Summary
Background
Serum hepatitis B virus (HBV) RNA may reflect intrahepatic HBV replication. Novel anti‐viral drugs have shown potent HBV RNA decline without concomitant hepatitis B surface antigen (HBsAg) decrease. How this relates to off‐treatment response is yet unclear.
Aim
To study the degree of on‐treatment viral antigen decline among patients with pronounced HBV RNA decrease in relation to off‐treatment sustained response and HBsAg loss.
Methods
HBV RNA, HBsAg and hepatitis B core‐related antigen (HBcrAg) were quantified in patients with chronic hepatitis B who participated in two randomised controlled trials of peginterferon‐based therapy. Sustained response (HBV DNA <2000 IU/mL) and/or HBsAg loss were assessed in patients with and without on‐treatment HBV RNA response (either >2 log HBV RNA decline or >1 log decline resulting in an undetectable value at on‐treatment week 24), stratified by concomitant HBsAg decline (<0.5/0.5‐1/>1 log).
Results
We enrolled 279 patients; 176 were hepatitis B e antigen (HBeAg)‐positive, and 103 were HBeAg‐negative. Sustained response was achieved in 20.4% of patients. At on‐treatment week 24, HBV RNA response was associated with higher sustained response rates (27.4% vs 13.0% in non‐responders, P = 0.004). However, among patients with an HBV RNA response (n = 135), 56.4% did not experience >0.5 log HBsAg decline. Among HBV RNA responders, sustained response was achieved in 47.6% of those with >1 log HBsAg decline (n = 20/42), vs 16.0% with <0.5 log decline (n = 12/75, P = 0.001). Similar results were obtained with HBcrAg and when response was defined as HBsAg loss.
Conclusions
In this cohort, many patients with HBV RNA response during peginterferon‐based treatment did not experience HBsAg and/or HBcrAg decline. The absence of concomitant decline in these viral antigens was associated with low rates of treatment response and HBsAg loss. Future trials should therefore consider kinetics of combined biomarkers to assess anti‐viral efficacy.
Trial registration, ClinicalTrials.gov: NCT00114361, NCT00146705.
1. INTRODUCTION
Chronic hepatitis B (CHB) infection is one of the main causes of end‐stage liver disease and hepatocellular carcinoma (HCC). 1 The optimal goal of anti‐viral treatment is the achievement of an off‐treatment sustained response to reduce the incidence of HCC and limit the progression of liver disease. 2 However, this remains difficult to achieve, as covalently closed circular DNA (cccDNA) persists in the hepatocytes. 3 , 4 , 5 On‐treatment maintained viral suppression is therefore a second‐best alternative.
While novel compounds are emerging, current therapeutic options are still limited to nucleo(s)tide analogues (NAs) and pegylated interferon (PEG‐IFN). Nucleo(s)tide analogues are well tolerated and suppress HBV replication effectively. 6 Nonetheless, nucleo(s)tide analogues do not directly affect cccDNA 7 and are therefore associated with a limited off‐treatment sustained response rate. 8 , 9 A finite duration of PEG‐IFN can result in higher sustained response rates, as it is able to inhibit HBV transcription and reduces the production of viral particles through targeting cccDNA. 10 However, sustained response is only achieved in a limited proportion of patients. 10 , 11 , 12 PEG‐IFN therapy is currently experiencing a revival, as it may be more effective when combined with novel anti‐virals. 13 , 14
Eradication of intrahepatic cccDNA is considered to be a crucial step in the clearance of the hepatitis B virus (HBV), and monitoring its kinetics during therapy is highly desirable. However, cccDNA can only accurately be quantified invasively by liver biopsy. Therefore, non‐invasive serological markers that correlate with intrahepatic replicative activity of HBV are needed to assess the efficacy of (novel) anti‐viral agents in CHB patients.
Recently, hepatitis B virus (HBV) RNA has emerged as a potential prognostic biomarker for treatment response, as it correlates with transcriptional activity of cccDNA and therefore may reflect intrahepatic replication activity. 15 , 16 , 17 , 18 Recent studies suggest that a decline in serum HBV RNA levels during treatment with nucleo(s)tide analogues or PEG‐IFN is associated with treatment response, although overall declines during treatment were limited. 19 , 20 , 21 , 22 , 23 , 24 Interestingly, recent phase 1 studies of novel capsid assembly modifiers have shown substantially stronger HBV RNA declines, which has been interpreted as a possible sign of a more potent effect on the intrahepatic HBV reservoir. 25
However, besides the observed decline in serum HBV RNA during capsid assembly modifiers therapy, little changes in hepatitis B surface antigen (HBsAg) and hepatitis B core‐related antigen (HBcrAg) concentrations were observed in these patients. 25 How this relates to long‐term off‐treatment response is yet unclear. 26 We therefore aimed to study the degree of on‐treatment HBsAg and HBcrAg decline among patients with pronounced HBV RNA decrease, both in relation to off‐treatment sustained response and HBsAg loss.
2. PATIENTS AND METHODS
2.1. Study population
For the current study, we enrolled chronic hepatitis B (CHB) patients who participated in two global randomised controlled trials (the 99‐01 and PARC study; trial registration numbers NCT00114361 and NCT00146705). Detailed information regarding inclusion criteria and study design have been described elsewhere. 27 , 28 In short, the 99‐01 study enrolled hepatitis B e antigen (HBeAg)‐positive patients (n = 266), who were randomised to treatment with PEG‐IFN alpha‐2b 100 µg/week plus lamivudine 100 mg/day or PEG‐IFN plus placebo for 52 weeks. 27 In the PARC study, HBeAg‐negative patients (n = 133) were treated with PEG‐IFN alpha‐2a 180 µg/week monotherapy or PEG‐IFN combination therapy with the addition of ribavirin 1000‐2000 mg for 48 weeks. 28 Response was assessed at 6 months after therapy discontinuation (end of follow‐up; EOF). For both studies, eligible patients had been HBsAg positive for at least 6 months, had a serum HBV DNA level of more than 10 000 copies/mL (equals ± 2000 IU/mL) and an elevated ALT greater than 1.5‐2 times the upper limit of normal (ULN) within 8 weeks before randomisation. The original study protocols were in line with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the medical ethical committees. Both the 99‐01 and PARC studies demonstrated that combination therapy was not superior to PEG‐IFN monotherapy, and data were therefore pooled for the current analysis. 27 , 28 For the current study we selected patients from the original studies if data were available for our primary outcome (sustained response) and a baseline HBV RNA measurement was available.
2.2. Serum HBV RNA, HBsAg and HBcrAg quantification
HBV RNA was quantified from serum samples using rapid amplification of complimentary DNA (cDNA)‐ends (RACE)‐based real‐time polymerase chain reaction (PCR) at a central laboratory (University Hospital Leipzig, Germany). The PCR technique has been described previously. 19 The lower limit of detection (LOD) for HBV RNA in this assay was 800 copies/millilitre (c/mL). 29 Quantitative HBsAg levels were assessed using Abbott Architect (Abbott, Abbott Park, IL). The assay's LOD for HBsAg levels was 0.05 IU/mL. HBcrAg was quantified using the Lumipulse® G HBcrAg assay (Fujirebio Europe). The LOD of HBcrAg measurements was 100 U/mL (2 log U/mL). 30 As HBcrAg partially depends on HBeAg‐status and is low or undetectable in most HBeAg‐negative patients during therapy, 30 the relationship between HBcrAg and response was assessed in the HBeAg‐positive subgroup only.
2.3. Endpoints
Primary outcomes were sustained response and HBsAg loss. Sustained response was defined as HBV DNA <2000 IU/mL 6 months after end of treatment. HBsAg loss was assessed at end of follow‐up and during long‐term follow‐up. 23 , 27 , 28 HBV RNA, HBsAg and HBcrAg levels were measured at baseline, on‐treatment week 12, on‐treatment week 24, end of treatment (EOT) and during follow‐up. HBV RNA response was defined as HBV RNA decline of either >2 log or an HBV RNA decline or >1 log which resulted in HBV RNA level below the LOD. HBsAg decline was categorised as <0.5 log, 0.5‐1 log and >1 log. HBcrAg decline was categorised as <1 log, 1‐3 log and >3 log. For this study, we assessed HBV RNA response and concomitant HBsAg or HBcrAg decline at on‐treatment week 24. For sensitivity analysis, HBV RNA response at different time points (on‐treatment week 12 and EOT) and in different subgroups (HBeAg‐status and type of treatment) was also assessed.
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS for Windows version 25.0 (SPSS Inc). Descriptive data were reported as percentages, means (±standard deviation; SD) and medians (with interquartile range; IQR) when appropriate. Data were tested for significance using chi‐squared test, Fisher's exact test or student's t‐test where appropriate. Univariate logistic regression analysis was used to estimate odds ratios (ORs) of HBsAg decline for sustained response in patients with HBV RNA response at on‐treatment week 24. Differences were considered statistically significant when P ≤ 0.05. Graphic representation of the results was performed using Graph Pad Prism version 5 for Windows (GraphPad Software).
3. RESULTS
3.1. Patient characteristics
This study included 279 patients; 176 HBeAg‐positive, 103 HBeAg‐negative. Baseline characteristics of these patients are displayed in Table 1. Mean baseline HBV RNA levels were 5.9 log c/mL (±1.6) in the overall study population. HBV RNA levels were higher in HBeAg‐positive patients; 6.8 log c/mL (±1.2) compared to 4.3 log c/mL (±0.9) in HBeAg‐negative patients. Baseline HBV RNA levels were already below the LOD in 12 patients (4.3%). Mean quantitative HBsAg levels were 4.2 log IU/mL (±0.7) at baseline. In HBeAg‐positive patients mean baseline HBcrAg levels were 8.3 log U/mL (±0.7). In the overall population, sustained response was achieved in 57 patients (20.4%) and HBsAg loss in 18 patients (6.5%).
TABLE 1.
Patient characteristics
| Characteristics | HBeAg‐positive (n = 176) | HBeAg‐negative (n = 103) |
|---|---|---|
| Age at inclusion, years (median, IQR) | 32 (23‐41) | 41 (33‐50) |
| Male (n, %) | 135 (76.7) | 74 (71.8) |
| Race (n, %) | ||
| Caucasian | 116 (65.9) | 98 (95.1) |
| Asian | 43 (24.4) | 3 (2.9) |
| Other | 17 (9.7) | 2 (1.9) |
| HBV genotype (n, %) | ||
| A | 53 (30.1) | 15 (14.6) |
| B | 18 (10.2) | 0 (0) |
| C | 35 (19.9) | 2 (1.9) |
| D | 64 (36.5) | 81 (78.6) |
| Other | 6 (3.4) | 5 (4.9) |
| Study treatment (n, %) | ||
| PEG‐IFN mono | 86 (48.9) | 52 (50.5) |
| PEG‐IFN + LAM | 90 (51.1) | NA |
| PEG‐IFN + RBV | NA | 51 (49.5) |
| Laboratory results at baseline | ||
| HBV RNA a (mean, ±SD) | 6.8 (1.2) | 4.3 (0.9) |
| HBsAg b (mean, ±SD) | 4.4 (0.7) | 3.8 (0.5) |
| HBV DNA b (mean, ±SD) | 8.3 (1.0) | 6.0 (1.2) |
| HbcrAg c (mean, ±SD) | 8.3 (0.7) | 5.1 (1.2) |
Abbreviations: HBcrAg, hepatitis B core‐related antigen; HBeAg, hepatitis B e antigen; HBsAg, quantitative hepatitis B surface antigen; HBV, hepatitis B virus; IQR, interquartile range; LAM, lamivudine; n, number; NA, not applicable; PEG‐IFN, peginterferon; RBV, ribavirin; SD, standard deviation.
Logarithmic scale, copies/mL.
Logarithmic scale, IU/mL.
Logarithmic scale, U/mL.
3.2. On‐treatment HBV RNA response is associated with sustained response and HBsAg loss
HBV RNA data at on‐treatment week 24 were available in 258/279 patients. Of those, 135 experienced an HBV RNA response (52.3%) at week 24 of therapy. Patients with an HBV RNA response had significantly higher rates of sustained response and HBsAg loss (Figure 1A). Among 135 patients with an HBV RNA response, sustained response was observed in 37 patients (27.4%) and HBsAg loss in 14 patients (10.4%). In contrast, of the 124 patients who did not experience an HBV RNA response, only 16 patients achieved sustained response (13.0%) and 1 patient achieved HBsAg loss (0.8%; P = 0.004 for sustained response and P = 0.001 for HBsAg loss).
FIGURE 1.
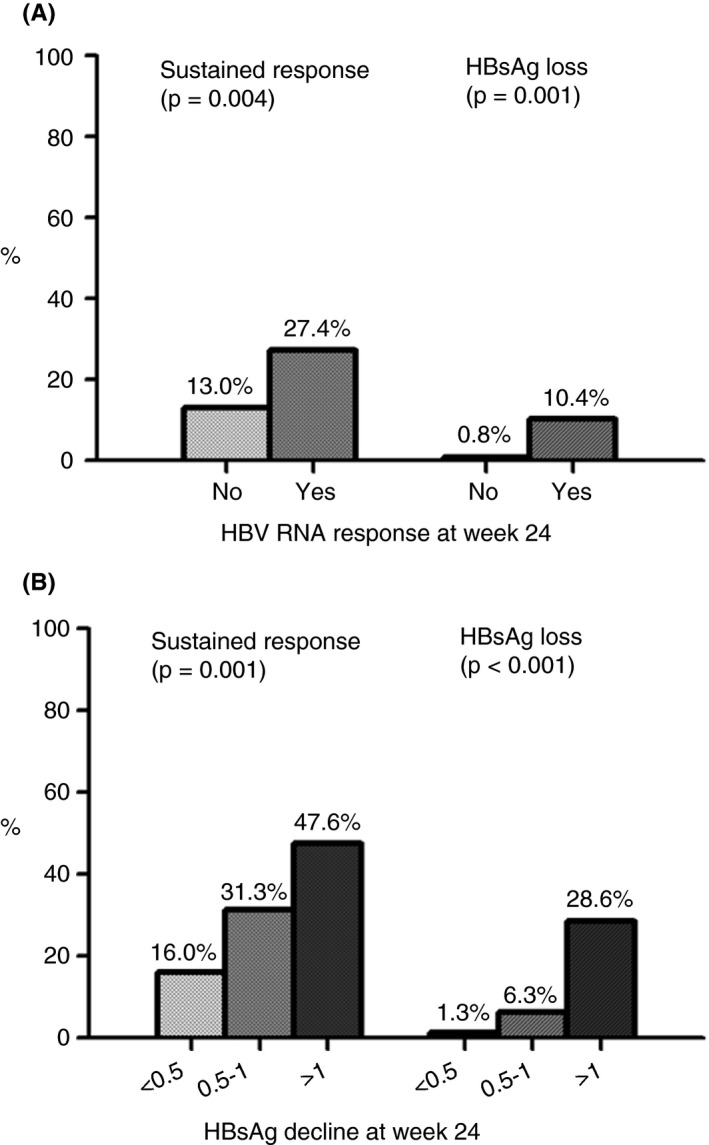
(A) Rates of sustained response (HBV DNA <2000 IU/mL) and hepatitis B surface antigen (HBsAg) loss in patients with and without hepatitis B virus (HBV) RNA response at on‐treatment week 24 (n = 258). (B) Rates of sustained response and HBsAg loss in subgroup of patients with HBV RNA response at on‐treatment week 24 (n = 133), stratified by HBsAg decline (<0.5, 0.5‐1 or >1 log). HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus
Similar results were obtained after stratification by HBeAg‐status. Among the HBeAg‐positive patients with an HBV RNA response (n = 85, 54.1%), sustained response was observed in 20 (23.5%) and HBsAg loss in 10 patients (11.8%). In contrast, of the 72 patients (45.9%) who did not experience an HBV RNA response, only seven (9.7%) achieved sustained response and none (0.0%) achieved HBsAg loss (P = 0.022 for sustained response and P = 0.003 for HBsAg loss). Among the HBeAg‐negative patients with an HBV RNA response (n = 50, 49.5%), 17 patients (34.0%) achieved sustained response and four patients (8.0%) achieved HBsAg loss. In contrast, of the 51 patients (50.5%) without an HBV RNA response, sustained response was observed in only nine patients (17.6%) and HBsAg loss in one patient (2.0%; P = 0.060 for sustained response and P = 0.162 for HBsAg loss).
Similar results were obtained when HBV RNA response was assessed at on‐treatment week 12 or at end‐of‐treatment.
3.3. Low response rates in the absence of HBsAg decline in patients with HBV RNA response
Among the 135 patients with an HBV RNA response at on‐treatment week 24, HBsAg data were available in 133 patients. Among patients with an HBV RNA response, a more prominent decline in HBsAg was observed in those who achieved sustained response, compared to those who did not (Figure 2A); mean HBsAg declines were 1.7 vs 0.6 log IU/mL (P = 0.001, Figure 1B), with an OR of 1.779 (P < 0.001).
FIGURE 2.
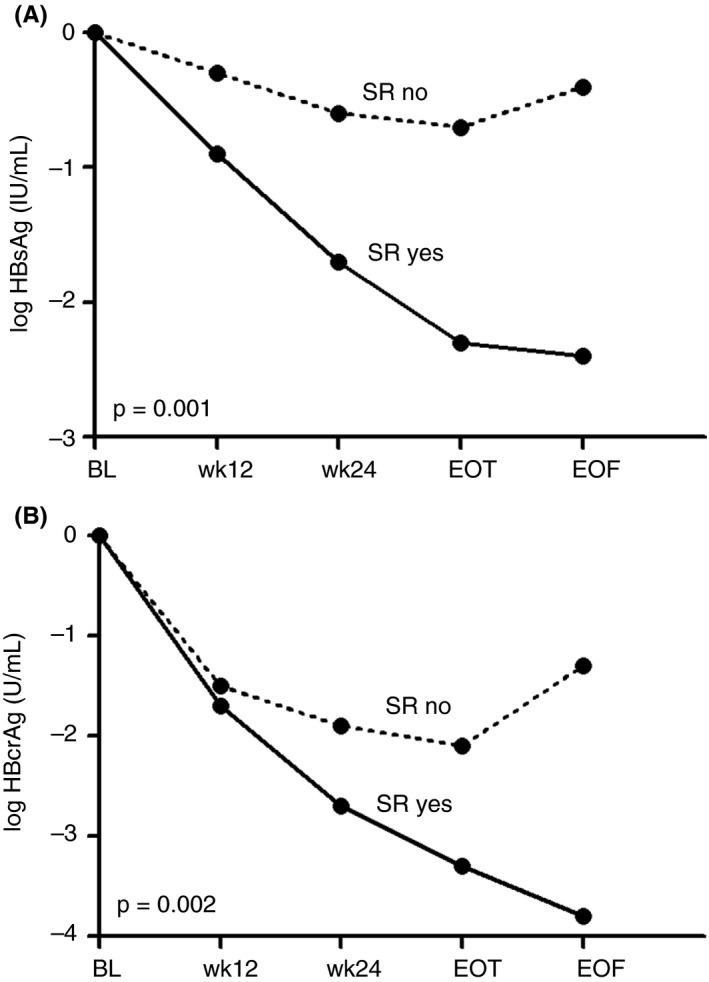
(A) Hepatitis B surface antigen (HBsAg) decline during treatment and follow‐up in patients with an hepatitis B virus (HBV) RNA response at on‐treatment week 24, stratified by patients with and without sustained response (HBV DNA <2000 IU/mL; SR). (B) Hepatitis B core‐related antigen (HBcrAg) decline during treatment and follow‐up in patients with an HBV RNA response at on‐treatment week 24, stratified by patients with and without SR. Assessed in patients with positive hepatitis B e antigen (HBeAg) at baseline. P‐value for comparison at week 24. BL, baseline; EOF, end of follow‐up; EOT, end of treatment; HBcrAg, hepatitis B core‐related antigen; HBsAg, hepatitis B surface antigen; SR, sustained response; wk, week
Among the 133 HBV RNA responders, 75 patients (56.4%) did not experience at least 0.5 log HBsAg decline at that same time point. Of those 75 patients, only 12 achieved sustained response (16.0%) and one achieved HBsAg loss (1.3%). Of the 42 patients (31.2%) with a concomitant HBsAg decline of more than 1 log, sustained response was achieved in 20 patients (47.6%) and HBsAg loss in 12 patients (28.6%; P ≤ 0.001).
Similar results were obtained when data were stratified according to HBeAg‐status at baseline. HBV RNA response at on‐treatment week 24 was observed in 85/157 HBeAg‐positive (54.1%) and in 50/101 HBeAg‐negative (49.5%) patients. Among the 85 HBeAg‐positive patients with an HBV RNA response, sustained response rates were 10.8% in patients with HBsAg decline of <0.5 log vs 42.4% in patients with >1 log HBsAg decline; HBsAg loss rates were 0.0% vs 30.3% (P = 0.006 for sustained response and P < 0.001 for HBsAg loss). Among the 50 HBeAg‐negative patients with an HBV RNA response, sustained response rates were 21.1% (with HBsAg decline <0.5 log) vs 66.7% (with HBsAg decline >1 log); and HBsAg loss rates were 2.6% vs 22.2% (P = 0.002 for sustained response and P = 0.037 for HBsAg loss). Findings were also consistent when analyses were limited to patients treated with PEG‐IFN monotherapy; sustained response rates were 18.0% in patients with HBsAg decline <0.5 log, vs 65.2% in patients with HBsAg decline >1 log; HBsAg loss rates were 2.0% vs 30.4% (P ≤ 0.001).
3.4. Low response rates in the absence of HBcrAg decline in HBeAg‐positive patients with HBV RNA response
In the 157 HBeAg‐positive patients HBV RNA response was observed in 85 patients (54.1%) at on‐treatment week 24. HBV RNA responders who achieved sustained response showed a more prominent on‐treatment decrease in HBcrAg than those who did not (Figure 2B); mean declines were 2.7 vs 1.9 log U/mL at week 24 (P = 0.002, Figure 2).
Of the 85 patients with an HBV RNA response at week 24, 79 had HBcrAg data available. A total of 14 patients (17.7%) did not experience at least 1 log HBcrAg decline, of whom only one (7.1%) achieved sustained response and HBsAg loss (Figure 3). Conversely, sustained response was observed in 69.2% (9/13) and HBsAg loss in 53.8% (7/13) of the patients with >3 log HBcrAg decline (P < 0.001 for both sustained response and HBsAg loss).
FIGURE 3.
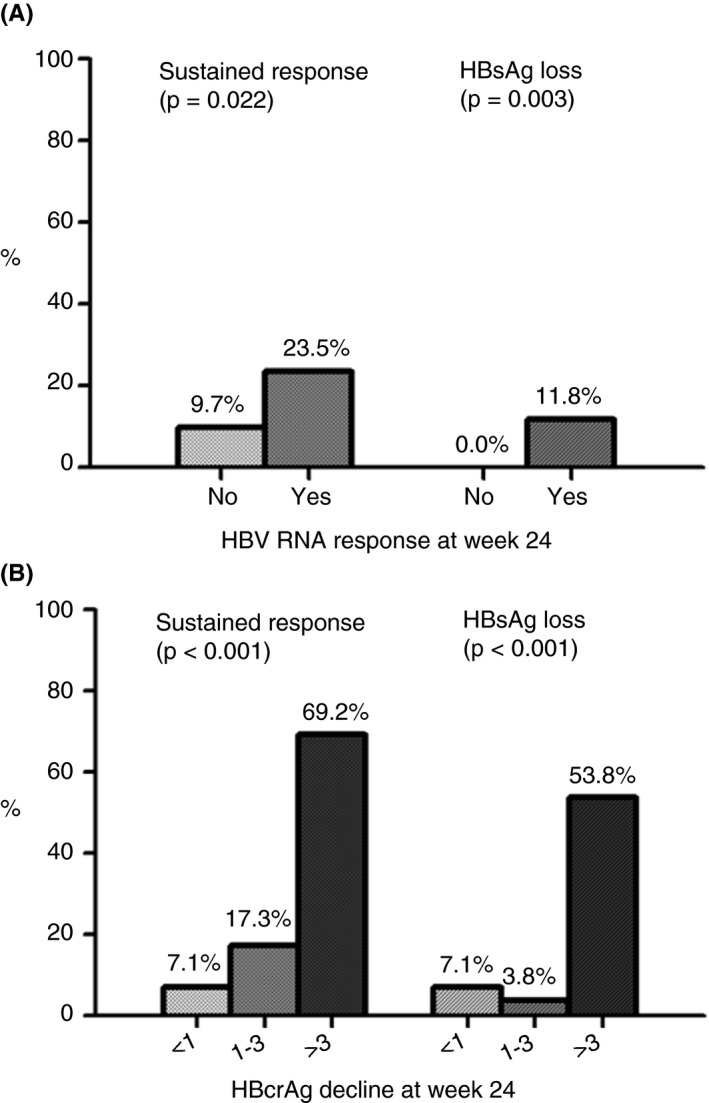
(A) Rates of sustained response (HBV DNA <2000 IU/mL) and hepatitis B surface antigen (HBsAg) loss in patients with and without hepatitis B virus (HBV) RNA response at on‐treatment week 24. Assessed in patients with positive hepatitis B e antigen (HBeAg) at baseline (n = 157). (B) Rates of sustained response and HBsAg loss in subgroup of patients with HBV RNA response at on‐treatment week 24 (n = 79), stratified by hepatitis B core‐related antigen (HBcrAg) decline (<1, 1‐3 or >3 log). Assessed in patients with positive HBeAg at baseline. HBcrAg, hepatitis B core‐related antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus
4. DISCUSSION
The current study, a pooled analysis of two randomised trials, confirms that on‐treatment HBV RNA decline is associated with higher rates of sustained off‐treatment response and HBsAg loss in patients treated with finite PEG‐IFN‐based treatment regimens. However, many patients with HBV RNA decrease did not experience a decline in the viral antigens HBsAg and/or HBcrAg. In our study, treatment response was predominantly observed in patients with both HBV RNA and viral antigen decline, whereas response rates in patients without concomitant viral antigen decrease were very low. These findings suggest that combinations of biomarkers should be used to ascertain a clinically relevant response to anti‐viral therapy and may have important implications for the interpretation of the anti‐viral efficacy of novel anti‐viral agents, such as capsid assembly modifiers.
In the past years, several viral biomarkers have been identified that may be used to estimate the probability of response to anti‐viral therapy, possibly through correlations with intrahepatic HBV transcriptional activity. Examples include serum levels of viral antigens, such as HBsAg, HBeAg and HBcrAg, and more recently also serum levels of HBV RNA. It is important to note that the intrahepatic cccDNA acts as a template for all of these biomarkers through transcription of four overlapping open reading frames (ORFs). Each ORF encodes for a subtype of polyadenylated RNAs, which subsequently serve as templates for the transcription of a number of proteins. 31 , 32 Their relative expression may be influenced by many factors, including host immune responses and anti‐viral therapy. Interpretation of kinetics of a single biomarker may therefore be misleading if changes in other biomarkers are not considered.
The potential advantage of HBV RNA as a biomarker is based on the assumption that serum HBV RNA levels reflect an early step in the HBV replication process. A decline of HBV RNA levels has therefore been postulated to directly reflect a decrease in HBV transcriptional activity, either through a reduction in the cccDNA template or inhibition of transcriptional activity. This phenomenon is elegantly demonstrated by studies that show potent HBV DNA, but limited HBV RNA, decreases with NA therapy, 21 , 33 since NA therapy does not influence HBV RNA production nor the cccDNA reservoir. 7 On the other hand, among the few patients experiencing significant HBV RNA declines during treatment with nucleo(s)tide analogues or PEG‐IFN, HBV RNA decline was associated with higher rates of off‐treatment sustained response. 18 , 20 , 21 , 24 , 33
Interestingly, two preliminary reports of recent studies in patients treated with novel capsid assembly modifiers have demonstrated superior HBV RNA declines. 13 , 25 Whether these declines also translate to higher rates of off‐treatment sustained response remains to be determined as off‐treatment data have not yet been reported. Importantly, both studies showed virtually no declines in viral antigens such as HBsAg and HBcrAg, despite the potent effects on HBV RNA. This apparent disconnection between the different markers may be accounted for by the relative short treatment duration of capsid assembly modifiers in experiment trials, as well as the different mode of action of the capsid assembly modifiers, which may have direct effects on HBV RNA production while not interfering with viral antigen production. The observed decrease in HBV RNA may therefore not reflect cccDNA decline. Until now, it has been unclear how such a response, i.e. a strong HBV RNA decline but with persistently high levels of viral antigens, relates to the prospect of subsequent treatment response.
Our study shows to the best of our knowledge for the first time that many patients experiencing HBV RNA decline during treatment with conventional anti‐viral agents do not experience concomitant decreases in HBsAg and HBcrAg. In this cohort, response rates were extremely low in this group, underscoring the complex interplay between these biomarkers. These findings may have important implications for studies evaluating novel anti‐viral agents, in particular the capsid assembly modifiers and other agents interfering with HBV RNA production, as it appears that HBV RNA decline itself might not be an adequate predictor of sustained response. We therefore argue that HBV RNA decline should not be used as a primary endpoint for treatment trials.
Strengths of our study include the large and very well‐characterised patient sample enrolled from two global randomised trials and the availability of data on a wide range of biomarkers. We were also able to assess both sustained response and HBsAg loss. However, despite the large number of patients, subgroup analysis did limit the number of available cases per group. Nevertheless, our findings were consistent when separately analysing patients according to HBeAg‐status or treatment regime. However, validation of our findings among these subgroups, as well as confirmation of our hypothesis on the applicability to novel agents, warrants further investigation.
In conclusion, HBV RNA decline without concomitant viral antigen (HBsAg and/or HBcrAg) decline is associated with low off‐treatment sustained response rates an HBsAg loss. Combinations of viral markers should be used to accurately assess response to anti‐viral therapy.
AUTHORSHIP
Guarantor of the article: None.
Author contributions: MS, SB, RdM and HLAJ conceived the study. SB and MS performed statistical analysis. SB and MS made graphic images, interpretation of data and revision of the manuscript. SB wrote the manuscript, which was revised by all authors.
ACKNOWLEDGEMENTS
We extend our sincere thanks and gratitude to N. Erler, PhD, statistician, for her advice, valuable comments and suggestions that benefited the statistical analysis of our study. All authors reviewed and approved the final manuscript.
Declaration of personal interests: SB received an unrestricted research grant from Gilead. AB has been received research grants from Roche, Gilead Sciences, Fujirebio, and Janssen. RdK has received honoraria for consulting/speaking from Gilead, Janssen, AbbVie and Norgine and received research grants form Gilead and Janssen. FvB has received research support and provided consultancy for Roche. TB has received research support, consulting and/or speaking fees from Gilead, Roche, Merck, AbbVie, Bristol‐Myers Squibb and Janssen. BH has received research support and consultancy fees from Intercept, Cymabay, Genfit, Mirum, Albireo, Calliditas and Chemomab. HJ has received research support, consulting and/or speaking fees from Gilead, Roche, Merck, AbbVie, Bristol‐Myers Squibb, Arbutus, Janssen and MedImmune. MS has received speaker's fees and research support from Roche, Innogenetics, BMS and Fujirebio. The other authors report no disclosures.
Brakenhoff SM, de Man RA, Boonstra A, et al. Hepatitis B virus RNA decline without concomitant viral antigen decrease is associated with a low probability of sustained response and hepatitis B surface antigen loss. Aliment Pharmacol Ther. 2020;53:314–320. 10.1111/apt.16172
The Handling Editor for this article was Professor Geoffrey Dusheiko, and it was accepted for publication after full peer‐review.
Funding information This study was sponsored by the Foundation for Liver and Gastrointestinal Research, Rotterdam, the Netherlands. HBcrAg testkits were provided free of charge by Fujirebio.
REFERENCES
- 1. World Health Organization . Hepatitis B factsheet. 2019, updated 18–07‐2019 [Access Date 18–05‐2020].
- 2. Martinez MG, Testoni B, Zoulim F. Biological basis for functional cure of chronic hepatitis B. J Viral Hepat. 2019;26:786‐794. [DOI] [PubMed] [Google Scholar]
- 3. Petersen J, Thompson AJ, Levrero M. Aiming for cure in HBV and HDV infection. J Hepatol. 2016;65:835‐848. [DOI] [PubMed] [Google Scholar]
- 4. Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA–the holy grail to hepatitis B cure. J Hepatol. 2016;64:S41‐S48. [DOI] [PubMed] [Google Scholar]
- 5. Kostyusheva A, Kostyushev D, Brezgin S, Volchkova E, Chulanov V. Clinical implications of hepatitis B Virus RNA and covalently closed circular DNA in monitoring patients with chronic hepatitis B today with a gaze into the future: the field is unprepared for a sterilizing cure. Genes (Basel). 2018;9:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buti M, Riveiro‐Barciela M, Esteban R. Long‐term safety and efficacy of nucleo(t)side analogue therapy in hepatitis B. Liver Int. 2018;38:84‐89. [DOI] [PubMed] [Google Scholar]
- 7. Lopatin U. Drugs in the pipeline for HBV. Clin Liver Dis. 2019;23:535‐555. [DOI] [PubMed] [Google Scholar]
- 8. Jeng WJ, Sheen IS, Chen YC, et al. Off‐therapy durability of response to entecavir therapy in hepatitis B e antigen‐negative chronic hepatitis B patients. Hepatology. 2013;58:1888‐1896. [DOI] [PubMed] [Google Scholar]
- 9. Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology. 2016;63:1481‐1492. [DOI] [PubMed] [Google Scholar]
- 10. Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha‐2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675‐684. [DOI] [PubMed] [Google Scholar]
- 11. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa‐2a, lamivudine, and the combination for HBeAg‐positive chronic hepatitis B. N Engl J Med. 2005;352:2682‐2695. [DOI] [PubMed] [Google Scholar]
- 12. Zhou Y, Yan R, Ru GQ, Yu LL, Yao J, Wang H. Pegylated‐interferon consolidation treatment versus nucleos(t)ide analogue consolidation treatment in non‐cirrhotic hepatitis B patients with hepatitis B e antigen seroconversion: an open‐label pilot trial. Hepatol Int. 2019;13:422‐430. [DOI] [PubMed] [Google Scholar]
- 13. Yuen MF, Gane EJ, Kim DJ, et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3–778 in patients with chronic HBV infection. Gastroenterology. 2019;156:1392‐1403.e7. [DOI] [PubMed] [Google Scholar]
- 14. Bazinet M, Pantea V, Placinta G, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa‐2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology. 2020;158:2180‐2194. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Jiang M, Xue J, Yan H, Liang X. Serum HBV RNA quantification: useful for monitoring natural history of chronic hepatitis B infection. BMC Gastroenterol. 2019;19:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giersch K, Allweiss L, Volz T, Dandri M, Lutgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol. 2017;66:460‐462. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Yu Y, Li G, et al. Natural history of serum HBV‐RNA in chronic HBV infection. J Viral Hepat. 2018;25:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 18. van Bommel F, van Bommel A, Krauel A, et al. Serum HBV RNA as a predictor of peginterferon alfa‐2a response in patients with HBeAg‐positive chronic hepatitis B. J Infect Dis. 2018;218:1066‐1074. [DOI] [PubMed] [Google Scholar]
- 19. van Bömmel F, Bartens A, Mysickova A, et al. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66‐76. [DOI] [PubMed] [Google Scholar]
- 20. Luo H, Tan N, Kang Q, et al. HBV pgRNA status can reveal the long‐term prognoses of chronic hepatitis B patients treated with nucleos(t)ide analogues. J Viral Hepat. 2020;27:323‐328. [DOI] [PubMed] [Google Scholar]
- 21. Huang YW, Takahashi S, Tsuge M, et al. On‐treatment low serum HBV RNA level predicts initial virological response in chronic hepatitis B patients receiving nucleoside analogue therapy. Antivir Ther. 2015;20:369‐375. [DOI] [PubMed] [Google Scholar]
- 22. Jansen L, Kootstra NA, van Dort KA, Takkenberg RB, Reesink HW, Zaaijer HL. Hepatitis B virus pregenomic RNA is present in virions in plasma and is associated with a response to pegylated interferon alfa‐2a and nucleos(t)ide analogues. J Infect Dis. 2016;213:224‐232. [DOI] [PubMed] [Google Scholar]
- 23. Farag MS, van Campenhout MJH, Pfefferkorn M, et al. Hepatitis B virus RNA as early predictor for response to PEGylated interferon alfa in HBeAg negative chronic hepatitis B. Clin Infect Dis. 2020: ciaa013. 10.1093/cid/ciaa013. [DOI] [PubMed] [Google Scholar]
- 24. Campenhout MJH, Bömmel F, Pfefferkorn M, et al. Serum hepatitis B virus RNA predicts response to peginterferon treatment in HBeAg‐positive chronic hepatitis B. J Viral Hepat. 2020;27:610‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuen M‐F, Agarwal K, Gane EJ, et al. Safety, pharmacokinetics, and antiviral effects of ABI‐H0731, a hepatitis B virus core inhibitor: a randomised, placebo‐controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5:152‐166. [DOI] [PubMed] [Google Scholar]
- 26. Sonneveld MJ. Core inhibitor therapy for chronic hepatitis B. Lancet Gastroenterol Hepatol. 2020;5:99‐100. [DOI] [PubMed] [Google Scholar]
- 27. Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa‐2b alone or in combination with lamivudine for HBeAg‐positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123‐129. [DOI] [PubMed] [Google Scholar]
- 28. Rijckborst V, ter Borg MJ, Cakaloglu Y, et al. A randomized trial of peginterferon alpha‐2a with or without ribavirin for HBeAg‐negative chronic hepatitis B. Am J Gastroenterol. 2010;105:1762‐1769. [DOI] [PubMed] [Google Scholar]
- 29. van Campenhout MJH, van Bommel F, Pfefferkorn M, et al. Host and viral factors associated with serum hepatitis B virus RNA levels among patients in need for treatment. Hepatology. 2018;68:839‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campenhout MJH, Rijckborst V, Brouwer WP, et al. Hepatitis B core‐related antigen monitoring during peginterferon alfa treatment for HBeAg‐negative chronic hepatitis B. J Viral Hepat. 2019;26:1156‐1163. [DOI] [PubMed] [Google Scholar]
- 31. Datta S, Chatterjee S, Veer V, Chakravarty R. Molecular biology of the hepatitis B virus for clinicians. J Clin Exp Hepatol. 2012;2:353‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diab A, Foca A, Zoulim F, Durantel D, Andrisani O. The diverse functions of the hepatitis B core/capsid protein (HBc) in the viral life cycle: implications for the development of HBc‐targeting antivirals. Antiviral Res. 2018;149:211‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo H, Tan N, Kang Q, et al. Hepatitis B virus pregenomic RNA status can reveal the long‐term prognoses of chronic hepatitis B patients treated with nucleos(t)ide analogues. J Viral Hepat. 2020;27:323‐328. [DOI] [PubMed] [Google Scholar]


