Abstract
X‐linked hypophosphataemia (XLH) and osteogenesis imperfecta (OI) are rare congenital disorders characterised by skeletal dysplasia. The two disorders may include dental anomalies potentially affecting individual well‐being. The aims of study were (a) to assess the oral health‐related quality of life (OHRQoL) in Danish adults with XLH or OI, and (b) to compare the results of the groups. A cross‐sectional study including 35 adults with XLH, 56 adults with OI type I and 17 adults with OI types III‐IV was conducted. The OHRQoL was assessed by the 49‐item version of the questionnaire Oral Health Impact Profile (OHIP). Summed domain scores (seven) were compared between XLH and OI groups. Prevalence of severe impact on OHRQoL (scores 3‐4) was compared between groups. The median scores in XLH group exceeded the medians in OI (P < .05) in the domains functional limitation (XLH:6.5; OI:4.0), pain (XLH:9.5; OI:5.0), psychological discomfort (XLH:5.5; OI:2.0), psychological disability (XLH:2.0; OI:0.0), handicap (XLH:2.0; OI:0.0) and total OHIP (XLH:35.0; OI:14.0). Differences in domains physical disability (XLH: 4.0; OI: 1.0) and social disability (XLH: 0.0; OI: 0.0) were not significant. Prevalence of severe impact on OHRQoL in the XLH group significantly exceeded the level in OI group in the domains functional limitation (XLH: 59%; OI: 35%), psychological discomfort (XLH: 38%; OI: 20%) and physical disability (XLH: 32%; OI: 13%). In conclusion, adults with XLH experience a higher negative impact on their OHRQoL than adults with OI. Only to a minor degree, individuals with OI types III‐IV experience a higher impact on OHRQoL than individuals with OI type I.
Keywords: disability, familial hypophosphataemic rickets, oral health, osteogenesis imperfecta, quality of life, tooth, X‐linked hypophosphataemia
1. BACKGROUND
The inherited diseases X‐linked hypophosphataemia (XLH) and osteogenesis imperfecta (OI) are rare systemic disorders, which both affect the skeleton leading to skeletal deformity and fractures, pain, mobility issues and functional limitation. Furthermore, the diseases may also affect dental tissues, oral health and oral health‐related well‐being.
XLH is characterised by an insufficient mineralisation of the bones and dental tissues due to abnormal renal phosphate wasting. 1 It is the most predominant of the inherited types of hypophosphataemia caused by mutations in the gene encoding for the phosphate‐regulating endopeptidase homolog, X‐linked (PHEX, MIM 300550). In children, the disease is called X‐linked hypophosphataemic rickets (XLHR, MIM 307800). 2 In Denmark, the prevalence of XLH is 4.8:100 000. 3 The typical clinical features of XLH in growing children are short, disproportionate stature, bone pain and skeletal deformities, for example bowing of weight‐bearing extremities and rachitic manifestations as metaphyseal swelling and rachitic rosary. In adulthood, patients often present with short disproportionate stature and skeletal deformities not sufficiently corrected during childhood. Adults with XLH often develop mineralising enthesopathies and osteoarthritis with increasing age, both associated with pain. In addition, patients with XLH may experience dental abnormalities, including spontaneous dental abscesses, 4 , 5 , 6 , 7 where the number of endodontically affected teeth increases with increasing age. 8
OI is a connective tissue disorder, characterised by bone fragility and an increased risk of low‐impact fractures. 9 Short stature, bone deformity, blue sclera, hearing loss and disturbances in the dental development also occur. Skeletal pain and certain physical disabilities are frequent in patients with OI. 10 , 11 , 12 OI is in most cases caused by defective collagen type 1, a protein constituting an essential part of the connective tissue. In Denmark, the prevalence of OI is estimated to be 11 per 100 000. 13 The most frequently used OI classification includes four clinical subtypes (I‐IV): Type I (mild and minor bone deformities), Type II (severe bone deformities and perinatal death), Type III (very short stature and severely progressing bone deformities) and Type IV (mildly affected growth and moderate bone deformities). 14 The classical dental aberration of OI is dentinogenesis imperfecta (DI). The clinical characteristics of DI are greyish or brownish discoloration of the dentition, obliteration of the dental cavum, shortness of dental roots and cervical constriction. 15 , 16 The prevalence of DI in Danish patients with OI is 25%. 17 Furthermore, Class III malocclusion with mandibular overjet is prevalent in patients with OI, especially Type III. 18 , 19 , 20
Most research on rare, inherited skeletal disorders has focused on the pathogenesis and clinical manifestations. During the last two decades, health‐related quality of life has also been investigated in studies on skeletal disorders. 21 Results of these studies indicate that XLH and OI impact on daily living and quality of life of affected individuals. 22 , 23 , 24 In addition, skeletal disorders with oro‐dental implications may also impact on the oral health‐related quality of life (OHRQoL). However, research on quality of life (QoL) with focus on oral health in the field of rare diseases is sparse. 25 , 26 XLH and OI are rare systemic diseases, which both impact on the skeleton but have different oro‐dental implications. The former is dominated by a high prevalence of endodontically affected teeth and frequent episodes of pain and the latter by the prevalent occurrence of aesthetically compromising DI and mandibular overjet. It could be relevant to investigate which of the two diseases have the greatest impact on OHRQoL, the condition with endodontically related pain implications or the condition dominated by an aesthetically compromised dentition. Furthermore, it may be questioned if OHRQoL is primarily related to number of teeth present in the oral cavity. This knowledge will be clinically relevant for dentists treating patients with these rare diseases as well as decision‐makers concerning the financial aspects such as reimbursement of patients with the two diseases.
The aims of the present study were as follows: (a) to assess OHRQoL in Danish adults with XLH or OI, (b) to make a comparison of OHRQoL between patients with XLH and OI, respectively and patients with mild OI vs moderate/severe OI and (c) to elucidate whether the number of teeth or the number of endodontically affected teeth impact on OHRQoL in the study population.
2. METHODS
2.1. Study population
The present dental study was conducted as a part of two separate cross‐sectional studies, each investigating a rare skeletal disorder in adult Danish individuals with XLH 1 or OI. 27 Patients were identified by search in medical files at university hospitals in Denmark, by contact to doctors at regional hospitals, and by contact to patient groups and families with one of these conditions as previously described. 1 , 27
In 49 children and adults, the XLH diagnosis was confirmed and they consented to participate in the main XLH study. 1 Thirty‐six adults in this group accepted to participate in the dental part of the study. The 36 adults with XLH (24 females (66.6%)) had a mean age of 41.6 years (SD = 15.8, range 19‐74). A detailed description of patient recruitment as well as genetic and biochemical verification of XLH has previously been published. 1 , 28
In 85 adults, the OI diagnosis was confirmed and they consented to participate in the main OI study. 27 A total of 75 of these patients also participated in the dental part of the study (OI type I: n = 56, type III: n = 7 and type IV: n = 12). The 75 adults with OI (40 females (53.3%)), had a mean age of 45.5 years (SD = 14.7, range 20‐77). Patients with OI were grouped into mild OI (OI type I) and moderate‐severe OI (types III‐IV). A detailed description of patient recruitment as well as genetic and clinical verification of OI has been published previously. 27
2.2. Study design
The dental parts of the studies were performed at Section of Pediatric Dentistry, Department of Dentistry and Oral Health, Aarhus University, Denmark. Intraoral clinical photographs and panoramic radiographs were obtained as previously described. 8 , 17 The total number of teeth in the oral cavity was counted. The number of endodontically affected teeth (teeth with radiographic signs of root‐filling or periapical osteolysis or both) were counted as previously described. 8 , 17 The presence of individuals with DI was assessed as previously described. 17 In the present study, occlusion was assessed on digital study models or plaster models and from clinical photographs. The presence or absence of mandibular overjet was recorded. A mandibular overjet was defined as a horizontal overjet ≤ 0 mm. During the participants' visit in the department, they were asked to fill out a questionnaire focusing on OHRQoL: The Danish version of the Oral Health Impact Profile (OHIP). OHIP is composed of 49 questions grouped into seven domains (functional limitation, physical pain, psychological impact, physical disability, psychological disability, social disability and handicap). 29 , 30 The questionnaire contains questions asking the respondents to score the frequency of specified symptom(s) or experience(s) ranging from ‘never’ to ‘daily’ or ‘always’ (scores 0‐4) (Table 1).
TABLE 1.
OHIP‐49 divided in the seven domains with an example of questions in individual domains
| Domain | Question numbers a | Example of question |
|---|---|---|
| Functional limitation (FuLimit) | 1‐9 | Have you felt that your sense of taste was worsened because of problems with your teeth, mouth or denture? |
| Physical pain (Pain) | 10‐18 | Have you had toothache? |
| Psychological discomfort (PsycDisc) | 19‐23 | Have you felt uncomfortable about the appearance of your teeth, mouth or denture? |
| Physical disability (PhysDisa) | 24‐32 | Have you been unable to brush your teeth properly due to problems with your teeth, mouth or denture? |
| Psychological disability (PsycDisa) | 33‐38 | Have you felt depressed because of problems with your teeth, mouth or dentures? |
| Social disability (SocDisa) | 39‐43 | Have you had difficulty doing your usual jobs because of problems with your teeth, mouth or dentures? |
| Handicap | 44‐49 | Have you experienced inability to enjoy the company of other people because of problems with your teeth, mouth or dentures? |
Questions which only focus on the usage of dentures have been omitted (question no. 9 and question no. 18).
The study was conducted in accordance with the Helsinki Declaration and approved by The Regional Scientific Ethical Committee of Southern Denmark (ID: M‐2678‐05), and by The Central Denmark Region Committees on Health Research Ethics (M‐20100108).
2.3. Statistical analyses
Data were analysed using Stata® 11.0 (StataCorpLP). Descriptive statistics were used to summarise and compare demographic data (age and gender) of the groups in the study population (XLH and OI). Furthermore, a comparison by the Wilcoxon rank‐sum test between groups on the total number of teeth and the number of endodontically affected teeth was included. The prevalence of DI and mandibular overjet in the respective groups was compared by the chi‐square test.
Overall OHIP score and domain scores for each participant were calculated by summing the response codes (0‐4) for the questions. If one or more of the questions in a domain were unanswered, the respective domain score as well as the overall OHIP score were recorded as missing and results were thus excluded from the analyses. The mean additive score of each domain as well as the mean overall OHIP score were calculated and indicated the severity of impact on OHRQoL in the respective domains. 31 In addition, the median additive scores were calculated, as data were not normally distributed. For the OHIP scale as a whole and for each of the seven domains, the number of answers reported as ‘very often’ or ‘fairly often’ (codes 3 or 4), was counted. The mean of these figures indicates the extent of severe impact on OHRQoL in the respective domains. The percentage of individuals responding to one or more questions with ‘very often’ or ‘fairly often’ (codes 3 or 4) was calculated and indicated the prevalence of severe impact on OHRQoL in the respective domains. 31
The median values of OHIP domain and total scores in the respective groups (XLH vs OI; OI mild vs OI moderate‐severe) were compared by the Wilcoxon rank‐sum test (Mann‐Whitney). For each of the OHIP domains and for the OHIP total, prevalence of severe impact on OHRQoL (scores 3‐4) in the respective groups (XLH or OI) was compared by logistic regression analysis with adjustment for the effect of gender and age. Potential interactions between the effect of gender and group (XLH or OI) and between gender and group were assessed in the regression analyses.
The association between each of the OHIP domain scores and the total number of teeth present in the oral cavity of the individual or the number of endodontically affected teeth was assessed by Spearman's rank correlation in the total group of participants (XLH and OI) and in each of the two subgroups (XLH or OI).
The null hypothesis said there is no difference in OHIP domain scores in the comparison between XLH and OI or in the comparison between mild OI and moderate/severe OI. Furthermore, it was hypothesised that neither the total number of teeth present nor the number of endodontically affected teeth impacted on the OHIP domain scores.
Values of P < .05 were considered statistically significant.
3. RESULTS
OHIP questionnaire data were obtained from 35 patients with XLH and 73 patients with OI. The mean age was 39.8 years in XLH group (95%CI: 34.2‐45.5) and 45.9 years (95%CI: 42.4‐49.4) in OI group; the difference in mean age was not statistically significant (P = .0568). The gender ratio (male%/female%) was 31/67 in XLH group and 45/55 in the OI group; the difference in gender ratio was not statistically significant (P = .173). The dental characteristics in terms of the total number of teeth and total number of endodontically affected teeth are shown in Table 2. There was no significant difference in total number of teeth in the XLH group compared with the OI group (P = .915). The number of endodontically affected teeth was higher in the XLH group compared with the OI group (P < .0001). DI was present in two of 55 individuals (4%) with mild OI, in all 17 individuals with moderate‐severe OI, and not present in the XLH group (Table 2). In one of the individuals with mild OI, DI was not assessed due to extensive tooth loss. Mandibular overjet was most prevalent in the group with moderate‐severe OI and present in only one individual in the XHL group (Table 2).
TABLE 2.
The total number of teeth present in the oral cavity, the number of endodontically affected teeth, the prevalence of dentinogenesis imperfecta (DI) and the prevalence of mandibular overjet; a comparison of total OI group vs XLH group and a comparison between OI subgroups
| N a | Teeth, total number | Endodontically affected teeth, number | DI | Mandibular overjet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean (SD) | 95% CI | Median | Mean (SD) | 95% CI | x patients b | x patients b | ||||||
| XLH | 35 | 27 |
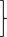 ns ns |
25.1 (7.87) | 22.4‐27.8 | 4 |
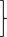 *
*
|
5.3 (4.89) | 3.7‐7.0 | 0 |
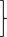 **
**
|
1 (4%) |
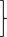 ns ns |
| OI all | 73 | 27 | 25.2 (6.13) | 23.8‐26.7 | 1 | 1.9 (2.22) | 1.4‐2.5 | 19 (26%) | 11 (16%) | ||||
| OI mild | 56 | 27.5 |
 ns ns |
25.6 (6.14) | 23.9‐27.2 | 2 |
 *
*
|
2.2 (2.30) | 1.6‐2.8 | 2 (4%) |
 **
**
|
4 (7%) |
 **
**
|
| OI severe | 17 | 27 | 24.2 (6.16) | 21.0‐27.3 | 0 | 1.06 (1.71) | 0.2‐1.9 | 17 (100%) | 7 (50%) | ||||
Missing data on DI in one patient with OI (one with mild OI).
Missing data on mandibular overjet in seven patients with XLH and four patients with OI (one with mild OI, three with moderate/severe OI).
Total number of patients in each group.
The number of patients with the specified diagnosis (DI or mandibular overjet) and the percentages of the total patient number (excl. patients with missing data) in each group.
P‐value < .05 in the comparison of medians by the Wilcoxon rank‐sum test (XLH vs OI and OI mild vs OI severe).
P‐value < .05 in the comparison of prevalence by the chi‐square test (XLH vs OI and OI mild vs OI severe).
The OHIP profile in terms of mean values of domain scores in the XLH and OI groups is illustrated in Figure 1. Reference data in Figure 1 are domain mean values in a group of 30 healthy Scandinavian recall patients in an OHIP reliability and validity study by Larsson et al 32
FIGURE 1.
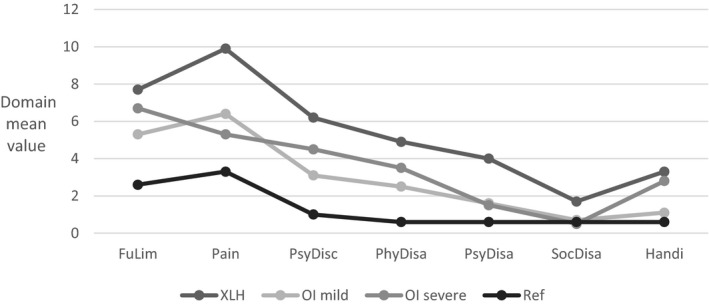
OHIP profile by means of domain scores in 35 adults with XLH, 56 adults with mild OI (type 1) and 17 adults with moderate‐severe OI (type 3‐4). Reference OHIP profile is obtained from domain mean values in a group of 30 healthy recall patients in a OHIP reliability and validity study by Larsson et al 32
When comparing the median values in the XLH and OI group, respectively the impact of XLH exceeds the impact of OI in the OHIP total score and in domains on functional limitation, pain, psychological discomfort, psychological disability and handicap (Figure 2). The number of missing values were in the XLH group, which had a total of 35 respondents, one (2.9%) in domains ‘functional limitations’, ‘pain’ and ‘psychological discomfort’, seven (20%) in domain ‘physical disability’, two (5.8%) in ‘psychological disability’, one (2.9%) in ‘social disability’ and zero in domain ‘handicap’. The number of missing values were in the OI group, which had a total of 73 respondents, seven (9.6%) in domain ‘functional limitations’, six (8.2%) in domain ‘pain’, three (4.1%) in ‘psychological discomfort’, 17 (23.3%) in domain ‘physical disability’, two (2.7%) in ‘psychological disability’, three (4.1%) in ‘social disability’ and eight (11%) in domain ‘handicap’.
FIGURE 2.
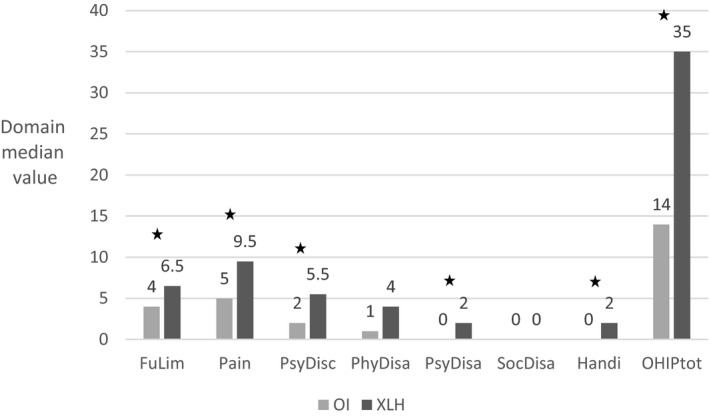
OHIP median domain and total scores, a comparison between 35 adults with XLH and 73 adults with OI.*P > .05
When comparing the median values in mild OI (type I) and moderate‐severe OI (types III‐IV), the impact of moderate‐severe OI significantly exceeds the impact of mild OI in only two domains: physical disability (OI type I: median = 1.0; OI types III‐IV: median = 2.5) and handicap (OI type I: median = 0.0; OI types III‐IV: median = 2.0). The number of missing values were in the mild OI group, which had a total of 56 respondents, five (8.9%) in domain ‘functional limitations’, three (5.4%) in domains ‘pain’ and ‘psychological discomfort’, 12 (21.4%) in domain ‘physical disability’, two (3.6%) in ‘psychological disability’, three (5.4%) in ‘social disability’ and five (8.9%) in domain ‘handicap’. The number of missing values were in the moderate‐severe OI group, which had a total of 17 respondents, two (11.8%) in domain ‘functional limitations’, three (17.6%) in domain ‘pain’, zero in domain ‘psychological discomfort’, five (29.4%) in domain ‘physical disability’, zero in domains ‘psychological disability’ and ‘social disability’, and three (17.6%) in domain ‘handicap’.
The Table S1 shows all data stratified according to type of disorder and gender.
In the total group studied (XLH and OI), the number of teeth in the oral cavity was negatively associated (P < .05) with the domain scores (the higher number of teeth, the lower OHIP scores) except for three domains (pain, social disability and handicap) (Table 3).
TABLE 3.
Association between OHIP domain scores and total number of teeth or the number of endodontically affected teeth in the total group of patients with XLH or OI
| FuLimit | Pain | PsycDisc | PhysDisa | PsycDisa | SocDisa | Handicap | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| ῥ | (teeth no) a | −0.3236* | 0.0185 | −0.2054* | −0.4110* | −0.2514* | −0.2049 | −0.1846 | −0.3040* |
| ῥ | (endo no) b | 0.4910* | 0.3331* | 0.4357* | 0.3938* | 0.5019* | 0.3952* | 0.5015* | 0.5064* |
Spearman rank correlation coefficient; OHIP domain scores vs number of teeth.
Spearman rank correlation coefficient; OHIP domain scores vs number of endodontically affected teeth.
P‐value < .05.
In each of the two groups (XLH or OI), the number of teeth in the oral cavity was negatively associated (P < .05) with the domain scores except for four domains, three being the same as mentioned above (XLH: pain, psychological disability, social disability and handicap) (OI: pain, psychological discomfort, social disability and handicap). In the total group (XLH and OI), the number of endodontically affected teeth was positively associated (P < .05) with the domain score in all domains (the higher number of endodontically affected teeth, the higher OHIP scores) (Table 3). The number of respondents (N) in the respective domains varied because of varying numbers of missing domain scores, the range of N being 67‐104. An assessment in each of the two groups (XLH or OI), showed a significant association between the number of endodontically affected teeth and domain score in all domains except one in each group (XLH: pain) (OI: physical disability). There was no significant association between gender and the level of domain score in any of the domains.
The prevalence of severe impact on OHRQoL in the XLH group significantly exceeded the prevalence in the OI group in four of the seven domains (Table 4). According to the logistic regression analysis, only the domain, psychological discomfort was significantly influenced by gender with males being most severely affected. Age did not significantly influence the results, and no significant interactions between group (XLH or OI) and gender or between group and age were found (Table 4). No significant differences were found in the comparison between the two OI subgroups.
TABLE 4.
Prevalence of individuals with severe impact on OHRQoL defined as one or more items scoring 3‐4 in the individual OHIP domains; a comparison between XLH group and the whole OI group adjusted for the effect of gender and age; OI female was the baseline
| OI | XLH | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| N a | No. score 3‐4 (%) b | N a | No. score 3‐4 (%) b | Baseline c | Group | Gender | Age | |
| FuncLimit | 66 | 23 (35)* | 34 | 20 (59)* | 0.21 (0.05;0.93) | 3.02 (1.24;7.40)* | 1.12 (0.50;2.61 | 1.02 (0.99;1.05) |
| Pain | 67 | 16 (24)(*) | 34 | 16 (47)(*) | 0.95 (0.22;4.05) | 2.46 (0.99;6.09)(*) | 0.84 (0.34;2.10) | 0.98 (0.95;1.01) |
| PsycDiscom | 70 | 14 (20)* | 34 | 13 (38)* | 0.18 (0.04;0.86 | 2.89 (1.10;7.59)* | 2.77 (1.08;7.09)* | 1.00 (0.97;1.03) |
| PhysDisa | 56 | 7 (13)* | 28 | 9 (32)* | 0.10 (0.02;0.65) | 3.48 (1.11;10.92)* | 1.18 (0.38;3.71) | 1.01 (0.97;1.04) |
| PsycDisa | 71 | 5 (7) | 33 | 3 (9) | 0.01 (0.001;0.21) | 1.52 (0.32;7.13) | 0.43 (0.08;2.31) | 1.04 (0.99;1.10) |
| SocDisa | 70 | 1 (1) | 34 | 1 (3) | 0.02 (0.001;1.90) | 2.10 (0.12;37.75) | 1.65 (0.10;28.20) | 0.99 (0.90;1.08) |
| Handicap | 65 | 9 (14) | 35 | 9 (26) | 0.05 (0.007;0.32) | 2.51 (0.85;7.44) | 0.70 (0.23;2.14) | 1.03 (0.99;1.06) |
Number of individuals who have answered all items of the specified domain and group.
Number of individuals who have answered ‘very often’ or ‘fairly often’ in one or more items of the specified domain and group.
Baseline denotes the constant, which is odds ratio (OR) for a female with OI to have scored 3‐4 in the specified domain.
P‐value < .05 in the comparison between XLH and OI by logistic regression analysing the probability of domain scores 3‐4 according to group (XLH or OI), gender and age. (*) P‐value = .052.
4. DISCUSSION
According to the present cross‐sectional study, adults with either XLH or OI experienced an impact on their OHRQoL; XLH had the most severe impact. When comparing individuals with mild OI and moderate‐severe OI, the moderate‐severely affected individuals experienced a slightly higher impact on their OHRQoL.
Both XLH and OI are characterised by skeletal dysplasia, and patients in both groups experience skeletal symptoms and limitations in their physical abilities. 11 , 33 However, the disorders have different aetiology and pathophysiology. XLH is characterised by disturbances in the phosphate homeostasis causing a defect mineralisation of bone and teeth 2 , 34 and OI being characterised by structural abnormalities of collagen, the major organic part of the bone. 10 XLH and OI differ in impact on the dentition and craniofacial structures. OI may include various degrees of dentinal dysplasia, DI, characterised by discoloration, pulp obliteration and an increased risk of dental fractures. However, DI is mainly associated with moderate‐severe OI, 16 which is in accordance with the results in the present cohort, where the prevalence of DI was 100% in moderate‐severe OI and close to zero in mild OI (Table 2). In contrast, XLH is associated with another type of dentinal dysplasia in addition to enamel dysplasia 5 , 6 predisposing for pulp necrosis, dental abscesses and thus the need for endodontic treatment. 8 In the present study, this is confirmed by the high prevalence of endodontically affected teeth in the XLH group compared to the OI group (Table 2). The median number of teeth present in XLH and OI was similar (N = 27), which is in line with the median numbers reported previously in a general adult Danish population examined in 1997, 2003 and 2008. 35 Thus, our results indicate that dental extractions are relatively rare irrespective of the high prevalence of DI in moderate‐severe OI or the high prevalence of endodontically affected teeth in XLH. However, the apparent low number of extractions may be due to selection bias caused by omission of edentulous individuals and patients' likely denial of participation in dental examinations if they have extensive decay and tooth loss. Furthermore, the location of absent teeth as well the type of prostheses if any might impact on OHRQoL. For example, absent maxillary incisors might influence social and psychological disability more than missing molars. Because of the limited sample sizes, it was decided not to adjust for these parameters in the comparison between groups. According to dental occlusion and craniofacial morphology, mandibular overjet and posterior cross‐bite were the dominant malocclusions in patients with moderate‐severe OI in the present cohort 18 (Table 2), which is in line with other studies. 19 , 20 Malocclusion in the OI group may be explained by the relative mandibular prognathism (unpublished data), which is significantly increased in comparison with the fairly normal values on inter‐maxillary relations in the XLH group as previously reported. 28
The OHIP profiles of both XLH and OI groups (Figure 1) qualitatively follow the profile in other studies using OHIP‐49 as the tool for assessment of OHRQoL. Examples are the Swedish OHIP validation study on groups with Sjögren syndrome, oral mucosal pain disorders or skeletal malocclusion, 32 and a Dutch study on patients with hypodontia receiving prosthodontic treatment in term of implants. 36 In common, all groups including the present cohorts of XLH and OI are characterised by reporting the highest score in the domains on functional limitation, and pain, followed by domains on psychological discomfort and physical disability; the lowest scores are in domains on psychological disability, social disability and handicap. However, the groups differ quantitatively concerning the level of the mean domain scores. In the OI and XLH groups of the present study, the scores exceeded the level of the reference group in all domains, except for the social domain. In relation to OHRQoL, this is an indication of minimal or no impact on social well‐being in contrast to the significant impact on oral health‐related functional limitation, pain and physical disability. This is in accordance with results from OI studies focusing on the general health‐related quality of life using the SF36 questionnaire, in which the mental component scores equalise the reference data in contrast to the physical component scores. 22 , 23 However, according to the OHIP instrument used in the present study, both XLH and OI seemingly influenced psychological discomfort as well as psychological disability. This indicates that oral and dental issues might be of special importance for the psychological well‐being of individuals with XLH or OI.
According to the comparison between the XLH and OI, patients with XLH experienced the highest impact on their OHRQoL in all domains, except for the social domain, which was low in both groups. In the study by Forestier‐Zhang et al 21 on the overall health‐related QoL, it was concluded that health‐related QoL was similar in XLH and OI. This discrepancy from the results of the present study on OHRQoL indicates that dental status and oral health have an isolated impact on QoL and that this finding is more prominent in XLH than in OI. Our study showed that the number of teeth in the oral cavity as well as the number of endodontically‐affected teeth might to some extent impact on OHRQoL irrespective of the underlying disease (Table 3). The more teeth, the lower scores in some of the OHIP domains. However, the values of the correlation coefficients are small, and the occurrence of a beta‐error in the statistical analyses cannot be excluded. The mean number of teeth did not differ between the two groups (XLH and OI) (Table 2); thus, this is not likely to explain OHRQoL differences between OI and XLH. In contrast, an increasing number of endodontically affected teeth seemed to be associated with increasing OHIP domain scores, and the endodontically affected teeth were more prevalent in XLH compared to the OI group (Table 2). It is reasonable to assume that the prevalent endodontic problems experienced by patients with XLH affect OHRQoL. This is supported by a previous study showing an obvious impact on OHRQoL in patients referred for endodontic treatment compared with patients referred for periodontal treatment. 37
In our study, prevalence of severe impact on OHRQoL was higher in the XLH group in the domains on functional limitation, pain, psychological discomfort and physical disability compared with the OI group (Table 4). In the OI group, participants with moderate‐severe OI had DI characterised by severe discoloration and a high prevalence of mandibular overjet (Table 2), which was not present in the XLH population except for one individual with mandibular overjet (Table 2). Mandibular overjet is indicative of a severe class III malocclusion, and according to a previous study, class III malocclusion has a greater impact on OHRQoL than normal class I occlusion. 38 However, the number of individuals with mandibular overjet or class III malocclusion in the present OI group was low, which makes comparison to the other class III groups challenging.
Due to the rarity of the disorders, the number of participants was limited. However, the present study population was relatively large compared with previous studies focusing on oral and dental issues in OI or XLH. 4 , 5 , 6 , 19 , 20 However, a risk of selection bias is present as not all Danish patients with the two diseases were enrolled and those not included may be the more mildly affected patient. To avoid too small study groups, the moderate and severe OI types were merged prior to data analysis. This grouping was supported by OI type 1 being characterised by quantitative deviations in type 1 collagen, whereas types 3 and 4 are characterised by qualitative deviations in type 1 collagen. 9 , 10 , 27 The wide range of age in the two groups might be a limitation. Due to the rarity of the diseases, this limitation had to be accepted to ensure a reasonable number of participants in the study. In addition, visual assessments of scatter plots of age vs OHIP domain scores demonstrated an apparent random effect of age on level of OHIP domain scores.
A strength of the present study was that the whole spectrum of patients diagnosed with OI or XLH was enrolled. In contrast, the missing control group is a limitation of the study, but the usage of reference OHIP data from a previous Scandinavian study on OHRQoL 32 served as a reasonable frame of reference for the OHIP data of the present study groups of OI and XLH.
A complete data set was not obtained for all participants, being a limitation of the study. However, questionnaire data were only totally missing in two participants (from the group with moderate‐severe OI). The majority of the missing data were related to single questions not answered by individual persons, which resulted in missing data on the specific domain. The greatest impact of missing data was in the domain ‘physical disability’ and the total OHIP score. However, it was a minor number of missing data and thus unlikely to have influenced our interpretation of the results.
Finally, to improve the OHRQoL in XLH and OI, we recommend a multidisciplinary approach with regular dental examinations to ensure early identification of dental problems allowing early preventative interventions aiming at preserving teeth and preventing endodontic treatments.
5. CONCLUSIONS
Adults with XLH experience a higher negative impact on their OHRQoL than adults with OI. Individuals with moderate‐severe OI only to a minor degree experience a higher impact on OHRQoL than individuals with mild OI. The impact in the XLH group seems to be associated with the high number of endodontically affected teeth.
ETHICS APPROVAL
The study was conducted in accordance with the Helsinki Declaration and approved by the Central Denmark Region Committees on Health Research Ethics (M‐20100108). The study complies with the STROBE Guidelines. All patients gave their written consent to participate in the study prior to inclusion.
CONFLICT OF INTEREST
Dr Gjørup reports personal fees from Kyowa Kirin, though non‐related to the submitted work. Dr Beck‐Nielsen reports Grants from Kyowa Kirin, personal fees from Kyowa Kirin, non‐financial support from Kyowa Kirin, personal fees from Novo Nordisk, personal fees from Pharma Cosmos, though non‐related to the submitted work. Dr Hald and Dr Haubek declare that they have nothing to disclose. The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
HG contributed to conception, design, clinical examinations and data acquisition, data analysis and interpretation, and prepared the first draft of the manuscript. SSB contributed to the conception of the study, recruited the participants with XLH, contributed to the interpretation of the data and critically revised the manuscript. JDH contributed to conception of the study, recruited the participants with OI, contributed to interpretation of the data and critically revised the manuscript. DH contributed to conception, design, data acquisition, analysis and interpretation, and critically revision of the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13114.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was supported by Central Denmark Region, Danish Osteoporosis Association (Osteoporoseforeningen Denmark), The Danish Association for Public Dentists (TNL/DOFT/ATO) and Care4BrittleBones.
Gjørup H, Beck‐Nielsen SS, Hald JD, Haubek D. Oral health‐related quality of life in X‐linked hypophosphataemia and osteogenesis imperfecta. J Oral Rehabil.2021;48:160–168. 10.1111/joor.13114
Funding information
Central Denmark Region; the Danish Osteoporosis Association (Osteoporoseforeningen, Denmark); the Danish Association for Public Dentists (ATO) and Care4BrittleBones. The role of the funding agencies was financial support, and funding agencies have not been involved in the design of the study, collection, analysis or interpretation of data or in writing the manuscript.
REFERENCES
- 1. Beck‐Nielsen SS, Brusgaard K, Rasmussen LM, et al. Phenotype presentation of hypophosphatemic rickets in adults. Calcif Tissue Int. 2010;87:108‐119. [DOI] [PubMed] [Google Scholar]
- 2. Hyp C. A gene (PEX) with homologies to endopeptidases is mutated in patients with X‐linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130‐136. [DOI] [PubMed] [Google Scholar]
- 3. Beck‐Nielsen SS, Brock‐Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160:491‐497. [DOI] [PubMed] [Google Scholar]
- 4. Souza MA, Soares Junior LA, Santos MA, Vaisbich MH. Dental abnormalities and oral health in patients with Hypophosphatemic rickets. Clinics (Sao Paulo). 2010;65:1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baroncelli GI, Angiolini M, Ninni E, Galli V, Saggese R, Giuca MR. Prevalence and pathogenesis of dental and periodontal lesions in children with X‐linked hypophosphatemic rickets. Eur J Paediatr Dent. 2006;7:61‐66. [PubMed] [Google Scholar]
- 6. Cremonesi I, Nucci C, D'Alessandro G, Alkhamis N, Marchionni S, Piana G. X‐linked hypophosphatemic rickets: enamel abnormalities and oral clinical findings. Scanning. 2014;36:456‐461. [DOI] [PubMed] [Google Scholar]
- 7. Chaussain‐Miller C, Sinding C, Wolikow M, Lasfargues JJ, Godeau G, Garabedian M. Dental abnormalities in patients with familial hypophosphatemic vitamin D‐resistant rickets: prevention by early treatment with 1‐hydroxyvitamin D. J Pediatr. 2003;142:324‐331. [DOI] [PubMed] [Google Scholar]
- 8. Andersen MG, Beck‐Nielsen SS, Haubek D, Hintze H, Gjorup H, Poulsen S. Periapical and endodontic status of permanent teeth in patients with hypophosphatemic rickets. J Oral Rehabil. 2012;39:144‐150. [DOI] [PubMed] [Google Scholar]
- 9. Glorieux FH. Osteogenesis imperfecta. Best Pract Res Clin Rheumatol. 2008;22:85‐100. [DOI] [PubMed] [Google Scholar]
- 10. Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377‐1385. [DOI] [PubMed] [Google Scholar]
- 11. Wekre LL, Froslie KF, Haugen L, Falch JA. A population‐based study of demographical variables and ability to perform activities of daily living in adults with osteogenesis imperfecta. Disabil Rehabil. 2010;32:579‐587. [DOI] [PubMed] [Google Scholar]
- 12. Bishop NJ, Walsh JS. Osteogenesis imperfecta in adults. J Clin Invest. 2014;124:476‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen PE Jr, Hauge M. Osteogenesis imperfecta: a genetic, radiological, and epidemiological study. Clin Genet. 1989;36:250‐255. [DOI] [PubMed] [Google Scholar]
- 14. Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shields ED, Bixler D, el‐Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol. 1973;18:543‐553. [DOI] [PubMed] [Google Scholar]
- 16. Barron MJ, McDonnell ST, Mackie I, Dixon MJ. Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis. 2008;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thuesen KJ, Gjorup H, Hald JD, et al. The dental perspective on osteogenesis imperfecta in a Danish adult population. BMC Oral Health. 2018;18:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bendixen KH, Gjorup H, Baad‐Hansen L, et al. Temporomandibular disorders and psychosocial status in osteogenesis imperfecta – a cross‐sectional study. BMC Oral Health. 2018;18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stenvik A, Larheim TA, Storhaug K. Incisor and jaw relationship in 27 persons with osteogenesis imperfecta. Scand J Dent Res. 1985;93:56‐60. [DOI] [PubMed] [Google Scholar]
- 20. Jensen BL, Lund AM. Osteogenesis imperfecta: clinical, cephalometric, and biochemical investigations of OI types I, III, and IV. J Craniofac Genet Dev Biol. 1997;17:121‐132. [PubMed] [Google Scholar]
- 21. Forestier‐Zhang L, Watts L, Turner A, et al. Health‐related quality of life and a cost‐utility simulation of adults in the UK with osteogenesis imperfecta, X‐linked hypophosphatemia and fibrous dysplasia. Orphanet J Rare Dis. 2016;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balkefors V, Mattsson E, Pernow Y, Saaf M. Functioning and quality of life in adults with mild‐to‐moderate osteogenesis imperfecta. Physiother Res Int. 2013;18(4):203‐211. [DOI] [PubMed] [Google Scholar]
- 23. Hald JD, Folkestad L, Harslof T, Brixen K, Langdahl B. Health‐related quality of life in adults with osteogenesis imperfecta. Calcif Tissue Int. 2017;101:473‐478. [DOI] [PubMed] [Google Scholar]
- 24. Che H, Roux C, Etcheto A, et al. Impaired quality of life in adults with X‐linked hypophosphatemia and skeletal symptoms. Eur J Endocrinol. 2016;174:325‐333. [DOI] [PubMed] [Google Scholar]
- 25. Wiemann S, Frenzel Baudisch N, Jordan RA, Kleinheinz J, Hanisch M. Oral symptoms and oral health‐related quality of life in people with rare diseases in Germany: a cross‐sectional study. Int J Environ Res Public Health. 2018;15:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanisch M, Bohner L, Sabandal MMI, Kleinheinz J, Jung S. Oral symptoms and oral health‐related quality of life of individuals with x‐linked hypophosphatemia. Head Face Med. 2019;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hald JD, Folkestad L, Harslof T, et al. Skeletal phenotypes in adult patients with osteogenesis imperfecta‐correlations with COL1A1/COL1A2 genotype and collagen structure. Osteoporos Int. 2016;27:3331‐3341. [DOI] [PubMed] [Google Scholar]
- 28. Gjørup H, Kjaer I, Sonnesen L, et al. Craniofacial morphology in patients with hypophosphatemic rickets: a cephalometric study focusing on differences between bone of cartilaginous and intramembranous origin. Am J Med Genet A. 2011;155A:2654‐2660. [DOI] [PubMed] [Google Scholar]
- 29. Gjørup H, Svensson P. OHIP‐(D) oral health impact profile. Et redskab til registrering af livskvalitet I forhold til det orofaciale område. Danish Dent J. 2006;110:8. [Google Scholar]
- 30. Slade GD, Spencer AJ. Development and evaluation of the oral health impact profile. Community Dent Health. 1994;11:3‐11. [PubMed] [Google Scholar]
- 31. Slade GD, Nuttall N, Sanders AE, Steele JG, Allen PF, Lahti S. Impacts of oral disorders in the United Kingdom and Australia. Br Dent J. 2005;198:489‐493. [DOI] [PubMed] [Google Scholar]
- 32. Larsson P, List T, Lundström I, Marcusson A, Ohrbach R. Reliability and validity of a Sweedish version of the Oral Health Impact Profile (OHIP‐S). Acta Odontol Scand. 2004;62:147‐152. [DOI] [PubMed] [Google Scholar]
- 33. Steele A, Gonzalez R, Garbalosa JC, et al. Osteoarthritis, osteophytes and enthesophytes affect biomechanical function in adults with X‐linked hypophosphatemia. J Clin Endocrinol Metab. 2020;105:e1798–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beck‐Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X‐linked hypophosphatemia‐related morbidity. Orphanet J Rare Dis. 2019;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirkevang LL, Vaeth M, Wenzel A. Ten‐year follow‐up observations of periapical and endodontic status in a Danish population. Int Endod J. 2012;45:829‐839. [DOI] [PubMed] [Google Scholar]
- 36. Filius MAP, Vissink A, Cune MS, Raghoebar GM, Visser A. Effect of implant therapy on oral health‐related quality of life (OHIP‐49), health status (SF‐36), and satisfaction of patients with several agenetic teeth: prospective cohort study. Clin Implant Dent Relat Res. 2018;20:592‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu P, McGrath C, Cheung GS. Quality of life and psychological well‐being among endodontic patients: a case‐control study. Aust Dent J. 2012;57:493‐497. [DOI] [PubMed] [Google Scholar]
- 38. Javed O, Bernabe E. Oral impacts on quality of life in adult patients with class I, II and III malocclusion. Oral Health Prev Dent. 2016;14:27‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


