Abstract
Background
The purpose of this study is to assess the body composition changes in men with recently diagnosed oligometastatic prostate cancer following neoadjuvant chemohormonal therapy. Further, we evaluated whether CT‐based body composition parameters are associated with biochemical recurrence or imaging progression.
Material and methods
Recently diagnosed castration‐naïve oligometastatic prostate cancer patients who received neoadjuvant docetaxel chemotherapy and androgen deprivation treatment (ADT) before prostatectomy and consolidation of local and oligometastatic disease (total eradication therapy), as part of a phase‐II prospective clinical trial were included. Body composition parameters including cross‐sectional areas of the psoas muscle, total, visceral, and subcutaneous adipose tissue were measured on serial CT scans obtained before and following completion of neoadjuvant treatment.
Results
A total of 22 prostate cancer patients were included (median age 58 years, median Gleason score 8). The median time intervals between commencement of neoadjuvant chemohormonal therapy and first and second follow‐up CTs were 3 and 12 months, respectively. Compared to the baseline scan, there were significant declines in psoas muscle cross‐sectional areas with estimated percentage declines of −13.9% (IQR: 7.6%–16.5%, p < .001) and −13.2% (IQR: 6%–11.2%, p < .001) on first and second follow‐up CTs. There were significant increases in subcutaneous adipose tissue following neoadjuvant chemohormonal therapy with percentage increases of +8.9% (IQR: 5.1%–21.5%, p = .002) and +18.9% (IQR: 6.1%–33.8%, p < .001), respectively. The median follow‐up was 34.5 months. The estimated 2‐year prostate‐specific antigen progression‐free and radiologic progression‐free survival were 95.5%. No significant association between baseline or percentage change in body composition parameters and disease progression were identified.
Conclusions
Our findings showed a significant reduction in muscle mass and an increase in subcutaneous adiposity in men treated with neoadjuvant docetaxel and ADT, more pronounced on the first follow‐up scan after completion of neoadjuvant treatment. Body composition parameters were not found to be significant predictors of disease progression in our cohort.
Keywords: androgen deprivation, body composition, castration sensitive, docetaxel, oligometastatic prostate cancer
1. INTRODUCTION
The clinical implication of body composition parameters has received great attention in the oncology field. 1 Many studies on patients with different types of cancers report a significant association between body composition and clinical outcomes. 2 Sarcopenia, characterized by a loss of skeletal muscle mass, has been shown to be negatively associated with treatment efficacy, chemotherapy‐related toxicities, and postoperative complications; these complications occasionally lead to impaired overall survival. 1 , 2 , 3 In addition, certain chemotherapeutic or chemohormonal regimens can alter the body composition of oncologic patients and result in changes in muscle mass or visceral or subcutaneous adipose tissue. 4
In recent years, several studies on castration‐resistant prostate cancer patients have investigated the prognostic value and impact of body composition on disease progression and clinical outcomes, particularly in those who have received docetaxel or androgen deprivation therapy (ADT) as first‐line treatment. 5 , 6 , 7 , 8 , 9 , 10 Data on the effect of neoadjuvant chemohormonal therapy on body composition and outcomes in patients with early‐stage or oligometastatic castration‐sensitive prostate cancer is scarce. 11 , 12 This study aims to assess the changes in body composition of recently diagnosed oligometastatic prostate cancer patients following neoadjuvant chemohormonal therapy with docetaxel and ADT. Second, we evaluate whether computed tomography (CT)‐based body composition parameters are associated with biochemical recurrence or disease progression in the same cohort of patients.
2. MATERIALS AND METHODS
2.1. Patients
This study is a retrospective analysis of a phase‐II prospective clinical trial (NCT02716974) on patients with recently diagnosed castration‐naïve oligometastatic prostate cancer who received neoadjuvant chemohormonal therapy before definitive prostatectomy and consolidation of local and oligometastatic disease (total eradication therapy). 13 , 14 Oligometastatic status was defined as five or fewer metastases including bone lesions and nonregional lymph nodes, visible on imaging including bone scan, contrast‐enhanced CT, or positron emission tomography. 13 The study was approved by the Institutional Review Board. Of 26 patients who met the clinical trial's eligibility criteria, 22 were included in our analysis (two patients did not complete therapy and lost follow‐up, two patients did not have available baseline images).
2.2. Treatment and follow‐up
All patients received systemic neoadjuvant chemohormonal therapy including up to six cycles of neoadjuvant docetaxel chemotherapy and ADT with luteinizing hormone‐releasing hormone agonists. Docetaxel was administered intravenously at a dose of 75, 55, or 35 mg/m2, based on the timing around ADT. Premedication included dexamethasone twice daily, beginning the day before chemotherapy, for 3 days. Dose reductions and treatment delays (up to 5 weeks from the standard 3‐week interval) were allowed to mitigate and manage toxicities.
Ten patients received concurrent Abiraterone + prednisone (duration: 1–16 weeks) in addition to docetaxel, initiated during the chemotherapy, and stopped before prostatectomy.
Patients underwent local tumor control with radical prostatectomy after completion of chemotherapy (all except one). Approximately at 12–16 weeks after prostatectomy, patients received radiation therapy to the prostatic/pelvic bed (local consolidation) and consolidative stereotactic radiation to the oligometastatic lesions (n = 21). In all patients except one, ADT was continued for 12 months. Patients were followed for evidence of biochemical recurrence or imaging progression/new metastases. Biochemical recurrence was defined as rising serum prostate‐specific antigen (PSA) ≥ 0.2 ng/ml followed by a second confirmatory level based on the American Urological Association and the European Association of Urology recommendations.
2.3. Assessment of body composition
Body composition was assessed on serial CT abdomen and pelvis exams performed (1) before initiation of neoadjuvant therapy, (2) within 1 month after the completion of neoadjuvant therapy, and (3) approximately 1 year after initiation of neoadjuvant therapy. The images were analyzed with Mirada XD (Mirada Medical).
Various parameters of body composition including cross‐sectional areas of the psoas muscle, total fat, visceral fat, and subcutaneous fat were measured at the level of L3‐L4 (Figure 1). Different tissue compartments were manually outlined, the demarcation of the tissue of interest was based on the following Hounsfield Unit (HU) thresholds: −29 to + 150 (skeletal muscle), −190 to −30 (adipose tissue, excluding visceral organs). Anonymized CT images were segmented blind to the clinical information, twice at two different time points. Any measurement discrepancy was addressed by a third review. Psoas muscle area, subcutaneous fat area, and visceral fat area were normalized for stature to derive psoas muscle index (PMI; cm2/m2), subcutaneous fat index (cm2/m2), and visceral fat index (cm2/m2), respectively. Visceral to subcutaneous adipose tissue ratios were calculated. Sarcopenia was defined as L3‐PMI < 5.7 cm2/m2. 15
Figure 1.
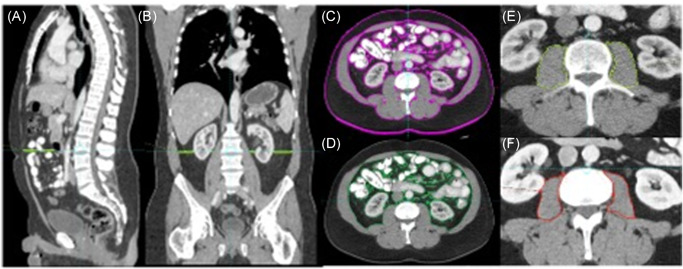
Body composition segmentation on computed tomography. Sagittal (A), Coronal (B) and axial (C–E) CT images at baseline; different tissue compartments measured at the level of L3 (L3‐L4) as follows: (C) Total fat cross‐sectional area: Visceral fat + subcutaneous fat (−190 to −30 HU), (D) visceral fat cross‐sectional area excluding visceral organs (−190 to −30 HU), (E) psoas muscle area (−29 to +150 HU). Axial CT (F) obtained after completion of neoadjuvant docetaxel and androgen deprivation showed a significant decrease in psoas muscle area compared to the baseline (26 cm2 at baseline vs. 21.7 cm2 at first follow‐up exam). CT, computed tomography; HU, Hounsfield unit[Color figure can be viewed at wileyonlinelibrary.com]
The mean muscle attenuation/muscle radiodensity, expressed as the mean Hounsfield Unit (HU), and indirectly correlated with fat infiltration in muscle, was measured. The following cut‐points have been used to define low muscle attenuation (myosteatosis): < 41 HU and < 33 HU in men with body mass index (BMI) < 25 and ≥ 25 kg/m2, respectively. 16
2.4. Statistical analysis
Continuous variables are expressed as the median and interquartile range (IQR). The relative percentage changes in body composition parameters including psoas muscle cross‐sectional area, and total, subcutaneous, and visceral adiposity were calculated as follows: (body composition parameter scan 2 [or 3]—body composition parameter scan 1 [or 2]) ÷ (body composition parameter scan 1 [or 2]). A nonparametric test for related groups of measurements was used to compare the changes in body composition parameters before and following completion of neoadjuvant chemohormonal therapy (baseline, two follow‐up scans). Friedman's two‐way analysis of variance test was applied to examine the differences between groups and significance values have been adjusted by the Bonferroni correction for multiple tests. Statistical significance was defined as p < .05. Analyses were performed using SSPSS version 18 (PASW).
3. RESULTS
3.1. Patient characteristics
A total of 22 patients with recently diagnosed oligometastatic prostate cancer were included. The median age was 58 years (IQR: 54.3–64.3, range: 48–75) and median Gleason score 8 (IQR: 8–9, range: 7–10).
The time interval between the baseline CT and initiation of chemotherapy was 1 month (IQR: 0–2 months). Patients received three to six cycles of neoadjuvant docetaxel therapy, among which 19 (86.4%) patients received four cycles. The median cumulative docetaxel dose was 180 mg/m2 (IQR: 180–220, range: 140‐390 mg/m2).
The median time interval between commencement of neoadjuvant therapy and first and second posttreatment follow‐up CTs were 3 months (IQR: 2–3) and 12 months (IQR: 10.5–17.5), respectively. The median follow‐up was 34.5 months (IQR: 29.2–40 months, range: 20–44 months). The characteristics of the included patients are summarized in Table 1.
Table 1.
Characteristics of the included patients
| Variables at time of diagnosis | ||
|---|---|---|
| Age (years) | 58 (54.3–64.3) | |
| Initial PSA (ng/ml) | 14 (7.2–44) | |
| Gleason score | 8 (8–9) | |
| BMI (kg/m2) | 29.3 (25.1–33.4) | |
| Normal (BMI < 25) | 5 (22.7%) | |
| Overweight (25 ≥ BMI > 30) | 6 (27.3%) | |
| Obese (BMI ≥ 30) | 11 (50%) | |
| Body composition before initiation of neoadjuvant therapy | ||
| Psoas muscle mass (cm2) | 27.5 (24.6–36.7) | |
| Psoas muscle index (cm2/m2) | 8.7 (8.11–11.3) | |
| Sarcopenia (PMI < 5.7 cm2/m2) | 2/22 (9.1%) | |
| Psoas muscle density (HU) | 51.9 (45.9–55.7) | |
| Total fat area (cm2) | 476.8 (389.8–685) | |
| Visceral adiposity (cm2) | 261.3 (142.4–326.9) | |
| Visceral adiposity index (cm2/m2) | 81.6 (46.4–102.9) | |
| Subcutaneous fat area (cm2) | 217 (159.8–340.9) | |
| Subcutaneous fat index (cm2/m2) | 72.4 (51.0–107.1) | |
| Visceral to subcutaneous fat ratio | 0.83 (0.65–1.41) | |
| Subcutaneous fat to muscle ratio | 9.0 (5.04–11.9) | |
| Follow‐up/outcome | ||
| Duration of neoadjuvant therapy (weeks) | 9 (9–11) | |
| Time from completion of neoadjuvant therapy to prostatectomy (weeks) | 9 (7–10) | |
| Biochemical recurrence | Rate (%) | 10 of 22 (45.5%) |
| Time to progression (months) | 27 (24.8–31.3), range 21–35 | |
| Imaging recurrence | Rate (%) | 4 of 22 (18.2%) |
| Time to progression (months) | 28.5 (22.8–30.5), range: 21‐–31 | |
Abbreviations: BMI, body mass index; HU, Hounsfield unit; PMI, psoas muscle index; PSA, prostate‐specific antigen.
3.2. Change in body composition parameters
The prevalence of sarcopenia before the treatment was 9.1% (2 of 22). Both sarcopenic patients were noted to have BMI > 30 kg/m2, thus the prevalence of sarcopenic obesity was 9.1%. The median PMI, psoas muscle density, subcutaneous fat index, and visceral fat index on baseline exam were 8.7 cm2/m2 (IQR: 8.11–11.3), 51.9 HU (IQR: 45.9–55.7), 72.4 cm2/m2 (51.0–107.1), and 81.6 cm2/m2 (46.4–102.9), respectively. No change in the prevalence of sarcopenia was observed on follow‐up CT exams, approximately 3 months and 1 year after commencement of neoadjuvant chemotherapy.
There were significant decreases in psoas muscle cross‐sectional areas following neoadjuvant therapy, with an estimated decline of −13.9% (IQR: 7.6%–16.5%, p < .001) and −13.2% (IQR: 6%–11.2%, p < .001) on first and second follow‐up exams relative to the baseline. In addition, we found a significant increase in subcutaneous adipose tissue compared to the baseline study with approximately +8.9% (IQR: 5.1%–21.5%, p = .002) and +18.9% (IQR: 6.1%–33.8%, p < .001) increase in subcutaneous adiposity on first and second follow‐up CTs, respectively. Similar trend was observed for total adipose cross‐sectional area. No significant difference in the visceral adipose tissue was observed after neoadjuvant therapy (p > .05). Table 2 summarized the percentage changes in body composition parameters between baseline and follow‐up exams.
Table 2.
Percentage changes in body composition parameters at baseline, first and second follow‐up exams after completion of docetaxel‐based neoadjuvant chemotherapy
| Baseline versus first follow‐up (n = 21) | Baseline versus second follow‐up (n = 20) | First versus second follow‐up (n = 19) | ||||
|---|---|---|---|---|---|---|
| Body composition parameters | % change | p Value | % change | p Value | % change | p Value |
| Psoas muscle area (cm2) | −13.9% (−7.6, −16.5) | <.001 | −13.2(−6, −11.2) | <.001 | 0(−8.0, +3.4) | 1 |
| Subcutaneous fat (cm2) | +8.9(+5.1, +21.5) | .002 | +18.9(+6.1, +33.8) | <.001 | +3.3(−0.2, +11.0) | .43 |
| Total adiposity (cm2) | +5.7(+0.8, +15.2) | .04 | +10.3(+2.7, +28.5) | <.001 | +2.1(−1.3, +8.3) | .43 |
| Visceral fat (cm2) | −1.6(−9.9, +18,7) | .56 | +8.4(−1.7, +23.8) | .56 | 0(−7.9, +12.2) | .56 |
None of the patients met the criteria for myosteatosis at baseline and first follow‐up, and one patient had myosteatosis at the time of second follow‐up exam. We found a significant gradual decrease in psoas muscle attenuation (HU) at second follow‐up exam compared to the baseline (p = .003). We did not find any significant association between age and baseline BMI and the percentage change in body composition parameters.
3.3. Disease progression according to body composition
All patients had biochemical remission (undetectable PSA) after radical prostatectomy. During the follow‐up period, biochemical recurrence occurred in 10 patients (45.5%) with a median time to biochemical progression of 27 months (IQR: 24.8–31.3; range: 21–35 months). Most biochemical recurrences occurred later than 2 years following treatment initiation, with 2‐year PSA progression‐free survival of 95.5%.
Four patients had radiologic evidence of progression including osseous (two patients) and lymph node metastasis (two patients), of which three of them showed concurrent biochemical recurrence at the time of radiologic progression. One patient with radiologic evidence of lymph node metastases without biochemical recurrence was found to have metastatic melanoma and passed away during the follow‐up period. The median time to radiologic progression was 28.5 months (IQR: 22.8–30.5; range: 21–31 months). The estimated 2‐year radiologic progression‐free survival was 95.5%.
Baseline body composition parameters including psoas muscle area/index, total, visceral, or subcutaneous fat indices area were not significant predictors of biochemical or radiologic progression. Similarly, we found no statistically significant association between the percentage change in body composition parameters and biochemical or radiologic progression. Patients with evidence of progression (biochemical or imaging) tend to have lower subcutaneous and visceral fat indices at baseline (62.2 vs. 78.8, p = .18 and 63.4 vs. 90.4, p = .23), and higher percentage increase in subcutaneous and visceral fat area on second follow‐up exam (+26.5% vs. +15.9%, p = .23 and +15.9% vs. +6.0%, p = .34), however, none reached statistical significance.
4. DISCUSSION
This is the first published study evaluating changes in body composition following neoadjuvant docetaxel and ADT in recently diagnosed oligometastatic castration‐sensitive prostate cancer patients who underwent total eradication therapy. We reported a significant reduction in psoas muscle cross‐sectional area and an increase in total and subcutaneous adipose tissue indices, all of which are most pronounced on first follow‐up scan after completion of treatment. Our findings on loss of lean muscle mass following taxane‐based chemotherapy are in‐line with some previously published studies on patients with different types of cancers 4
Systemic neoadjuvant therapy remains the mainstay of treatment in metastatic castration‐sensitive prostate cancer, either with ADT alone or in combination with a chemotherapeutic agent. 17 Several studies support the advantage of multidisciplinary total eradication treatments including combined neoadjuvant chemohormonal and local and/or metastasis‐directed therapies in patients with oligometastatic hormone‐sensitive disease to maximize cure rate. 5 , 13 , 14 , 18 Docetaxel‐based chemotherapy is one of the standard first‐line agents in chemohormonal therapy of patients with metastatic disease and those with high‐risk nonmetastatic prostate cancer. 5 , 17 Androgens promote anabolism in the musculoskeletal system while generally repressing adiposity. Thus androgen‐deprivation treatments alter the body composition parameters, result in loss of lean body mass and increase in adipose tissue. 12 , 19 Docetaxel accumulates in adipose tissue, resulting in a higher volume of distribution. It has been suggested that higher body fat composition is associated with improved survival in oncologic patients, possibly due to energy metabolism and better distribution and tolerance to chemotherapeutic agents. 20
The association of body composition with efficacy of first‐line treatment and survival outcome in prostate cancer have been investigated in several studies, 5 , 6 , 7 , 8 , 9 , 10 , 21 , 22 predominantly in patients with castration‐resistant metastatic disease, as well as three studies on patients with localized disease. 10 , 21 , 22 A detailed summary of these studies is provided in Table 3. 5 , 6 , 7 , 8 , 9 , 10 , 21 , 22 In almost all related studies except one, 23 the body composition was assessed at baseline (before the initiation of therapy). Thus, the effects of percentage change in body composition following therapy and survival outcomes remain to be evaluated.
Table 3.
Summary of studies investigating the association of body composition parameters and outcome in prostate cancer
| Author, year | Design | Patient | Time of assessment | Findings | |
|---|---|---|---|---|---|
| characteristics | |||||
| (Treatment) | |||||
| Pak et al., 2019 21 | Retrospective | 2042 | Preoperative | Low psoas muscle index is associated with increased risks of biochemical recurrence, distant metastasis, and overall mortality regardless of the BMI. | |
| localized PCa | |||||
| (radical prostatectomy) | |||||
| Mason et a., 2018 22 | Retrospective | 698 | Preoperative | Prevalence sarcopenia (55.6%) | |
| localized PCa | |||||
| (radical prostatectomy) | Sarcopenia (Skeletal muscle index < 55 cm2/m2) was not found to be independently associated with perioperative complications or oncologic outcomes. | ||||
| McDonald et al., 2017 10 | Retrospective | 171 | Before treatment | Lower subcutaneous adipose tissue density was associated with a lower rate of biochemical failure following definitive treatment. | |
| high‐risk PCaa | |||||
| (definitive RT+ADT) | |||||
| Kashiwagi et al., 2020 11 | Retrospective | 178 | Before treatment | High psoas muscle volume index is associated with longer overall survival. | |
| metastatic hormone‐naïve PCa | |||||
| (ADT) | |||||
| Pak et al., 2020 9 | Retrospective | 230 CRPCa | Before treatment | High skeletal muscle mass (median, 49.9 cm2/m2) is associated with reduced risk of disease progression and mortality in patients treated with Docetaxel but not those who received ADT. | |
| (docetaxel‐44, ADT‐86) | |||||
| Stangl‐Kremser et al., 2019 8 | Retrospective | 186 | Before treatment | Prevalence sarcopenia (82.8%) | |
| Low skeletal muscle volume is an independent prognostic factor for tumor progression. | |||||
| CRPCa | *Patients with high visceral to‐subcutaneous fat ratio tend to have shorter overall survival (p = .06) | ||||
| (docxetaxel+prednisone, prior ADT) | |||||
| Ohtaka et al., 2019 15 | Retrospective | 77 | Before treatment | Prevalence sarcopenia (34%) | |
| CRPCa | |||||
| (docetaxel) | Sarcopenia (Psoas muscle index < 5.7 cm2/m2) is an independent predictor of poor tolerance to docetaxel. | ||||
| Thekkekara et al., 2018 22 | Retrospective | 59 | Before and after treatment | Prevalence sarcopenia (57.6%) | |
| metastatic CSPCa | Significant loss of muscle mass and increase in adipose burden without changes in BMI following treatment. | ||||
| (ADT ± upfront docetaxel) | No significant change in body composition of patients who received docetaxel+ADT (n = 25) compared to those who received ADT alone (n = 34). | ||||
| Lee et al., 2018 5 | Retrospective | 282 | Before treatment | Subcutaneous fat index (>39.9 cm2/m2) is associated with higher progression‐free and cancer‐specific survival rates. | |
| CRPCa | |||||
| (docetaxel+ADT) | *Patients with high skeletal muscle index (52.4 cm2/m2) tend to have higher progression‐free and cancer‐specific survival rates (p = .08) | ||||
| Cushen et al., 2016 6 | Retrospective | 63 | Before treatment | Prevalence sarcopenia (47%) | |
| metastatic CRPCa | Docetaxel toxicity: Sarcopenia and low muscle attenuation are associated with neutropenia. Measurements of adiposity were not predictive of docetaxel toxicity. | ||||
| (docetaxel+ADT) | Survival: Neither sarcopenia nor sarcopenic obesity was associated with overall survival. High volume of visceral fat and BMI < 25 kg/m2 are associated with reduced survival. | ||||
| Wu et al., 2015 7 | Retrospective | 333 | Within 1 month from treatment initiation | High visceral to subcutaneous fat area ratio was associated with poor prognosis. | |
| metastatic CRPCa | |||||
| (docetaxel) | High visceral fat to muscle ratio and high BMI were associated with increased duration from starting docetaxel to death. | ||||
Abbreviations: ADT, androgen deprivation therapy; BMI, body mass index; CRPCa, castration‐resistant prostate cancer; CSPCa, castration‐sensitive prostate cancer; PCa, prostate cancer; PSA, prostate‐specific antigen; RT, radiotherapy.
High‐risk defined as PSA > 20 ng/ml, Gleason score ≥ 8, or extra‐prostatic extension.
In a retrospective study on 2042 patients with localized prostate cancer who underwent radical prostatectomy with median follow‐up of 94.3 months, Pak et al. 21 showed that PMI is independently associated with risk of biochemical recurrence, distant metastasis, and mortality. The 10‐year progression‐free survival, cancer‐specific and overall survival rates increased substantially from the lowest to the highest PMI quartiles. 21 Although the findings remain controversial, most studies on patients with castration‐resistant prostate cancer showed high skeletal muscle mass, PMI, and subcutaneous fat index at the time of diagnosis of castration‐resistant disease are associated with the efficacy of docetaxel treatment and higher progression‐free and cancer‐specific survival. 5 , 9 High pretreatment visceral to subcutaneous fat ratio has been shown to be associated with poor prognosis. 7 , 8
Published data on patients with metastatic hormone‐naïve prostate cancer are limited to a recent retrospective study 11 and an abstract. 23 Kashiwagi et al. 11 studied 178 metastatic hormone‐naïve prostate cancer patients who received primary ADT and showed a higher psoas muscle ratio is a predictor of longer overall survival (HR = 0.45, p = .02) but not progression‐free survival. The respective median follow‐up, progression‐free survival, and overall survival in the above study were 32 (range: 0–190 months), 28 months, and 80 months, with all‐cause death, occurred in 33.7% of patients. 11 In a published abstract on 59 metastatic castration sensitive prostate cancer patients who received ADT with or without upfront docetaxel, Thekkekara et al. 23 reported a significant decrease in skeletal muscle index, increase in fat‐mass‐index, and prevalence of sarcopenia following treatment. They reported a shorter time to progression in sarcopenic patients (p = .046).
In our cohort of recently diagnosed castration naïve oligometastatic prostate cancer, we found no significant association between baseline or percentage change in body composition parameters and disease progression following neoadjuvant chemohormonal therapy. Potential explanation for lack of significant association are (1) different patient population with favorable prognostic features, for example, oligometastatic disease, lower prevalence of sarcopenia, (2) relatively small number of patients, (3) shorter follow‐up period, and (4) lower rate of progression, surgical complications or death in our cohort compared to prior studies. Thus, further prospective studies with a larger sample size and longer follow‐up are needed to assess whether changes in body composition are associated with survival outcomes and other measures of quality of life (physical, social and mental health). In addition, the effect of lifestyle modification, exercise training, nutritional intervention, and pharmacotherapy in maintaining muscle mass during neoadjuvant chemohormonal therapy in this patient population needs to be investigated in future studies.
Aggressive neoadjuvant therapy and prostatectomy in patients with metastatic disease represent a promising approach, 13 but there may be significant consequences to the overall well‐being of the patients and quality of life, as borne out by the observed changes in body composition. Thus, patients choosing to undergo such aggressive therapies should be counseled regarding the implications of those changes in body composition.
5. CONCLUSION
A significant reduction in PMI and increase in total and subcutaneous adipose tissue were observed in patients with recently diagnosed oligometastatic castration‐sensitive prostate cancer following completion of neoadjuvant docetaxel and ADT. However, our study did not show a statistically significant association between body composition parameters (baseline or percentage change after therapy) and disease progression.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Sheikhbahaei S, Reyes DK, Rowe SP, Pienta KJ. CT‐based assessment of body composition following neoadjuvant chemohormonal therapy in patients with castration‐naïve oligometastatic prostate cancer. The Prostate. 2021;81:127‐134. 10.1002/pros.24088
REFERENCES
- 1. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer. 2016;57:58‐67. [DOI] [PubMed] [Google Scholar]
- 3. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015;205(3):W255‐W266. [DOI] [PubMed] [Google Scholar]
- 4. Hopkins JJ, Sawyer MB. Interactions of lean soft‐tissue and chemotherapy toxicities in patients receiving anti‐cancer treatments. Cancer Chemother Pharmacol. 2018;82(1):1‐29. [DOI] [PubMed] [Google Scholar]
- 5. Lee JS, Lee HS, Ha JS, et al. Subcutaneous fat distribution is a prognostic biomarker for men with castration‐resistant prostate cancer. J Urol. 2018;200(1):114‐120. [DOI] [PubMed] [Google Scholar]
- 6. Cushen SJ, Power DG, Murphy KP, et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate‐resistant prostate cancer treated with docetaxel. Clin Nutr ESPEN. 2016;13:e39‐e45. [DOI] [PubMed] [Google Scholar]
- 7. Wu W, Liu X, Chaftari P, et al. Association of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective review. PLOS One. 2015;10(3):0122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stangl‐Kremser J, Suarez‐Ibarrola R, Andrea DD, et al. Assessment of body composition in the advanced stage of castration‐resistant prostate cancer: special focus on sarcopenia. Prostate Cancer Prostatic Dis. 2020;23(2):309‐315. [DOI] [PubMed] [Google Scholar]
- 9. Pak S, Kim MS, Park EY, Kim SH, Lee KH, Joung JY. Association of body composition with survival and treatment efficacy in castration‐resistant prostate cancer. Front Oncol. 2020;10:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald AM, Fiveash JB, Kirkland RS, et al. Subcutaneous adipose tissue characteristics and the risk of biochemical recurrence in men with high‐risk prostate cancer. Urol Oncol. 2017;35(11):663‐e621. [DOI] [PubMed] [Google Scholar]
- 11. Kashiwagi E, Shiota M, Masaoka H, et al. Relationship between body composition and hormone sensitivity for androgen deprivation therapy in patients with metastatic prostate cancer. Prostate Int. 2020;8(1):22‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL. Body composition changes during androgen deprivation therapy for prostate cancer: a 2‐year prospective study. Crit Rev Oncol Hematol. 2008;68(2):172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reyes DK, Rowe SP, Schaeffer EM, et al. Multidisciplinary total eradication therapy (TET) in men with newly diagnosed oligometastatic prostate cancer. Med Oncol. 2020;37(7):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohit Gupta AS, Hiten Patel, Diane Reyes, et al. Definitive therapy for men with newly‐diagnosed oligometastatic prostate cancer: initial surgical outcomes from a phase II study. J Urol. 2019;201(4):MP22‐14. [Google Scholar]
- 15. Ohtaka A, Aoki H, Nagata M, et al. Sarcopenia is a poor prognostic factor of castration‐resistant prostate cancer treated with docetaxel therapy. Prostate Int. 2019;7(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee CM, Kang J. Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2020;11:1270‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao A, Vapiwala N, Schaeffer EM, Ryan CJ. Oligometastatic prostate cancer: a shrinking subset or an opportunity for cure? Am Soc Clin Oncol Educ Book. 2019;39:309‐320. [DOI] [PubMed] [Google Scholar]
- 18. Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6(11):8491‐8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599‐603. [DOI] [PubMed] [Google Scholar]
- 20. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer‐counterpoint. Cancer Res. 2018;78(8):1906‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pak S, Park SY, Shin TJ, et al. Association of muscle mass with survival after radical prostatectomy in patients with prostate cancer. J Urol. 2019;202(3):525‐532. [DOI] [PubMed] [Google Scholar]
- 22. Mason RJ, Boorjian SA, Bhindi B, et al. The association between sarcopenia and oncologic outcomes after radical prostatectomy. Clin Genitourin Cancer. 2018;16(3):e629‐e636. [DOI] [PubMed] [Google Scholar]
- 23. Thekkekara RJ, Pathak S, Yadav U, et al. Lean and fat‐mass changes following upfront docetaxel compared to androgen deprivation monotherapy in metastatic castration‐naïve prostate cancer (abstract). J Clin Oncol. 2018;36(15_suppl):e17020. [Google Scholar]


