Abstract
Aim
To evaluate the efficacy and safety of once‐weekly subcutaneous semaglutide, a glucagon‐like peptide‐1 (GLP‐1) analogue, versus once‐daily sitagliptin as add‐on to metformin in patients with type 2 diabetes (T2D) in a multiregional clinical trial.
Materials and Methods
In the 30‐week, randomized, double‐blind, double‐dummy, active comparator SUSTAIN China trial, 868 adults with T2D inadequately controlled on metformin (HbA1c 7.0%‐10.5%) were randomized to receive once‐weekly semaglutide 0.5 mg (n = 288), semaglutide 1.0 mg (n = 290) or once‐daily sitagliptin 100 mg (n = 290). The primary and confirmatory secondary endpoints were change from baseline to week 30 in HbA1c and body weight, respectively.
Results
The trial enrolled ~70% (605/868) of the patients in China, and the remaining patients from four other countries, including the Republic of Korea. Both doses of semaglutide were superior to sitagliptin in reducing HbA1c and body weight after 30 weeks of treatment. The odds of achieving target HbA1c of less than 7.0% (53 mmol/mol), weight loss of 5% or higher, or 10% or higher, and the composite endpoint of HbA1c less than 7.0% (53 mmol/mol) without severe or blood glucose‐confirmed symptomatic hypoglycaemia no weight gain, were all significantly higher with both semaglutide doses compared with sitagliptin. The safety profile for semaglutide was consistent with the known class effects of GLP‐1 receptor agonists (RAs). Consistent efficacy and safety findings were seen in the Chinese subpopulation.
Conclusions
Once‐weekly semaglutide was superior to sitagliptin in improving glycaemic control and reducing body weight in patients with T2D inadequately controlled on metformin. The safety and tolerability profiles were consistent with those of semaglutide and other GLP‐1 RAs. Semaglutide is an effective once‐weekly treatment option for the Chinese population.
Keywords: GLP‐1 analogue, glycaemic control, incretin therapy, phase III study, randomized trial, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a complex, multifactorial disease, with a steadily increasing prevalence influenced by increasing obesity and physical inactivity. In China, T2D prevalence was 116.4 million (10.9%) in 2019 and is expected to increase to 140.5 million by 2030, representing a large health burden for society. 1 , 2 The focus of T2D management is to optimize glycaemic control to reduce the risk of both microvascular and macrovascular complications. 3 , 4 , 5 , 6
Despite the wide range of treatment options available, 7 , 8 less than 20% of Chinese patients with T2D achieve the recommended target of HbA1c of less than 7%, 9 , 10 , 11 and many are therefore at risk of developing diabetes complications including cardiovascular disease. The prevalence of obesity (body mass index [BMI] ≥ 28 kg/m2) among the Chinese population is 24.3%. 12 It is known that a weight loss of 5% or more is associated with improved glycaemic control in patients with T2D, and weight loss is therefore a cornerstone of T2D treatment. 7 , 13 , 14 The pathophysiology of T2D in East Asians involves reduced insulin secretory capacity and less insulin resistance compared with Caucasians. 15 , 16 , 17
The most recent guidelines and position statements issued in collaboration by the American Diabetes Association and the European Association for the Study of Diabetes, 8 as well as guidelines from the Chinese Diabetes Society (CDS), 7 emphasize an individually tailored choice of glucose‐lowering treatment for managing T2D. Besides optimizing glycaemic control, several other factors influence treatment choice, including the effect on weight, risk of hypoglycaemia and cardiovascular status. Currently, the CDS recommends the addition of glucose‐lowering agents, including glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, after first‐line metformin treatment and lifestyle modification in patients with T2D.
Semaglutide is a once‐weekly long‐acting GLP‐1 RA approved for the treatment of T2D under the trade name Ozempic®. The GLP‐1 moiety of semaglutide is modified by the addition of a fatty diacid chain and two amino acid substitutions. These modifications prolong its half‐life through enhanced binding to albumin and inhibition of degradation by DPP‐4, facilitating once‐weekly dosing. 18 The efficacy and safety of semaglutide have been established in more than 10 000 patients in the SUSTAIN clinical trial programme. Semaglutide has consistently shown superior reductions in HbA1c and body weight versus placebo and a range of active comparators such as sitagliptin, exenatide extended release, insulin glargine, dulaglutide, canagliflozin and liraglutide. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Furthermore, semaglutide was also associated with a significant 26% reduction in the risk of major adverse cardiovascular events compared with placebo in a preapproval cardiovascular outcomes trial (CVOT). 30
Chinese regulatory guidelines require that the efficacy and safety of semaglutide is evaluated in a clinical trial including a large proportion of Chinese subjects to obtain regulatory approval in China. Thus, the present trial evaluated the efficacy and safety of once‐weekly treatment with semaglutide (0.5 and 1.0 mg) versus sitagliptin 100 mg once‐daily in patients with T2D inadequately controlled on metformin, with ~70% (605/868) of patients enrolled in China and the remaining patients enrolled in four other countries including the Republic of Korea (~13% of patients). The present trial was designed to resemble one of the global SUSTAIN trials (SUSTAIN 2 20 ), to allow for a comparison of semaglutide in Chinese patients with the results obtained in a global population.
2. MATERIALS AND METHODS
2.1. Trial design
This was a 30‐week, randomized, double‐blind, double‐dummy, active‐controlled, multicentre and multiregional, parallel‐group trial. Participants were selected and screened by the investigators and randomized across 65 sites in Brazil, the China region (consisting of mainland China, Taiwan and Hong Kong), the Republic of Korea, South Africa and Ukraine in a 2:2:1:1 manner to receive one of: semaglutide 0.5 mg and sitagliptin placebo, semaglutide 1.0 mg and sitagliptin placebo, sitagliptin 100 mg and semaglutide placebo (0.5 mg), or sitagliptin 100 mg and semaglutide placebo (1.0 mg). Randomization was performed using an interactive web response system at the randomization visit. The randomization scheme was stratified by region (the China region vs. other countries). The trial comprised a screening period (weeks −2 to 0), a 30‐week treatment period and a 5‐week follow‐up period (Figure S1). The trial was conducted in accordance with the Declaration of Helsinki 31 and the International Conference on Harmonisation Good Clinical Practice, 32 and all patients gave informed consent prior to inclusion in the study. Brief details of the study are available at clinicaltrials.gov (NCT03061214).
2.2. Participants
Eligible participants were aged 18 years or older, diagnosed with T2D with HbA1c 7.0%‐10.5% (53‐91 mmol/mol) (both inclusive), and treated with metformin monotherapy at a stable dose of 1500 mg or higher, or a maximum tolerated dose of 1000 mg or higher for 60 days before screening. Key exclusion criteria included treatment with glucose‐lowering agent(s) other than metformin for 60 days before screening, history of pancreatitis (acute or chronic), a screening calcitonin value of 50 ng/L or higher, history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, a cancer diagnosis in the previous 5 years, impaired renal function (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2), heart failure (NYHA class IV) and acute coronary or cerebrovascular event within 90 days before randomization. Full details of the inclusion/exclusion criteria are provided in Tables S1 and S2.
2.3. Drug administration
Semaglutide and semaglutide placebo were administered by once‐weekly subcutaneous injections in the thigh, abdomen or upper arm, and were to be taken on the same day of the week irrespective of meals. Participants followed a fixed‐dose escalation regimen starting from a dose of 0.25 mg and the dose doubled every 4 weeks until the maintenance dose was achieved. Doses were not to be changed during the trial after the semaglutide maintenance dose had been reached. Sitagliptin and sitagliptin placebo were provided as tablets and were to be administered orally once‐daily irrespective of meals. Metformin dose or dosing frequency was not to be changed during the treatment period, unless rescue medication was needed. Rescue medication was offered if fasting plasma glucose (FPG) exceeded predefined criteria (Table S3).
2.4. Endpoints
The primary endpoint was change from baseline to week 30 in HbA1c. The confirmatory secondary endpoint was change from baseline to week 30 in body weight. Supportive secondary efficacy endpoints were the responder endpoints: the proportions of participants at week 30 who achieved targets of HbA1c less than 7.0% (53 mmol/mol) or 6.5% or less (48 mmol/mol); a composite endpoint of HbA1c less than 7.0% (53 mmol/mol) without severe or blood glucose (BG)‐confirmed symptomatic hypoglycaemia and no weight gain; and weight loss of 5% or higher, or 10% or higher. Furthermore, supportive efficacy endpoints were: change from baseline to week 30 in FPG; self‐measured plasma glucose (SMPG, mean seven‐point profile and mean postprandial increment over all meals) fasting insulin; fasting C‐peptide; fasting glucagon; fasting pro‐insulin; fasting pro‐insulin to insulin ratio; homeostatic model assessment of β‐cell function (fasting HOMA‐β) and insulin resistance (fasting h HOMA‐IR); BMI; waist circumference; systolic and diastolic blood pressure; fasting lipids, high‐sensitivity C‐reactive protein and patient‐reported outcomes including the Short Form‐36v2 (SF‐36v2) health survey and Diabetes Treatment Satisfaction Questionnaire status (DTSQs).
Supportive secondary safety endpoints were: treatment‐emergent adverse events (AEs); severe or BG‐confirmed symptomatic hypoglycaemic episodes; change from baseline to week 30 and/or follow‐up in haematology and biochemistry variables (including lipase and amylase); calcitonin; pulse; electrocardiogram readings and physical examination; and the occurrence of antisemaglutide antibodies. All patients were required to have fundus photography or dilated fundoscopy before enrolment and at end‐of‐treatment (Table S5). An external event adjudication committee performed validation of selected AEs (Table S6). All endpoints were prespecified in the trial protocol.
2.5. Statistical analysis
Sample size calculations were based on demonstration of non‐inferiority of HbA1c with a margin of 0.3% when comparing both semaglutide doses with the pooled sitagliptin group. With an assumed standard deviation of 1.1%, a 90% marginal power would be obtained with 228 patients randomized in each of the semaglutide groups if the true treatment difference (semaglutide vs. sitagliptin) was as high as −0.03%. Hence, at least 286 patients should be randomized to each group and 858 patients in total based on an assumed discontinuation rate of 20%. Furthermore; with 286 subjects per group, a marginal power of at least 90% for demonstrating HbA1c superiority, for any dose of the two dose level comparisons, is obtained if the true treatment difference is as high as −0.30%‐point. Of these patients, 600 were to be randomized in the China region (consisting of mainland China, Taiwan and Hong Kong). The trial was powered according to the total population.
A total of six confirmatory hypotheses relating to HbA1c non‐inferiority/superiority and body weight superiority were to be tested hierarchically for the total population (Figure S2). All analyses performed for the total population were also prespecified for the Chinese population but not performed under multiplicity control.
The analyses of the confirmatory endpoints and other change from baseline endpoints were based on the full analysis set (FAS) using data from the ‘on‐treatment without rescue medication’ observation period in a mixed model for repeated measures (MMRM). The model included all postbaseline measurements collected at scheduled visits up to and including week 30 data as dependent variables. Treatment and the China region or other countries were included in the model as fixed effects and baseline responses as covariates, all nested within visit. An unstructured covariance matrix was employed for measurements within the same patients. From this model, the two by dose level estimated treatment differences (ETDs) between semaglutide versus sitagliptin at week 30 were presented together with associated two‐sided 95% confidence intervals (CIs) and unadjusted two‐sided P‐values (nominal α = 0.05) for testing of non‐inferiority and superiority. Responder endpoints (yes/no) at week 30 were analysed using logistic regression with treatment as a fixed effect adjusted for region and relevant baseline response(s).
Sensitivity analyses including pattern mixture models addressing the impact of missing values were performed for change in HbA1c and body weight.
We performed post hoc subgroup analyses (the China region vs. other countries, all countries and by sex) using the above MMRM model addressing the confirmatory endpoints and adding treatment by relevant subgroups as the interaction term to inspect potential heterogeneity in ETDs across subgroups at week 30. No control for multiplicity was performed.
3. RESULTS
3.1. Participant disposition and baseline characteristics
Of the 1159 patients screened, 868 were randomly assigned to treatments, with 288 patients in the semaglutide 0.5 mg group, 290 patients in the semaglutide 1.0 mg group and 290 patients in the pooled sitagliptin group, exposed to trial product. One patient in the semaglutide 0.5 mg group was not exposed to the trial product and was excluded from the safety analysis set (Figure 1). A total of 805 patients (92.7%) completed the trial; 768 patients (88.5%) completed treatment and 99 patients (11.4%) discontinued treatment prematurely, of which 54 (6.2%) because of AEs. Rescue medication was provided to nine patients (3.1%) in the semaglutide 0.5 mg group, four patients (1.4%) in the semaglutide 1.0 mg group and 19 patients (6.6%) in the sitagliptin group. Baseline characteristics were balanced across the three treatment groups (Table 1). The trial population was predominantly of Asian ethnicity (85.0%) and from Asian countries (~70% from region China and ~13% from the Republic of Korea), and included more men than women. The baseline characteristics for the Chinese population are provided in Table S8.
FIGURE 1.
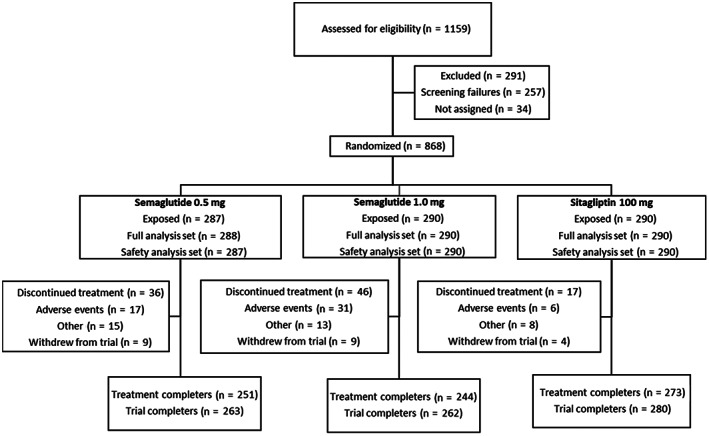
Flow of participation through the trial. Note: Data for randomized patients (n = 868) are from the full analysis set. Number of patients randomized in the China region, which includes China, Hong Kong and Taiwan, (N = 605), the Republic of Korea (N = 110), Brasil (N = 75), South Africa (N = 45) and Ukraine (N = 33)
TABLE 1.
Baseline characteristics
| Semaglutide 0.5 mg (N = 288) | Semaglutide 1.0 mg (N = 290) | Sitagliptin 100 mg (N = 290) | |
|---|---|---|---|
| Age (years) at randomization | 53.0 (11.4) | 53.0 (10.6) | 53.1 (10.4) |
| HbA1c (%) at randomization | 8.1 (0.9) | 8.1 (0.9) | 8.1 (0.9) |
| FPG concentration (mmol/L) at randomization | 9.30 (2.67) | 9.29 (2.22) | 9.05 (2.21) |
| Diabetes duration (years) at randomization | 6.3 (5.4) | 6.7 (4.9) | 6.1 (5.2) |
| Body weight (kg) at randomization | 77.6 (16.4) | 76.1 (16.3) | 75.5 (14.7) |
| BMI (kg/m2) at randomization | 28.2 (5.0) | 27.9 (5.0) | 27.3 (4.7) |
| eGFR (MDRD [mL/min/1.73m2]) at randomization | 109.0 (59–196) | 110.0 (61–274) | 109.0 (60–222) |
|
Sex Female Male |
128 (44.4%) 160 (55.6%) |
136 (46.9%) 154 (53.1%) |
105 (36.2%) 185 (63.8%) |
|
Ethnicity Hispanic or Latino Not Hispanic or Latino |
24 (8.3%) 264 (91.7%) |
28 (9.7%) 262 (90.3%) |
30 (10.3%) 260 (89.7%) |
|
Race Asian White Black or African American |
243 (84.4%) 30 (10.4%) 8 (2.8%) |
251 (86.6%) 28 (9.7%) 8 (2.8%) |
244 (84.1%) 31 (10.7%) 9 (3.1%) |
|
Concomitant illness and medical history reported at screening Hypertension Hyperlipidaemia Hepatic steatosis Dyslipidaemia Diabetic retinopathy |
151 (52.4%) 94 (32.6%) 52 (18.1%) 71 (24.7%) 50 (17.4%) |
152 (52.4%) 97 (33.4%) 52 (17.9%) 84 (29.0%) 46 (15.9%) |
147 (50.7%) 85 (29.3%) 51 (17.6%) 67 (23.1%) 52 (17.9%) |
|
Diabetes medications at randomization Biguanides |
287 (99.7%) a |
289 (99.7%) b |
290 (100%) |
|
Other concomitant medications at randomization (≥5% in any group) Statins Calcium channel blockers Antiplatelet drugs excluding heparin ARBs ACE inhibitors β‐Blockers Herbal and traditional medicine |
91 (31.6%) 53 (18.4%) 53 (18.4%) 56 (19.4%) 22 (7.6%) 22 (7.6%) 19 (6.6%) |
92 (31.7%) 49 (16.9%) 44 (15.2%) 50 (17.2%) 27 (9.3%) 33 (11.4%) 22 (7.6%) |
91 (31.4%) 57 (19.7%) 56 (19.3%) 46 (15.9%) 26 (9.0%) 18 (6.2%) 17 (5.9%) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; MDRD, Modification of Diet in Renal Disease.
All data are from the full analysis set. Data are mean (SD); median (range); or N (%).
Hypertension and diabetic retinopathy data were collected as medical history whereas hyperlipidaemia and dyslipidaemia data were collected as concomitant illnesses at screening.
All except two patients met the inclusion criteria related to the background metformin treatment (a stable dose of metformin [≥1500 mg or maximum tolerated dose ≥1000 mg] for a period of 60 days prior to screening). These two patients discontinued the trial product prematurely because of the violation of inclusion criteria.
3.2. Efficacy
At week 30, mean HbA1c (baseline 8.1%) was reduced by 1.4% with semaglutide 0.5 mg, 1.7% with semaglutide 1.0 mg and 0.9% with sitagliptin (Table 2). The ETD versus sitagliptin was −0.51 (−0.66; −0.36) with semaglutide 0.5 mg and –0.85 (−1.00; −0.70) with semaglutide 1.0 mg (P < .0001 confirming non‐inferiority and superiority for both semaglutide doses vs. sitagliptin; Table 2 and Figure 2). Semaglutide was also superior to sitagliptin with respect to the confirmatory secondary endpoint of change in body weight from baseline to week 30. Body weight was reduced, from 76.4 kg at baseline, by 2.9 kg (3.9%) with semaglutide 0.5 mg and 4.2 kg (5.7%) with semaglutide 1.0 mg versus 0.4 kg (0.5%) with sitagliptin, with an ETD of −2.48 kg for semaglutide 0.5 mg and −3.79 kg for semaglutide 1.0 mg versus sitagliptin (both P < 0.0001; Table 2 and Figure 2). For both endpoints, all sensitivity analyses resulted in similar and statistically significant ETDs with corresponding 95% CIs (Figures S5 and S6). The cumulative distribution for the confirmatory endpoints is shown in Figures S3 and S4.
TABLE 2.
Study outcomes by treatment group at week 30
| Overall baseline (SD) | Semaglutide 0.5 mg (N = 288) | Semaglutide 1.0 mg (N = 290) | Sita 100 mg (N = 290) | |||
|---|---|---|---|---|---|---|
| Change from baseline at week 30 | ETD vs. sitagliptin (95% CI) | Change from baseline at week 30 | ETD vs. sitagliptin (95% CI) | Change from baseline at week 30 | ||
| Glycaemic outcomes | ||||||
| Mean HbA1c (%) | 8.1 (0.9) | −1.4 | −0.51 (−0.66; −0.36) a | −1.7 | −0.85 (−1.00; −0.70) a | −0.9 |
| Mean fasting plasma glucose (mmol/L) | 9.2 (2.4) | −2.0 | −0.98 (−1.28; −0.67) a | −2.5 | −1.47 (−1.77; −1.17) a | −1.0 |
| Seven‐point self‐measured plasma glucose (mmol/L) | ||||||
| Mean | 10.8 (2.4) | −2.4 | −0.68 (−0.97; −0.39) a | −2.9 | −1.24 (−1.53; −0.95) a | −1.7 |
| Increment b | 3.1 (1.9) | −1.1 | −0.39 (−0.66; −0.13) a | −1.2 | −0.51 (−0.77; −0.24) a | −0.7 |
| Body weight‐related outcomes | ||||||
| Mean body weight (kg) | 76.4 (15.8) | −2.9 | −2.48 (−3.06; −1.90) a | −4.2 | −3.79 (−4.37; −3.21) a | −0.4 |
| Mean body weight (%) | NA | −3.9 | −3.46 (−4.22; −2.69) a | −5.7 | −5.28 (−6.04; −4.51) a | −0.5 |
| Mean BMI (kg/m2) | 27.8 (4.9) | −1.1 | −0.92 (−1.13; −0.70) a | −1.6 | −1.40 (−1.62; −1.19) a | −0.2 |
| Mean waist circumference (cm) | 96.1 (12.0) | −2.7 | −1.88 (−2.67; −1.08) a | −4.2 | −3.35 (−4.14; −2.56) a | −0.8 |
| Blood pressure and pulse rate | ||||||
| Mean systolic blood pressure (mmHg) | 128.8 (14.6) | −3.4 | −2.3 (−4.4; −0.2) a | −6.6 | −5.5 (−7.6; −3.4) a | −1.1 |
| Mean diastolic blood pressure (mmHg) | 80.3 (9.3) | −0.9 | 0.1 (−1.3; 1.4) | −1.5 | −0.6 (−1.9; 0.7) | −1.0 |
| Mean pulse rate (beats per minute) | 77.6 (10.4) | 3.5 | 3.4 (2.0; 4.8) a | 3.9 | 3.7 (2.3; 5.1) a | 0.1 |
Abbreviations: BMI, body mass index; CI, confidence interval; ETD, estimated treatment difference; N, number of patients contributing to analysis; N/A, not applicable; Sita, sitagliptin.
A total of 868 patients were randomized and 867 patients were exposed to treatment. The analysis of the primary and secondary confirmatory endpoints was based on data from 868 patients in a mixed model for repeated measures (see statistical analysis section). All data are from the on‐treatment without rescue medication observation period for the full analysis set, with the exception of pulse rate, which is from the on‐treatment observation period for the safety analysis set. Data are mean (SD), mean or ETD (95% CI).
Statistically significant;
Mean postprandial glucose increment (over all meals).
FIGURE 2.
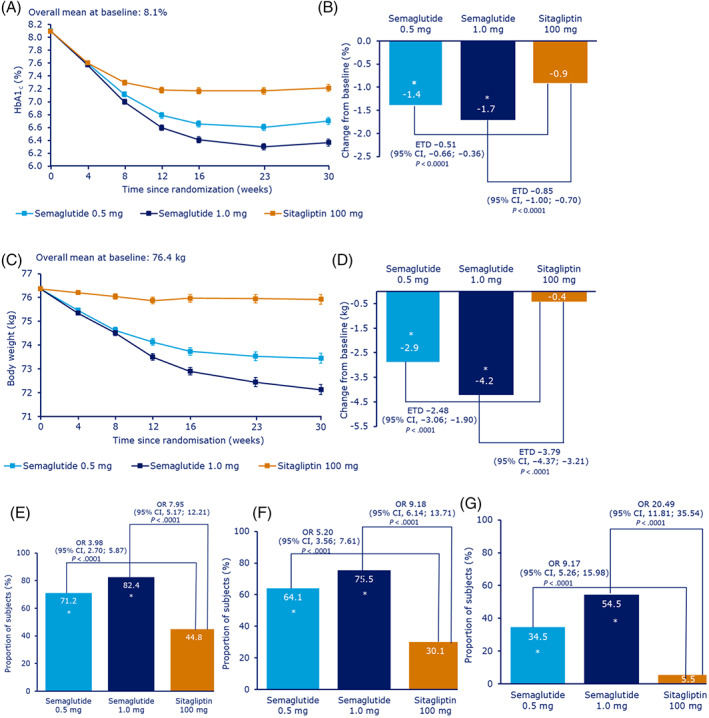
Primary and secondary efficacy endpoints (HbA1c and body weight) change from baseline to week 30. Change in A, mean HbA1c by week, B, change in mean HbA1c after 30 weeks, C, change in mean body weight by week, D, change in mean body weight after 30 weeks, E, proportion of patients achieving the HbA1c target of less than 7.0%, F, proportion of patients achieving HbA1c less than 7.0% without severe or BG‐confirmed symptomatic hypoglycaemia and no weight gain and G, the proportion of patients achieving a weight loss of 5% or more. For all estimated change data (panels A–D), data are mean estimate (± standard error) ‘On‐treatment without rescue medication’ data. The post‐baseline responses were analyzed using a mixed model for repeated measurements with treatment and the China region/other as fixed factors and baseline value as covariate, all nested within visit. Mean estimates are adjusted according to observed baseline distribution. All site visits, except screening visit, were to be completed in fasting state. In panel B, the non‐inferiority p‐value was calculated as two times one‐sided p‐value from a t‐distributed test statistic comparing the treatment contrast with 0.3 rather than zero as in a superiority test. For panels E–G, data are ‘On‐treatment without rescue medication’ data. The binary endpoints were analyzed using a logistic regression model with treatment and the China region/other as fixed factors and baseline values as covariates. Before analysis, missing data for individual components were imputed from a mixed model for repeated measurements with treatment and the China region/other as fixed factors and baseline values as covariates, all nested within visit, and subsequently dichotomized. All site visits, except screening visit, were to be completed in fasting state. BG, blood glucose; ETD, estimated treatment difference; CI, confidence interval; OR, odds ratio; *statistically significant
Consistent results for HbA1c and body weight were seen in the Chinese and Korean subpopulations of the trial and are shown in Table S9 and Figures S17–S20.
Greater proportions of patients achieved targets of HbA1c less than 7.0% (71% and 82% vs. 45%), 6.5% or less (55% and 68% vs. 28%) and the composite endpoint of HbA1c less than 7.0% (53 mmol/mol) without severe or BG‐confirmed symptomatic hypoglycaemia and no weight gain (64% and 76% vs. 30%) (Figures 2 and S7) with semaglutide 0.5 and 1.0 mg than with sitagliptin. The reductions in mean FPG, mean seven‐point SMPG and incremental SMPG were significantly greater with both doses of semaglutide than with sitagliptin (Table 2). Significant reductions were seen in other markers of β‐cell glucose sensitivity and insulin resistance with semaglutide compared with sitagliptin (Figure S8).
Weight loss responses of 5% or more (35% and 55% vs. 6%) and 10% or more (8% and 16% vs. 0.3%) were achieved by greater proportions of patients with semaglutide 0.5 and 1.0 mg than with sitagliptin (Figures 2 and S7). BMI and waist circumference were also significantly reduced with both doses of semaglutide compared with sitagliptin (Table 2).
Overall, patients randomized to semaglutide had favourable changes in blood pressure and lipid concentrations (Table 2 and Figure S9). Total cholesterol and systolic blood pressure were both significantly reduced with both doses of semaglutide compared with sitagliptin. No difference between treatments was seen for diastolic blood pressure. For very low‐density lipoprotein (VLDL) cholesterol and triglycerides, the reductions were significant with semaglutide 1.0 mg compared with sitagliptin but not with semaglutide 0.5 mg.
Patient‐reported outcomes as evaluated using the DTSQs showed an improved treatment satisfaction with both doses of semaglutide vs sitaglipin (Figure S10), and the SF‐36v2 showed an improved overall treatment satisfaction score with both doses of semaglutide vs sitaglipin (Figure S11).
In a post hoc subgroup analysis, no significant treatment interaction was seen for change in HbA1c for the China region compared with other countries (P = .86) at week 30. A significant treatment interaction between the China region versus other countries was observed for change in absolute body weight (kg), but not for relative body weight (%) (P = .025 and P = .065, respectively; Table S10). No significant treatment interaction was seen for either change in HbA1c (P = .70) or change in body weight (P = .17) when looking across countries (Table S11). The semaglutide effect on HbA1c and body weight was independent of sex (Tables S12 and S13).
3.3. Safety and tolerability
The overall proportion of patients with AEs was comparable across treatment groups (69%‐75%). The majority of the AEs were mild and moderate in severity (Table 3). Serious AEs were reported in low numbers and with no differences in reporting pattern across treatment groups and with no evident clustering of event types. Two patients died during the trial: one with semaglutide 0.5 mg (cardiovascular death) and one with semaglutide 1.0 mg (undetermined cause of death), both assessed as unlikely related to the trial product by the investigator.
TABLE 3.
Treatment‐emergent adverse events summary by system organ class and incidence of hypoglycaemia
| Semaglutide 0.5 mg (N = 287) | Semaglutide 1.0 mg (N = 290) | Sitagliptin 100 mg (N = 290) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | E | N | (%) | E | N | (%) | E | |
| Any treatment emergent adverse events a | 209 | 72.8 | 729 | 216 | 74.5 | 788 | 199 | 68.6 | 596 |
| Serious adverse events b | 18 | 6.3 | 23 | 18 | 6.2 | 23 | 12 | 4.1 | 15 |
| Fatal adverse events | 1 | 0.3 | 1 | 1 | 0.3 | 1 | 0 | ||
| Severe adverse events b | 4 | 1.4 | 5 | 11 | 3.8 | 14 | 7 | 2.4 | 7 |
| Moderate adverse events b | 50 | 17.4 | 84 | 50 | 17.2 | 95 | 35 | 12.1 | 60 |
| Mild adverse events b | 201 | 70.0 | 640 | 199 | 68.6 | 679 | 186 | 64.1 | 529 |
| Gastrointestinal adverse events | 108 | 37.6 | 219 | 129 | 44.5 | 294 | 55 | 19.0 | 87 |
| Adverse events leading to premature discontinuation | 17 | 5.9 | 26 | 31 | 10.7 | 45 | 6 | 2.1 | 8 |
| Gastrointestinal adverse events | 10 | 3.5 | 13 | 21 | 7.2 | 29 | 1 | 0.3 | 1 |
| Adverse events occurring in ≥5% of patients in one or more treatment groups by preferred term | |||||||||
| Diarrhoea | 58 | 20.2 | 94 | 49 | 16.9 | 99 | 20 | 6.9 | 24 |
| Upper respiratory tract infection | 28 | 9.8 | 37 | 38 | 13.1 | 43 | 42 | 14.5 | 63 |
| Nausea | 22 | 7.7 | 24 | 39 | 13.4 | 54 | 5 | 1.7 | 7 |
| Lipase increased | 22 | 7.7 | 30 | 13 | 4.5 | 15 | 22 | 7.6 | 30 |
| Decreased appetite | 21 | 7.3 | 21 | 23 | 7.9 | 23 | 4 | 1.4 | 4 |
| Diabetic retinopathy | 21 | 7.0 | 20 | 15 | 5.2 | 15 | 10 | 3.4 | 10 |
| Nasopharyngitis | 17 | 5.9 | 21 | 9 | 3.1 | 12 | 11 | 3.8 | 15 |
| Vomiting | 14 | 4.9 | 16 | 19 | 6.6 | 22 | 3 | 1.0 | 5 |
| Abdominal discomfort | 8 | 2.8 | 9 | 15 | 5.2 | 16 | 1 | 0.3 | 1 |
| Other adverse events | |||||||||
| Severe or BG‐confirmed hypoglycaemia c | 2 | 0.7 | 3 | 6 | 2.1 | 7 | 4 | 1.4 | 5 |
| EAC‐confirmed mild acute pancreatitis | 0 | 1 | 0.3 | 1 | 0 | ||||
| EAC‐confirmed malignant neoplasms | 0 | 0 | 2 | 0.7 | 2 | ||||
Abbreviations: %, percentage of patients experiencing at least one event; BG, blood glucose; E, number of events; EAC, Event Adjudication Committee; N, number of patients experiencing at least one event. Data are ‘On‐treatment’ data from the safety analysis set, with the exception of diabetic retinopathy and EAC‐confirmed malignant neoplasms, which are ‘In‐trial’ data for the safety analysis set.
Treatment‐emergent AEs are events with onset date (or increase in severity) during the on‐treatment observation period, including the 5‐week follow‐up period.
The investigator was to assess the AE with regards to severity (mild, moderate, severe) and seriousness (serious/non‐serious) according to the definitions in the trial protocol (Table S7).
Severe or blood glucose‐confirmed symptomatic hypoglycaemia were defined as severe according to the American Diabetes Association classification 33 (episode requiring assistance of another person to actively administer carbohydrate or glucagon or take other corrective actions) or confirmed by a glucose value of <3.1 mmol/L (56 mg/dL) with symptoms consistent with hypoglycaemia.
More patients with semaglutide 0.5 mg (5.9%) and 1.0 mg (10.7%) experienced AEs leading to premature treatment discontinuation compared with sitagliptin (2.1%) (Table 3 and Figure S12). The most frequently reported type of AEs and most common reason for discontinuation in semaglutide‐treated patients were gastrointestinal AEs (GIAEs). GIAEs occurred mainly during the first 8‐12 weeks of the trial during the dose escalation period (Figure S13). The most frequently reported GIAEs were diarrhoea followed by nausea and vomiting; all were more frequently reported among semaglutide‐treated patients.
The proportion of patients with severe or BG‐confirmed hypoglycaemia was low and, overall, was similar across treatment groups (Table 3). No episodes of severe hypoglycaemia were reported.
Events of diabetic retinopathy were reported by 19 and 14 patients in the semaglutide 0.5 and 1.0 mg groups, respectively, and by 10 patients in the sitagliptin group. Most of the events were non‐serious, mild in severity, reported at routine end‐of‐trial eye examinations and did not require treatment. Amylase and lipase levels increased in all three treatment groups and at week 30 the increase in amylase and lipase was greater with both doses of semaglutide than with sitagliptin (Figures S14 and S15). One Event Adjudication Committee (EAC)‐confirmed event of mild acute pancreatitis was reported in the semaglutide 1.0 mg group, and the patient recovered from the event. With both doses of semaglutide there was a greater increase in pulse rate compared with sitagliptin (Table 2 and Figure S16). Four semaglutide‐treated patients developed antisemaglutide antibodies after treatment initiation and a cross‐reaction with human GLP‐1 occurred in one of these patients. No antisemaglutide antibodies had an in vitro neutralizing effect on semaglutide. Neoplasm‐related AEs were reported by nine, 14 and 10 patients in the semaglutide 0.5, 1.0 mg and sitagliptin groups, respectively. No neoplasms in the semaglutide groups were reported as malignant (Table S4).
No clinically relevant changes in other safety laboratory variables (including eGFR, liver variables, calcitonin, creatine kinase and haematology variables) and other biochemistry values during physical examinations or electrocardiograms were reported (data not shown).
4. DISCUSSION
In this trial, once‐weekly semaglutide 0.5 and 1.0 mg were found to be superior to sitagliptin in reducing HbA1c and body weight in patients with T2D inadequately controlled on metformin therapy.
The trial was undertaken to obtain approval for semaglutide in China, with ~70% of patients enrolled in China. Consistent with the results in the total population, significantly larger reductions in HbA1c and body weight were seen with semaglutide in the Chinese subpopulation. Consistent improvements in glycaemic control and body weight with semaglutide were also seen in the Korean subpopulation (~13% of patients) in this trial.
The magnitude of the reductions in HbA1c and body weight observed with semaglutide in this trial in both the Chinese and Korean subpopulations were consistent with those observed in the global SUSTAIN programme. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 The results were also consistent with a pooled analysis across the SUSTAIN 1–5 and 7 trials, 33 showing clinically relevant reductions in HbA1c with semaglutide that were largely consistent across all race groups (Caucasian, Asian, black/African, American).
Absolute weight loss was smaller in the Asian subpopulation treated with semaglutide compared with other race groups in a pooled analysis across the SUSTAIN 1–5 and 7 trials. 34 Similarly, the absolute weight loss obtained by patients in this trial was smaller than in the global SUSTAIN 2 trial with a similar trial design 20 ; this could be explained by a lower baseline body weight for patients included in the present trial (the relative [%] weight loss was of a similar magnitude to that of the global trials) as well as the different trial durations (56 weeks for SUSTAIN 2 vs. 30 weeks for the present trial). The post hoc analysis in this trial also showed some heterogeneity in treatment differences according to regional subgroups (the China region vs. other countries) for absolute weight loss. A similar pattern of smaller absolute weight loss was also seen in the two trials with Japanese patients. 28 , 29
A significantly higher proportion of patients reached the target of HbA1c of less than 7.0% with both doses of semaglutide (71% and 82%) compared with sitagliptin (45%), consistent with the results reported in the global SUSTAIN programme. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 These substantial HbA1c target results are of major importance in reducing microvascular complications in T2D. 3 , 4 , 5 , 6 A similar proportion of patients reaching HbA1c of less than 7.0% with semaglutide was also observed in the Chinese population.
Large proportions, 35% and 55% of the patients in the semaglutide 0.5 and 1.0 mg groups, respectively, achieved a clinically meaningful weight loss of 5% or more, an important factor in T2D treatment associated with a multitude of beneficial physiological effects including improved glycaemic control. A similar proportion of patients reaching a weight loss of 5% or more with semaglutide was also observed in the Chinese population.
Semaglutide also resulted in marked improvements in cardiometabolic risk markers compared with sitagliptin including significantly greater reductions in waist circumference and BMI, systolic blood pressure, high‐sensitivity C‐reactive protein and improvements in most lipid variables. In line with the observed improvements in cardiometabolic risk markers, semaglutide has been associated with a significant 26% reduction in cardiovascular risk compared with placebo in a preapproval CVOT. 30
For patient‐reported outcomes, semaglutide 0.5 and 1.0 mg were associated with improved treatment satisfaction (DTSQs). For SF36v2, all three treatment groups showed improvements in most of the domains; however, there were no statistically significant differences in the individual domains between either of the semaglutide doses versus sitagliptin except for a statistically significant improvement in the overall physical component summary score (SF36v2) with semaglutide 0.5 mg, but not with semaglutide 1.0 mg compared with sitagliptin.
Semaglutide was well tolerated in this 30‐week trial, with a safety profile consistent with the known class effects of GLP‐1 RAs. The results of this trial showed low rates of serious AEs for both semaglutide and sitagliptin. The reported higher incidence of GIAEs with semaglutide was expected, with similar rates as those seen across the global SUSTAIN programme. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 The discontinuation rate was, as expected, higher with both doses of semaglutide than with sitagliptin, also as reported in the global SUSTAIN 2 trial with a similar trial design. 35 The primary reason for this was GIAEs, which mainly occurred in the first 12 weeks during dose escalation. The occurrence of BG‐confirmed hypoglycaemia was low and similar among treatment groups, in line with the glucose‐dependent mechanism of action of both GLP‐1 RAs and DPP‐4 inhibitors. 36 These results, combined with the consistently low rates of hypoglycaemia reported across the global SUSTAIN trials, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 can help mitigate fear of hypoglycaemia as a barrier to achieving glycaemic control.
In this trial, the baseline incidence of diabetic retinopathy at screening was higher than in previous SUSTAIN trials. Events of diabetic retinopathy were more frequent with both doses of semaglutide compared with sitagliptin, but with no dose dependency observed for semaglutide. Most of the events were discovered in relation to the routine end‐of‐trial eye examination, and were mild, non‐proliferative and did not require treatment. The use of semaglutide has previously been associated with a higher rate of retinopathy‐related complications compared with placebo, which is consistent with the phenomenon of early worsening of pre‐existing diabetic retinopathy secondary to an initial, rapid improvement in glycaemic control. 30 , 37 The possible effect of semaglutide on diabetic eye disease is being investigated further in the ongoing FOCUS trial (NCT03811561).
In line with previous reports, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 there was a greater increase in amylase and lipase with both semaglutide doses compared with sitagliptin in this trial, but only one event of pancreatitis was reported. Previous findings have indicated that increased lipase and amylase levels during treatment with GLP‐1 RAs are not indicative of pancreatitis. 38 Consistent safety findings were also seen in the Chinese population.
The commercially available GLP‐1 RAs in China currently include once‐daily (exenatide, liraglutide, lixisenatide, benaglutide) and once‐weekly (exenatide extended release, dulaglutide and loxenatide) formulations. Once‐weekly GLP‐1 RAs may improve patient adherence and health‐related quality‐of‐life compared with daily formulations. 39 Furthermore, selected GLP‐1 RAs (including semaglutide) offer additional cardiovascular‐protective benefits and reduce the risk of major adverse cardiovascular events 40 , 41 , 42 in people with uncontrolled diabetes and cardiovascular disease.
This trial has several strengths, including the head‐to‐head comparison of semaglutide with a well‐established glucose‐lowering medication in a trial with a randomized double‐blind, double dummy‐controlled design. A high proportion of patients completed the trial. Our study also has limitations: although the trial duration was sufficiently long to assess the primary outcome, the long‐term effects and persistence require longer studies to assess fully. As with any randomized controlled trial with multiple eligibility criteria and frequent intensive follow‐ups between healthcare professionals and patients, the population of this trial is possibly more homogeneous and adherent to treatment as opposed to a real‐world, heterogenous population with T2D.
In summary, this trial confirmed a favourable risk‐benefit profile of semaglutide 0.5 and 1.0 mg in the overall population as well as in Chinese patients with T2D in line with the results from other SUSTAIN trials. This trial brings important ethnic comparative data of semaglutide indicating that once‐weekly semaglutide may be a suitable treatment option for Chinese patients with T2D.
CONFLICT OF INTEREST
LJ reports receiving personal fees from Novo Nordisk during the conduct of this study. ZN is an employee of Novo Nordisk. SR is an employee of and stockholder at Novo Nordisk. TVS is an employee of and shareholder at Novo Nordisk. XD, YiL, YuL, SL, ML and GY have nothing to disclose. FGE reports receiving grants from Novo Nordisk outside the submitted work.
AUTHOR CONTRIBUTIONS
All authors contributed to the conduct of the study and the writing and critical revision of the manuscript at all stages of development. All authors approved the final version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14232.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGEMENTS
We thank all the patients, investigators and trial‐site staff members who were involved in the conduct of the trial; Umut Erhan and Azadeh Houshmand‐Øregaard, Novo Nordisk, for review and input to the manuscript; and Lene Lerche, Novo Nordisk, for medical writing assistance. The trial was funded by Novo Nordisk A/S, Denmark.
Ji L, Dong X, Li Y, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as add‐on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30‐week, double‐blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23:404–414. 10.1111/dom.14232
Funding information The trial was funded by Novo Nordisk A/S, Denmark.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 9th ed. Brussels, Belgium; 2019. [Google Scholar]
- 2. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner RC, Holman RR, Stratton IM, et al. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854‐865. [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854‐865. [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 7. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. [DOI] [PubMed] [Google Scholar]
- 8. Davies M, D'Alessio D, Fradkin J, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(10):925.e11‐22. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . 6. Glycemic targets. Diabetes Care. 2020;43(Suppl 1):S66‐S76. [DOI] [PubMed] [Google Scholar]
- 11. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2018 executive summary. Endocr Pract. 2018;24(1):91‐120. [DOI] [PubMed] [Google Scholar]
- 12. Hou X, Lu J, Weng J, et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS One. 2013;8(3):e57319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Look AHEAD Research Group , Wing RR. Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the look ahead trial. Arch Intern Med. 2010;170(17):1566‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2018;41:S1‐S159. [DOI] [PubMed] [Google Scholar]
- 15. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in east Asians. Curr Diab Rep. 2015;15(6):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yabe D, Kuwata H, Seino Y. The journey to understanding incretin systems: theory, practice and more theory. J Diabetes Investig. 2019;10(5):1171‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6(5):495‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58(18):7370‐7380. [DOI] [PubMed] [Google Scholar]
- 19. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 20. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 21. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 22. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 23. Rodbard H, Lingvay I, Reed J, et al. Efficacy and safety of semaglutide once‐weekly vs placebo as add‐on to basal insulin alone or in combination with metformin in subjects with type 2 diabetes (SUSTAIN 5) [abstract]. Diabetologia. 2016;59(Suppl 1):364‐365. [Google Scholar]
- 24. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 25. Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once‐weekly semaglutide versus daily canagliflozin as add‐on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double‐blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834‐844. [DOI] [PubMed] [Google Scholar]
- 26. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add‐on to SGLT‐2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356‐367. [DOI] [PubMed] [Google Scholar]
- 27. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1‐3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–109. [DOI] [PubMed] [Google Scholar]
- 28. Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab 2018;20(2):378‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab. 2018;20(5):1202‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 31. World Medical Association . WMA Declaration of Helsinki ‐ Ethical Principles for Medical Research Involving Human Subjects. Last amended by the 64th WMA General Assembly, Fortaleza, Brazil: World Medical Association; 2013.
- 32. International Conference on Harmonisation . ICH Harmonised Tripartite Guideline for Good Cinical Practice. Geneva, Switzerland: International Council for Harmonisation; 1996. [Google Scholar]
- 33.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo‐Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeSouza C, Cariou B, Garg S, Lausvig N, Navarria A, Fonseca V. Efficacy and safety of semaglutide for type 2 diabetes by race and ethnicity: a post hoc analysis of the sustain trials. J Clin Endocrinol Metab. 2020;105(2):dgz072. [DOI] [PubMed] [Google Scholar]
- 35. Ahrén B, Comas LM, Kumar H, et al. Efficacy and Safety of Once‐weekly Semaglutide vs Sitagliptin as add‐on to Metformin and/or Thiazolidinediones After 56 Weeks in Subjects With Type 2 Diabetes (SUSTAIN 2). European Association for the Study of Diabetes, 52nd meeting, ePoster #7672016.
- 36. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metab. 2016;18(3):203‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinberg WM, Nauck MA, Zinman B, et al. LEADER 3‐lipase and amylase activity in subjects with type 2 diabetes baseline data from over 9000 subjects in the leader trial. Pancreas. 2014;43(8):1223‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once‐weekly medications for diabetes. Diabetes Obesity Metabol. 2011;13(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 40. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 42. Novo Nordisk A/S . Ozempic® (semaglutide), US prescribing information (PI). Plainsboro, NJ, USA: Novo Nordisk Inc.; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


