Abstract
Introduction:
Vaso-occlusive crisis (VOC) is one of the main causes of hospital admission in patients with sickle cell disease (SCD). Ketamine is often used as an adjuvant to opioids to control sickle cell crisis; however, there is a lack of evidence about its safety and efficacy for VOC in SCD patients.
Objective:
To synthesize evidence from published reports about the efficacy and safety of ketamine in the management of acute painful VOC in both pediatric and adult SCD patients.
Methods:
A systematic literature search of PubMed, Scopus, Web of Science, EBSCO and Cochrane Library was conducted, up to March 2019. Studies reporting the analgesic effects and side effects of ketamine in the management of acute painful VOC in pediatric and adult SCD patients were included. The primary outcome measure was improvement in pain scale, and the secondary outcomes were reduction in opioid utilization and side effects. Studies were narratively summarized in this review.
Results:
Fourteen studies (with a total of 604 patients) were included in the final analysis. Several case reports and case series showed that ketamine significantly reduced pain scales and opioid utilization in both populations. The only randomized controlled trial available showed that ketamine was noninferior to morphine in reducing pain scores, but had a higher incidence of nonlife-threatening, reversible adverse effects. However, a retrospective study of 33 patients showed a higher pain score in the ketamine group with an acceptable short-term adverse effect.
Conclusion:
Ketamine has a potentially comparable efficacy with other opioids in reducing the pain during VOC in SCD patients. However, it also likely has a higher rate of transient adverse events. Owing to the lack of published randomized controlled trials, current evidence is not sufficient to confirm the safety and efficacy of ketamine. Future well-designed randomized controlled trials are strongly recommended.
Keywords: Ketamine, pain, sickle cell disease, systematic review, vaso-occlusive crisis
INTRODUCTION
Sickle cell disease (SCD) is one of the most common genetic disorders, affecting >300,000 children every year worldwide, with an expected increase in prevalence in the coming years.[1,2] SCD is characterized by chronic anemia, organ damage and acute manifestations such as stroke, acute chest syndrome and severe bacterial infections.[3] Acute vaso-occlusive crisis (VOC) is the hallmark complication of SCD and is responsible for >90% of acute hospital admissions in SCD.[4,5] These pain episodes occur as a result of tissue ischemia, caused by vessel occlusion by sickled red blood cells. They are common in the extremities, back, joints, abdomen and chest.[6]
Traditional pain control in VOC involves intravenous (IV) hydration combined with nonsteroidal anti-inflammatory drugs and opioids, often delivered through a patient-controlled analgesia pump.[7] Opioids modulate analgesia through mu receptor activation and substance P inhibition in presynapses. Prolonged opioid utilization abnormally activates N-methyl D-aspartate (NMDA) receptors in central neurons and stimulates synapses between nociceptive C fibers and neurons in the spinal cord. This causes hyperalgesia and opioid tolerance, which lead to opioid-refractory pain.[8,9,10]
Ketamine was developed as a general anesthetic in the 1960s. After a decade of its approval by the US Food and Drug Administration, researchers found that ketamine antagonizes NMDA receptors in the central nervous system, which produces a dissociative anesthesia effect.[11] Further, ketamine has been shown to modulate hyperalgesia and opioid-related tolerance in the management of chronic pain in malignancy, neuropathic pain and postoperative pain.[12,13,14] During SCD crisis, ketamine infusions have been found to reduce pain in pediatrics and adults.[15,16,17] Ketamine can be given through the oral, IV, intramuscular, subcutaneous, epidural, transdermal or intraarticular route, but the IV route is the most common route.[18]
Although the conclusive clinical evidence on its use in VOC is lacking, clinicians from different disciplines continue to use ketamine as an adjunct to opioids in managing acute and chronic pain.[19,20] Some argue that with less effective drug choices, one may substitute for less studied approaches such as ketamine infusions. Therefore, this systematic review was conducted to answer the following research question: in patients with acute SCD crises, does the use of ketamine reduce pain, opioid utilization and side effects compared with those on opioids alone/controls?
METHODS
This systematic review was conducted in accordance with the PRISMA guidelines.[21]
Literature search
We searched PubMed, Scopus, Web of Science Core Collection, EBSCO (all databases) and Cochrane Library to identify relevant studies from 1970 up to 31st March 2019, when the search was carried out. MESH terms and the free-text criteria were used for each database using the following keywords: (ketamine OR “N-methyl-D-aspartate receptor” OR NMDA OR “ketamine hydrochloride”) AND (“Sickle cell disease” OR “SCD” OR “sickling disorder” OR “sickle hemoglobin” OR (HbS) OR “vaso-occlusive crises” OR “sickle cell crisis” OR “vaso-occlusive episode” OR “vaso-occlusive crisis” OR (VOC) OR (pain). No filters were applied for language and country of origin.
Eligibility criteria
We included studies that met the following criteria:
Study design
Randomized controlled trials and observational studies (including case reports, case series, single-arm studies, and comparative studies). Data from conference abstracts were also included.
Population
Children and adult patients with acute painful SCD crises.
Intervention
Ketamine at any dose and by any route.
Comparator
Opioids or any control.
Outcomes
Primary outcome measured was the pain scale and secondary outcomes were opioid utilization and rate of side effects.
We excluded animal studies, reviews and studies with other types of pain or complications in SCD patients other than VOC.
Study selection
The records retrieved from the searches were imported into Endnote X7 (Clarivate Analytics, Philadelphia, PA, USA). The same software was used for managing references and omitting duplicates. Both authors independently and in duplicate screened titles, abstracts and full texts for eligible articles, assessed risk of bias and extracted data from each eligible study using a standardized Excel spreadsheet. Disagreements or uncertainties were resolved by consensus with a third independent reviewer.
Data extraction and outcomes
Data extraction was carried out by both authors, with a third reviewer resolving any disagreement. The data collected were study characteristics (first author's name, publication year and study design), sample population characteristics (number of participants, mean age and gender), interventions (route of administrations and dose) and the related outcomes including pain scale, opioid utilization and side effects rate.
Quality assessment
The risk of bias of the included randomized clinical trials was assessed according to the Cochrane Handbook of Systematic Reviews of Interventions.[22] We evaluated the quality of comparative observational studies using the Newcastle Ottawa Scale.[23] Each included study was assessed based on reporting three essential domains: (a) Selection of the study subjects, (b) comparability of groups on demographic characteristics and important potential confounders and (c) ascertainment of the prespecified outcome (exposure/treatment). For case reports and case series, we used the Methodological Index for Non-Randomized Studies, a validated 12-point scale wherein the first 8 points that account for selection, ascertainment, causality, and reporting were used.[24]
Quantitative evidence synthesis
All included studies were grouped according to the study design and are presented in a table format. Results are reported and discussed narratively.
RESULTS
Search strategy results
In the initial database search, 1497 records were identified. Of them, 1058 records remained after removing duplicates. After title and abstract screening, 20 articles were found to be initially eligible for inclusion, and their full texts were screened. Of these, 14 articles met the inclusion criteria and were included for further analysis. The PRISMA flow diagram of study selection is shown in Figure 1.
Figure 1.
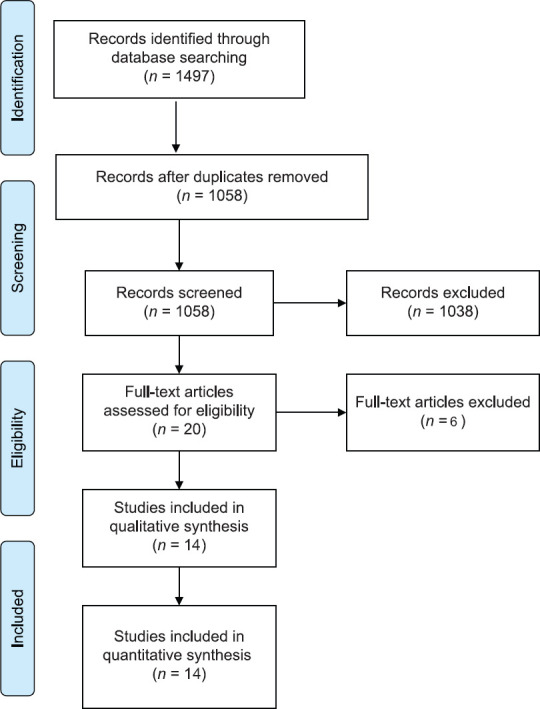
PRISMA flow diagram
Baseline characteristics and risk of bias
Fourteen studies, including one clinical trial, one retrospective study, five case reports, four case series and three single-arm observational studies, with a total of 604 patients were included in this systematic review. All articles were published in English between 2011 and 2018. The age of participants ranged from 7.5 to 42 years. Two studies included only pediatric patients,[15,25] three studies included pediatric and young adult patients,[7,26,27] and 9 studies included adult patients.[16,17,28,29,30,31,32,33,34] The numeric rating scale was used to assess pain in 11 studies. The basic characteristics of the included studies are shown in Table 1. The included randomized clinical trial was at low risk in terms of selection bias, performance bias, detection bias, attraction bias and reporting bias.[25] The included retrospective study, case series and case reports showed a moderate to a high quality of evidence. The summary of the risk of bias of both included trial and observational studies is presented in Supplementary Tables 1-3.
Table 1.
Summary of the qualitative and quantitative studies
| First author, year of publication | Study design | Participants | Interventions | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| n | Age | Sex (male/female) | Pain scale | Primary outcomes | Side effects | |||
| Lubega et al., 2018[25] | Randomized controlled trial | 120/120 (240) | 11.8 (3.5) | 85/155 | IV ketamine 1 mg/kg versus IV morphine 0.1 mg/kg as an infusion over 10 min | NRS | Pain scales and opioid consumption were reduced after ketamine take | Nystagmus and dysphoria were the commonest side effects |
| Neri et al., 2014[7] | Retrospective study | 33 | 15.6 (7.5-21.4) | 11/22 | Ketamine 0.1 mg/kg/h and opioids versus opioid PCA | NRS | Ketamine reduced pain scales and opioid use | Vivid dreams, delusions and dizziness |
| Nobrega et al., 2017[26] | Single-arm observational study | 85 | 15 (13-17) | 39/41 | Ketamine infusion | NRS, Wong-baker faces or FLACC | Significant reduction in pain scales | No side effects were reported |
| Sheehy et al., 2017[27] | Single-arm observational study | 181 | - | - | Ketamine infusion 0.05-1 mg/kg/h | NRS, Wong-baker faces or FLACC | Significant reduction in pain scale | No side effects were reported |
| Chu et al., 2013[28] | Single-arm observational study | 30 | - | - | Ketamine infusion | - | Reduction in pain scale and opioid use | - |
| Palm et al., 2018[17] | Case series | 5 | 25-42 | 1/4 | Ketamine, up to 5 µg/kg/min | NRS | Reductions in pain scale and opioid use | Reduced adverse events after ketamine |
| Hassel et al., 2017[29] | Case series | 10 | - | - | Ketamine infusion | NRS | 91.7% of ketamine infusions reduced pain intensity scores and opioid intake | 11.1% of ketamine infusions caused side effects |
| Tawfic et al., 2014[16] | Case series | 9 | 27±11 | 1/8 | Ketamine, 0.2-0.25 mg/kg/h, plus 5 mg boluses | NRS | Improvement of pain and IV opioid use | Nausea and vomiting |
| Zempsky et al., 2010[15] | Case series | 5 | 13.4±2.96 | 1/4 | Ketamine infusion, 0.06-0.2 mg/kg/h | NRS | Improvement of pain, decreased opioid use in 40% of patients | Hypertension, unresponsiveness, nystagmus and dysphoria |
| Gimovsky et al., 2017[30] | First case report | 1 | 25 | Female | Ketamine, 10 mg/h | NRS | No decrease in pain, but opioid requirements decreased | No serious maternal or neonatal adverse effects |
| Second case report | 1 | 29 | Female | Ketamine, 10-25 mg/h during the first day | NRS | Pain resolved | No serious maternal or neonatal adverse effects | |
| Jennings et al., 2013[31] | Case report | 1 | 38 | Female | Ketamine 15 mg oral every 6 h versus morphine | NRS | Reduction in pain scale and opioid use | No side effects lead to discontinuation |
| Uprety et al., 2013[32] | Case report | 1 | 31 | Male | Ketamine infusion | NRS | Reduction in pain scale and opioid use | No side effects lead to discontinuation |
| Meals et al., 2011[33] | Case report | 1 | 31 | Male | Low dose ketamine infusion | - | Pain scale was reduced | No side effects lead to discontinuation |
| Kerr et al., 2011[34] | Case report | 1 | 33 | Male | Ketamine, started at 0.08 mg/kg/h, titrated to 18 mg/h over 48 h | VRS | Significant decrease in pain score | Minimal discomfort |
NRS – Numeric Rating Scale; FLACC – Face, legs, activity, cry and consolability; PCA – Patient-controlled analgesia; IV – Intravenous; VRS – Verbal Rating Scale
Supplementary Table 1.
Risk of bias assessment of the included randomized controlled trial (Lubega et al., 2018)
| Parameter | Risk of bias | Reason/quotations |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | A computer program was used to generate the randomization sequence by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Block randomization with a block of 10 used to randomly assign participants to either receive ketamine or morphine in equal numbers for the two groups |
| Blinding of participants and personnel (performance bias) | Low risk | The mixed drug was labeled with the patient study number and delivered to the research assistant in a transparent syringe (all drugs are colorless liquids) |
| Blinding of outcome assessment (detection bias) | Low risk | Concealment was achieved by making sure that each syringe was labeled according to sequence-generated codes earlier presented as a list of sequential random treatment codes. The labeled syringes were brought in an opaque carrier envelope to the clinic and handed to the attending nurse who retrieved them with their sticker code number, similar to computer generated number sequence, becoming the patients study number |
| Incomplete outcome data (attrition bias) | Low risk | The clinical data from all patients were used for ITT analysis |
| Selective reporting (reporting bias) | Low risk | Protocol is not available, but it is expected that all major outcomes were reported |
| Other bias | Unclear |
ITT – Intention to treat
Supplementary Table 3.
The methodological quality of the included case reports and case series
| Study | Selection Patient(s) represent(s) the whole experience of the investigator | Ascertainment | Causality | Reporting Case(s) described with sufficient details | Total score | ||||
|---|---|---|---|---|---|---|---|---|---|
| The exposure adequately ascertained | The outcome adequately ascertained | Alternative causes that may explain the observation ruled out | There was a challenge/rechallenge phenomenon | There was a dose- response effect | Follow-up long enough | ||||
| Nobrega et al., 2017 | * | * | * | * | - | * | * | * | 7 |
| Sheehy et al., 2017 | * | * | * | - | - | * | * | * | 6 |
| Chu et al., 2013 | * | * | * | * | - | * | * | * | 7 |
| Palm et al., 2018 | * | * | * | - | - | * | * | * | 6 |
| Hassel et al., 2017 | * | * | * | - | - | * | * | * | 6 |
| Tawfic et al., 2014 | * | * | * | - | - | * | * | * | 6 |
| Zempsky et al., 2010 | * | * | * | - | - | * | * | * | 6 |
| Gimovsky et al., 2017 | * | * | * | - | - | * | * | * | 6 |
| Jennings et al., 2013 | * | * | * | - | * | * | * | 6 | |
| Uprety et al., 2013 | * | * | * | - | - | * | * | * | 6 |
| Meals et al., 2011 | * | * | * | - | - | * | * | * | 6 |
| Kerr et al., 2011 | * | * | * | - | - | * | * | * | 6 |
If yes |(*= 1 point) If no (- = no points)
Supplementary Table 2.
Methodological quality of the included observational study based on the Newcastle Ottawa scale for assessing the quality of epidemiological studies
| Study | Selection | Comparability Control for 2 important factorsc,d | Exposure | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposurea | Outcome was not present at start of studyb | Assessment of outcome | Follow-up long enoughe | Adequacy of follow-up of cohortf | |||
| Neri et al., 2014 | * | * | * | - | * | * | * | * | 7 |
aIf the exposure data was obtained from prescription database or medical record, a point was assigned, bIf the study design is prospective study, a point was assigned, cIf adjusted for age, a point was assigned, dIf adjusted for any other additional factors, a point was assigned, eIf the completeness of follow-up was 80% or more, a point was assigned, if follow up, a point was assigned
Qualitative evidence from published case reports
Five case reports were published about the role of ketamine in VOC between 2011 and 2017. A case report of a 31-year-old male by Uprety et al. showed an improvement in the patient's pain and opioid utilization after ketamine infusion.[32] Jennings et al. included a 38-year-old female with sickle cell thalassemia, administrated with oral ketamine on day 7 of her admission due to VOC, since the introduction of ketamine regimen her daily dose of morphine decreased significantly without apparent side effect.[31] Further, Kerr et al. included a 33-year-old male, administrated with subanesthetic IV ketamine, and reported an improvement of VOC. However, the patient experienced psychomimetic side effects after ketamine injection.[34] Meals et al. included a 31-year-old male and showed that the patient's pain was greatly diminished after the ketamine administration. Moreover, the required morphine dose was reduced on the 1st day after ketamine injection. Somnolence and horizontal nystagmus occurred immediately after the boluses of ketamine and lasted for <10 min.[33] Gimovsky et al. included two pregnant women, treated with ketamine for SCD. The first patient showed no pain reduction, while the pain was resolved in the second patient.[30] No hemodynamically significant side effects were reported by Uprety et al., Jennings et al. and Meals et al.
Qualitative evidence from published case series
Four case series, enrolling a total of 29 patients, assessed the role of ketamine in the management of VOC between 2010 and 2018. In adults, the most recent study by Palm et al. included five patients with sickle cell crisis and reported that ketamine significantly reduced the mean numeric pain scale score (7.2 vs. 6.4) and opioid utilization (median reduction of 90 mg morphine equivalents per patient).[17] Another case series by Hassell et al. included 10 patients and showed that ketamine infusion reduced pain scores from an average of 7.88 to 4.25 out of 10 and reduced opioid use. About 11.1% of ketamine injections caused side effects, including hallucinations and vomiting that led to discontinuation.[29] A case series by Tawfic et al. reported an improvement in pain scores after adding ketamine–midazolam injection (P = 0.01). In addition, the morphine requirement was significantly lower after adding this regimen. One patient developed psychotomimetic manifestation after starting ketamine.[16]
In 2010, a case series by Zempsky et al. included five children treated with low-dose ketamine infusion for sickle cell VOC. Two of the five patients achieved clinically significant pain control. One additional patient had a significant reduction in opiate utilization. Two patients experienced adverse effects including dysphoria, unexpected nystagmus, hypertension and unresponsiveness.[15]
Qualitative evidence from observational studies
Three single-arm observational studies assessed the role of ketamine in VOC, with a total of 296 patients. Sheehy et al. reported that subanesthetic ketamine infusions were associated with significant reductions in the mean pain scores from baseline (mean pain scores 6.64 [95% confidence interval (CI): 6.38–6.90]) to those recorded on the day after discontinuation of ketamine (mean pain scores 4.38, 95% CI [4.06–4.69], P < 0.001). No side effects were reported.[27] Nobrega et al. included 85 children with VOC-associated pain and showed that ketamine produced a significant decrease in pain scores and opioid consumption.[26] Another study enrolled 30 patients, administered with ketamine infusion for pain crisis and showed that the opioid requirement was significantly lower after ketamine compared to before ketamine was started.[28]
Quantitative evidence from comparative studies
A recent controlled trial by Lubega et al., in their double-blinded, prospective randomized study, compared high-dose ketamine (1 mg/kg) versus morphine (0.1 mg/kg) in 240 children with VOC (120 in each arm) and found that the incidence of adverse effects was higher in participants receiving ketamine.[25] However, all the events were transient and nonlife threatening. This may be explained by the high dose of ketamine used in this study. A retrospective study of 33 children with VOC by Neri et al. compared two admissions for all participants where they received low dose ketamine (0.1 mg/kg) and opioids in one admission versus opioids alone in another admission. The study did not find ketamine to have any opioids sparing effect (i.e., reducing the required dose of opioids to achieve satisfactory pain control). It was attributed by the authors to the higher severity of crises in ketamine admissions. In terms of the safety profile, the study found that ketamine has acceptable short-term adverse effects.
DISCUSSION
This systematic review evaluated the published literature on the use of ketamine infusion to control pain during VOC in sickle cell patients. The findings of the included studies highlight that ketamine has promising efficacy for reducing pain during VOCs, which was comparative with other opioids. However, compared to opioids, a higher rate of adverse events was noted in the ketamine group, although these were mild and transient.
The reduction of pain after ketamine infusion confirms the analgesic utility of ketamine in these patients. Another interesting finding in some published reports was that ketamine infusion had an opioid sparing effect.[17,29,31,33] In contrast, one study did not find such effect, which the authors attributed to the inclusion of patients with more severe pain crises.[7] Notably, Palm et al. suggested that ketamine may have an additional benefit in chronically ill patients due to its anti-depressive effects.[17]
Interestingly, Nobrega et al. found that the analgesic effects of ketamine in SCD patients were age and sex dependent (i.e., females and older patients experienced more pain reduction). These variables, along with pain location and infusion duration, independently predicted pain score reduction in a multivariate analysis.[26] Although this finding is intriguing, it is in line with those of animal and human studies wherein the effects of NMDA receptor antagonists were found to vary with age and sex.[35,36,37]
Of note, the study by Neri et al. concluded that pain scores were higher in the ketamine group.[7] However, Lubega et al. showed that ketamine was noninferior to morphine in reducing pain scores.[25] This difference may be explained by the different doses in the two studies (0.1 and 1 mg/kg in the studies by Neri and Lubega et al., respectively) and the higher severity of the VOC in the ketamine group in the study by Neri et al. In terms of safety, Neri et al. study showed that ketamine infusion of 0.1 mg/kg/h was safe in the short-term. Further, compared with ketamine bolus in other studies, the infusion method was associated with more favorable adverse effects. However, the long-term adverse effects could not be evaluated in this study because of its design.
In the study by Lubega et al., a higher incidence of transient nonlife threatening adverse effects was observed, with nystagmus, dysphoria and salivation being the most commonly reported. All of the adverse effects were resolved after stopping the infusion and were not life threatening. The higher rate of adverse effects was attributed to the high dose of ketamine used in the study. The commonly reported adverse events in the reviewed case reports/series were dysphoria, nystagmus and psychotomimetic manifestations. However, most of these events were transient and not-life threatening, although they sometimes caused discontinuation or unblinding in the reviewed studies. Further evaluation of the safety of ketamine in this population is needed in large-scale clinical trials.
Limitations
The literature available on the studied topic is limited: Mostly, case reports/series are available, and only two quantitative studies are available, including a randomized trial wherein ketamine was used as a monotherapy rather than as an adjuvant therapy. In addition, the data available is heterogeneous, with variable doses and durations of infusions for ketamine, thereby limiting the ability to establish clear clinical recommendations about the safe use of ketamine in SCD VOC patients.
Future research directions
The evidence summarized in this systematic review strongly indicates the need for randomized controlled trials comparing ketamine with opioids for VOC of SCD patients. To the best of the authors' knowledge, currently there are few ongoing randomized controlled trials on this topic: Young et al.,[38] The AKTSS study (ClinicalTrials.gov Identifier: NCT03296345), KSickle study (ClinicalTrials.gov Identifier: NCT02801292) and one in our center (ClinicalTrials.gov Identifier: NCT03431285). The findings of these randomized controlled trials will provide strong evidence for the safety and efficacy of ketamine for VOC in SCD patients. We recommend that the correlation between the efficacy and the dose and duration of administration should be tested to optimize the use of ketamine in these patients. In addition, investigating the possible factors that may influence the efficacy of ketamine such as gender, location of pain and duration of infusion is also recommended.
CONCLUSION
This systematic review showed that ketamine has a potentially comparable efficacy with other opioids in reducing pain during VOC in SCD patients. However, in those receiving higher ketamine dose, a higher rate of transient adverse events has been reported compared with the comparator group. Further, randomized trials are needed to establish the safety and efficacy of ketamine in VOC of SCD patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank Dr. Mohammed Aljumaan, Consultant of the Emergency Department and a Toxicologist, who acted as an independent reviewer and assisted in resolving disagreements or uncertainties that greatly improved the manuscript.
REFERENCES
- 1.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: Modelling based on demographics, excess mortality, and interventions.Osrin D, editor. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: A HuGE review. Am J Epidemiol. 2000;151:839–45. doi: 10.1093/oxfordjournals.aje.a010288. [DOI] [PubMed] [Google Scholar]
- 3.Brousse V, Makani J, Rees DC. Management of sickle cell disease in the community. BMJ. 2014;348:g1765. doi: 10.1136/bmj.g1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: A critical reappraisal. Blood. 2012;120:3647–56. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 5.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: Frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- 6.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, et al. Pain site frequency and location in sickle cell disease: The PiSCES project. Pain. 2009;145:246–51. doi: 10.1016/j.pain.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neri CM, Pestieau SR, Young H, Elmi A, Finkel JC, Darbari DS. Low-dose ketamine for children and adolescents with acute sickle cell disease related pain: A single center experience. J Anesth Clin Res. 2014;05:1–5. [Google Scholar]
- 8.Dunlop R, Bennett KC. Pain management for sickle cell disease in children and adults. In: Dunlop R, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2006. p. CD003350. [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–61. PMID: 21412369. [PubMed] [Google Scholar]
- 10.Chu LF, Angst MS, Clark D. Opioid-induced Hyperalgesia in Humans. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 11.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–75. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar H, Haywood C, Jr, Molokie R. Sickle cell disease in the United States: Looking back and forward at 100 years of progress in management and survival? Am J Hematol. 2010;85:346–53. doi: 10.1002/ajh.21676. doi: 10.1002/ajh.21676. PMID: 20425797. [DOI] [PubMed] [Google Scholar]
- 13.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine PG. Low-dose ketamine in the management of opioid nonresponsive terminal cancer pain. J Pain Symptom Manage. 1999;17:296–300. doi: 10.1016/s0885-3924(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 15.Zempsky WT, Loiselle KA, Corsi JM, Hagstrom JN. Use of low-dose ketamine infusion for pediatric patients with sickle cell disease-related pain. Clin J Pain. 2010;26:163–7. doi: 10.1097/AJP.0b013e3181b511ab. [DOI] [PubMed] [Google Scholar]
- 16.Tawfic QA, Faris AS, Kausalya R. The role of a low-dose ketamine-midazolam regimen in the management of severe painful crisis in patients with sickle cell disease. J Pain Symptom Manage. 2014;47:334–40. doi: 10.1016/j.jpainsymman.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Palm N, Floroff C, Hassig TB, Boylan A, Kanter J. Low-dose ketamine infusion for adjunct management during vaso-occlusive episodes in adults with sickle cell disease: A case series. J Pain Palliat Care Pharmacother. 2018;32:20–6. doi: 10.1080/15360288.2018.1468383. [DOI] [PubMed] [Google Scholar]
- 18.Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32:298–306. doi: 10.4103/0970-9185.168149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler FK, Kotwal RS, Buckenmaier CC, Edgar EP, O'Connor KC, Montgomery HR, et al. A triple-option analgesia plan for tactical combat casualty care: TCCC Guidelines Change 13-04. J Spec Oper Med. 2014;14:13–25. doi: 10.55460/CBRW-A2G1. [DOI] [PubMed] [Google Scholar]
- 20.Martinez V, Derivaux B, Beloeil H Regional Anaesthesia and the Pain Committee of the French Society of Anaesthesiology and Intensive Care. Ketamine for pain management in France, an observational survey. Anaesth Crit Care Pain Med. 2015;34:357–61. doi: 10.1016/j.accpm.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–8. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): Development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 25.Lubega FA, DeSilva MS, Munube D, Nkwine R, Tumukunde J, Agaba PK, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: A randomized controlled trial. Scand J Pain. 2018;18:19–27. doi: 10.1515/sjpain-2017-0140. [DOI] [PubMed] [Google Scholar]
- 26.Nobrega R, Sheehy KA, Lippold C, Rice AL, Finkel JC, Quezado ZM. Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res. 2018;83:445–54. doi: 10.1038/pr.2017.197. [DOI] [PubMed] [Google Scholar]
- 27.Sheehy KA, Lippold C, Rice AL, Nobrega R, Finkel JC, Quezado ZM. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: A single-center cohort study. J Pain Res. 2017;10:787–95. doi: 10.2147/JPR.S131156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel Gowhari, Aileen Chu, Julie Golembiewski, Robert E. Molokie; Low-Dose Ketamine Infusion In Adult Patients With Sickle Cell Disease – Impact On Management Of Acute Painful Episodes. Blood. 2013;122:2249. [Google Scholar]
- 29.Hassell K, Ngongo W, Montgomery R, Hornick L. (374) Ketamine infusion as an analgesic adjunct in the management of severe pain in patients with sickle cell disease. J Pain. 2017;18:S68. [Google Scholar]
- 30.Gimovsky AC, Fritton K, Viscusi E, Roman A. Evaluating the use of ketamine for pain control with sickle cell crisis in pregnancy. A Case Rep. 2018;10:20–2. doi: 10.1213/XAA.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 31.Jennings CA, Bobb BT, Noreika DM, Coyne PJ. Oral ketamine for sickle cell crisis pain refractory to opioids. J Pain Palliat Care Pharmacother. 2013;27:150–4. doi: 10.3109/15360288.2013.788599. [DOI] [PubMed] [Google Scholar]
- 32.Uprety D, Baber A, Foy M. Ketamine infusion for sickle cell pain crisis refractory to opioids: D case report and review of literature. Ann Hematol. 2014;93:769–71. doi: 10.1007/s00277-013-1954-3. [DOI] [PubMed] [Google Scholar]
- 33.Meals CG, Mullican BD, Shaffer CM, Dangerfield PF, Ramirez RP. Ketamine infusion for sickle cell crisis pain in an adult. J Pain Symptom Manage. 2011;42:e7–9. doi: 10.1016/j.jpainsymman.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Kerr C, Holahan T, Milch R. The use of ketamine in severe cases of refractory pain syndromes in the palliative care setting: A case series. J Palliat Med. 2011;14:1074–7. doi: 10.1089/jpm.2010.0424. [DOI] [PubMed] [Google Scholar]
- 35.Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-d-aspartate receptor antagonists: Dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–83. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Coyle CM, Laws KR. The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum Psychopharmacol Clin Exp. 2015;30:152–63. doi: 10.1002/hup.2475. [DOI] [PubMed] [Google Scholar]
- 38.Young JR, Sawe HR, Mfinanga JA, Nshom E, Helm E, Moore CG, et al. Subdissociative intranasal ketamine plus standard pain therapy versus standard pain therapy in the treatment of paediatric sickle cell disease vaso-occlusive crises in resource-limited settings: Study protocol for a randomised controlled trial. BMJ Open. 2017;7:e017190. doi: 10.1136/bmjopen-2017-017190. [DOI] [PMC free article] [PubMed] [Google Scholar]


