanofi, Merck Sharp & Dohme, AstraZeneca, Novartis, Eli Lilly and Bristol‐Myers Squibb. NHM has no disclosures to declare. CP has received honoraria as a speaker or advisor for AstraZeneca, Otsuka, Merck Sharp & Dohme, Boehringer Ingelheim, Eli Lilly, Vifor and Novartis; she is a member of the CREDENCE trial steering committee and has received honoraria from Janssen to compensate for time spent working on the trial steering committee; she is also a director at Certa Therapeutics.
AUTHOR CONTRIBUTIONS
All the authors participated in the design, literature analysis and data interpretation. All the authors participated in the manuscript preparation, critically reviewed the draft manuscript and approved the final version of the manuscript for publication. All the authors take full responsibility for the accuracy of the content.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14251.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Syed Abdul Haseeb (MS, CMPP) of MediTech Media, Asia Pacific, which was funded by AstraZeneca Ltd, in accordance with Good Publication Practice (GPP3) guidelines. The development of this consensus statement was supported by an unrestricted educational grant from AstraZeneca Ltd, who had no influence on the content.
Khoo CM, Deerochanawong C, Chan SP, et al. Use of sodium‐glucose co‐transporter‐2 inhibitors in Asian patients with type 2 diabetes and kidney disease: An Asian perspective and expert recommendations. Diabetes Obes Metab. 2021;23:299–317. 10.1111/dom.14251
Funding information The development of this consensus statement was supported by an unrestricted educational grant from AstraZeneca Ltd, who had no influence on the content.
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- 1. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018;71(6):884‐895. [DOI] [PubMed] [Google Scholar]
- 3. Said SM, Nasr SH. Silent diabetic nephropathy. Kidney Int. 2016;90(1):24‐26. [DOI] [PubMed] [Google Scholar]
- 4. Polonia J, Azevedo A, Monte M, Silva JA, Bertoquini S. Annual deterioration of renal function in hypertensive patients with and without diabetes. Vasc Health Risk Manag. 2017;13:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang G, Luk AOY, Tam CHT, et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 2019;95(1):178‐187. [DOI] [PubMed] [Google Scholar]
- 6. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73:S291‐S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toyama T, Furuichi K, Ninomiya T, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all‐cause mortality, and renal events in diabetic patients: meta‐analysis. PLoS One. 2013;8(8):e71810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225‐232. [DOI] [PubMed] [Google Scholar]
- 9. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, UKPDS Study Group . Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832‐1839. [DOI] [PubMed] [Google Scholar]
- 10. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390(10105):1888‐1917. [DOI] [PubMed] [Google Scholar]
- 12. Chandie Shaw PK, Baboe F, van Es LA, et al. South‐Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch‐European diabetic patients. Diabetes Care. 2006;29(6):1383‐1385. [DOI] [PubMed] [Google Scholar]
- 13. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators . Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057‐2063. [DOI] [PubMed] [Google Scholar]
- 14. Wu AY, Kong NC, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia. 2005;48(1):17‐26. [DOI] [PubMed] [Google Scholar]
- 15. Balkau B, Deanfield JE, Despres JP, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116(17):1942‐1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng AY, Kong AP, Wong VW, et al. Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia. 2006;49(8):1777‐1784. [DOI] [PubMed] [Google Scholar]
- 17. Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross‐sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(suppl 1):53‐61. [DOI] [PubMed] [Google Scholar]
- 18. Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31(5):893‐898. [DOI] [PubMed] [Google Scholar]
- 19. Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young‐onset type 2 diabetes in Asia (the JADE programme): a cross‐sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935‐943. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association . Standards of medical care in diabetes‐2019. Diabetes Care. 2019;42(suppl 1):S1‐S193.30559224 [Google Scholar]
- 21. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 22. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383‐393. [DOI] [PubMed] [Google Scholar]
- 23. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861‐869. [DOI] [PubMed] [Google Scholar]
- 24. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851‐860. [DOI] [PubMed] [Google Scholar]
- 25. Viberti G, Wheeldon NM, MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators . Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure‐independent effect. Circulation. 2002;106(6):672‐678. [DOI] [PubMed] [Google Scholar]
- 26. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577‐1589. [DOI] [PubMed] [Google Scholar]
- 27. Wong MG, Perkovic V, Chalmers J, et al. Long‐term benefits of intensive glucose control for preventing end‐stage kidney disease: ADVANCE‐ON. Diabetes Care. 2016;39(5):694‐700. [DOI] [PubMed] [Google Scholar]
- 28. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884‐894. [DOI] [PubMed] [Google Scholar]
- 29. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;(4):CD007004. [DOI] [PubMed] [Google Scholar]
- 30. Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double‐masked, cross‐over study. Diabetes Care. 2005;28(9):2106‐2112. [DOI] [PubMed] [Google Scholar]
- 31. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 32. Heerspink HJL, Stefansson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436‐1446. [DOI] [PubMed] [Google Scholar]
- 33. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 34. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 35. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 36. International Diabetes Federation . IDF Diabetes Atlas, 8th edition. Updated 2017. https://www.idf.org/component/attachments/attachments.html?id=1405&task=download. Accessed June 2020.
- 37. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease ‐ a systematic review and meta‐analysis. PLoS One. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13(6):621‐630. [DOI] [PubMed] [Google Scholar]
- 39. Wen CP, Cheng TY, Tsai MK, et al. All‐cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173‐2182. [DOI] [PubMed] [Google Scholar]
- 40. Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51(3):373‐384. [DOI] [PubMed] [Google Scholar]
- 41. Mooyaart AL, Valk EJ, van Es LA, et al. Genetic associations in diabetic nephropathy: a meta‐analysis. Diabetologia. 2011;54(3):544‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young BA, Katon WJ, Von Korff M, et al. Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the PATHWAYS study. J Am Soc Nephrol. 2005;16(1):219‐228. [DOI] [PubMed] [Google Scholar]
- 43. Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND study. Cardiorenal Med. 2012;2(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yokoyama H, Oishi M, Kawai K, Sone H, Japan Diabetes Clinical Data Management Study Group . Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in type 2 diabetes: JDDM study 16. Diabet Med. 2008;25(12):1426‐1432. [DOI] [PubMed] [Google Scholar]
- 45. Brugnara L, Novials A, Ortega R, De Rivas B. Clinical characteristics, complications and management of patients with type 2 diabetes with and without diabetic kidney disease (DKD): a comparison of data from a clinical database. Endocrinol Diabetes Nutr. 2018;65(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 46. Hsieh YM, Lee WJ, Sheu WH, Li YH, Lin SY, Lee IT. Inpatient screening for albuminuria and retinopathy to predict long‐term mortality in type 2 diabetic patients: a retrospective cohort study. Diabetol Metab Syndr. 2017;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oellgaard J, Gaede P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long‐term renal benefits. Kidney Int. 2017;91(4):982‐988. [DOI] [PubMed] [Google Scholar]
- 48. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951‐964. [DOI] [PubMed] [Google Scholar]
- 49. National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850‐886. [DOI] [PubMed] [Google Scholar]
- 50. Malaysian Clinical Practice Guidelines . Management of type 2 diabetes mellitus. Updated 2015. http://www.acadmed.org.my/view_file.cfm?fileid=763. Accessed May 2018.
- 51. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guideline Development Group . Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant. 2015;30(suppl 2):ii1‐ii142. [DOI] [PubMed] [Google Scholar]
- 53. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium‐glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752‐772. [DOI] [PubMed] [Google Scholar]
- 54. Inzucchi SE, Zinman B, Wanner C, et al. SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12(2):90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. FDA Drug Safety Communication . FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin#:~:text=The%20U.S.%20Food%20and%20Drug,(Farxiga%2C%20Xigduo%20XR). Accessed July 2020.
- 56. Gilbert RE, Thorpe KE. Acute kidney injury with sodium‐glucose co‐transporter‐2 inhibitors: a meta‐analysis of cardiovascular outcome trials. Diabetes Obes Metab. 2019;21(8):1996‐2000. [DOI] [PubMed] [Google Scholar]
- 57. Cahn A, Melzer‐Cohen C, Pollack R, Chodick G, Shalev V. Acute renal outcomes with sodium‐glucose co‐transporter‐2 inhibitors: real‐world data analysis. Diabetes Obes Metab. 2019;21(2):340‐348. [DOI] [PubMed] [Google Scholar]
- 58. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care. 2017;40(11):1479‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kopyt NP. Slowing progression along the renal disease continuum. J Am Osteopath Assoc. 2005;105(4):207‐215. [PubMed] [Google Scholar]
- 60. Haneda M, Seino Y, Inagaki N, et al. Influence of renal function on the 52‐week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38(1):66‐88. [DOI] [PubMed] [Google Scholar]
- 61. Ito D, Inoue K, Sumita T, et al. Long‐term effects of ipragliflozin on diabetic nephropathy and blood pressure in patients with type 2 diabetes: 104‐week follow‐up of an open‐label study. J Clin Med Res. 2018;10(9):679‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jian X, Yang QL, Xiao S, Jing Z, Hu SD. The effects of a sodium‐glucose cotransporter 2 inhibitor on diabetic nephropathy and serum oxidized low‐density lipoprotein levels. Eur Rev Med Pharmacol Sci. 2018;22(12):3994‐3999. [DOI] [PubMed] [Google Scholar]
- 63. Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double‐blind, placebo‐controlled study on long‐term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long‐term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Osonoi T, Gouda M, Kubo M, Arakawa K, Hashimoto T, Abe M. Effect of canagliflozin on urinary albumin excretion in Japanese patients with type 2 diabetes mellitus and microalbuminuria: a pilot study. Diabetes Technol Ther. 2018;20(10):681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sugiyama S, Jinnouchi H, Yoshida A, et al. Renoprotective effects of additional SGLT2 inhibitor therapy in patients with type 2 diabetes mellitus and chronic kidney disease stages 3b‐4: a real world report from a Japanese specialized diabetes care center. J Clin Med Res. 2019;11(4):267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takashima H, Yoshida Y, Nagura C, et al. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: a randomized open‐label prospective trial. Diab Vasc Dis Res. 2018;15(5):469‐472. [DOI] [PubMed] [Google Scholar]
- 67. Pollock C, Stefansson B, Reyner D, et al. Albuminuria‐lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7(6):429‐441. [DOI] [PubMed] [Google Scholar]
- 68. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long‐term renal function. Kidney Int. 2011;80(3):282‐287. [DOI] [PubMed] [Google Scholar]
- 69. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2015;313(6):603‐615. [DOI] [PubMed] [Google Scholar]
- 71. Imai E, Haneda M, Chan JC, et al. Reduction and residual proteinuria are therapeutic targets in type 2 diabetes with overt nephropathy: a post hoc analysis (ORIENT‐proteinuria). Nephrol Dial Transplant. 2013;28(10):2526‐2534. [DOI] [PubMed] [Google Scholar]
- 72. Liu X, Zhai T, Ma R, Luo C, Wang H, Liu L. Effects of uric acid‐lowering therapy on the progression of chronic kidney disease: a systematic review and meta‐analysis. Ren Fail. 2018;40(1):289‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431‐437. [DOI] [PubMed] [Google Scholar]
- 74. Mahaffey K, Li J, Badve S, et al. The effects of canagliflozin on uric acid and gout in patients with type 2 diabetes in the CANVAS programme. EASD. Barcelona, Spain. Diabetologia. 2019;(Supp 1):340‐341. [Google Scholar]
- 75. Chan JC, Wat NM, So WY, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004;27(4):874‐879. [DOI] [PubMed] [Google Scholar]
- 76. Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end‐stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63(4):1499‐1507. [DOI] [PubMed] [Google Scholar]
- 77. Yang XL, So WY, Kong AP, et al. End‐stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia. 2006;49(10):2299‐2308. [DOI] [PubMed] [Google Scholar]
- 78. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 81. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 82. Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA‐REG OUTCOME((R)) trial. J Diabetes Investig. 2019;10(3):760‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691‐704. [DOI] [PubMed] [Google Scholar]
- 84. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28(1):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
- 88. Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub‐analysis from DECLARE TIMI‐58 trial. Circulation. 2019;139(22):2516‐2527. [DOI] [PubMed] [Google Scholar]
- 89. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139(22):2528‐2536. [DOI] [PubMed] [Google Scholar]
- 90. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD‐REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 91. Muskiet MHA, Wheeler DC, Heerspink HJL. New pharmacological strategies for protecting kidney function in type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7(5):397‐412. [DOI] [PubMed] [Google Scholar]
- 92. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium‐glucose cotransporter‐2 inhibitors. Kidney Int. 2018;94(1):26‐39. [DOI] [PubMed] [Google Scholar]
- 94. Kawanami D, Matoba K, Takeda Y, et al. SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci. 2017;18(5):1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


