Abstract
Continuous subcutaneous insulin infusion (CSII) treatment may improve long-term glycemic outcomes and enhance quality of life compared with a multiple daily injection (MDI) insulin regimen for people with type 1 diabetes. As the number of people treated with CSII via a tubeless insulin pump is increasing, there is growing interest in the long-term glycemic outcomes of this treatment option across diverse populations. This multicenter, retrospective study evaluated glycemic control in 156 adults with type 1 diabetes initiating tubeless insulin pump therapy following transition from either MDI or CSII with a tubed insulin pump. In this study, use of the tubeless insulin pump over 12 months was associated with significant improvement in A1C in adults with type 1 diabetes, most notably in those with an A1C ≥9.0% and those previously treated with MDI.
Multiple studies have demonstrated clinical and quality of life benefits of continuous subcutaneous insulin infusion (CSII) compared with a multiple daily injection (MDI) insulin regimen for the treatment of type 1 diabetes (1–14). Although clinical evidence shows that MDI users may benefit from switching to CSII therapy, the overall adoption of CSII in the United States has been low historically, with an estimated 20–40% of people using this technology (15,16). More recent data from the U.S. T1D Exchange Clinic Registry indicate that the prevalence of CSII use is increasing at the specialty endocrinology centers participating in this registry, with an estimated 63% of patients using CSII in 2018, compared with 50% in 2012 (17,18). However, a known limitation of this registry is that it is not population-based and likely overestimates frequency of device use in the overall U.S. population. Even with greater rates of diabetes technology adoption, only 21% of adults participating in the T1D Exchange Clinic Registry attained an A1C <7.0% as recommended by the American Diabetes Association (ADA) (19). Therefore, there is value in gaining a better understanding of the effects of various type 1 diabetes therapies on glycemic control in real-world clinical settings.
The Omnipod® Insulin Management System (Insulet Corp., Acton, MA) is a tubeless insulin pump that consists of the Pod, a small, adhesive, waterproof (IP28) wearable insulin patch pump with automated cannula insertion, and the personal diabetes manager (PDM), a handheld device used to wirelessly control and monitor the Pod (20). CSII treatment with a differentiated system such as the tubeless insulin pump offers unique features that may benefit some users in managing their treatment (21). For example, the Pod is waterproof, and there is no tubing to become snagged or pulled out. As a result, it can be worn during swimming, bathing, and sports activities, without the need to disconnect and disrupt insulin delivery, as there may be with a tubed pump (22). With the automated cannula insertion, a consistent insertion distance and angle is achieved. The user never has to see or handle the needle, and there are fewer components than with tubed pumps (20,21). Lastly, the tubeless form factor of the Pod and the wireless PDM may be more discreet and easier to hide for wearing and managing (21,23). These features may help patients overcome barriers that may be associated with insulin pump therapy and promote treatment adherence (10,23). Initial evidence has indicated that tubeless insulin pump use has resulted in improved quality of life and patient satisfaction (8,24–26). There is also evidence that tubeless insulin pump therapy may be associated with improved glycemic outcomes (1,8,10,26–28).
As the number of people using tubeless insulin pump therapy increases, there is a growing need to understand the long-term clinical outcomes of this therapy in various populations. The objective of this study was to evaluate the effect of 12 months of treatment with the tubeless insulin pump on glycemic control in adults with type 1 diabetes following transition from their prior form of insulin therapy (MDI or CSII with a tubed insulin pump).
Research Design and Methods
Study Design
This was a multicenter, retrospective electronic health record–based clinical investigation.
Patients at participating sites in the United States who were ≥18 years of age, diagnosed with type 1 diabetes for ≥1 year as of 1 November 2013, and receiving treatment with either MDI or CSII with a tubed insulin pump before transitioning to the Omnipod system from November 2013 through July 2016 were eligible for inclusion.
Eligible patients were required to have an A1C measurement at Omnipod initiation (baseline) and at 1-year follow-up. A1C values were available in the electronic health records at the respective sites. All sites used an NGSP-standardized A1C methodology. Given variable outpatient follow-up in the real-world clinical setting, baseline A1C was defined as the A1C value closest to the initiation date, ranging from 3 months before to 1 month after initiation; follow-up A1C was defined as the A1C value closest to 12 months after initiation, ranging from 9 to 15 months post-initiation. Patients who became pregnant during the follow-up period were excluded. Institutional review board approval of the protocol was obtained from participating sites. Given the retrospective, de-identified data collection, the study was exempted from informed consent requirements.
Outcome Measures
The primary end point was change in A1C from the time of transition (baseline) to 12 months after initiation of the tubeless insulin pump. The change in A1C at 3 and 6 months post-tubeless insulin pump initiation and the change in A1C stratified by baseline A1C and by prior treatment modality (MDI or CSII with tubed insulin pump) were also analyzed. Secondary outcomes included change in body weight from baseline to 12 months post-tubeless insulin pump initiation.
Statistical Analysis
Outcome measures were summarized as mean and SD across patients at the multiple time points of interest (baseline and 3, 6, and 12 months post-initiation of the tubeless pump). A dependent t test for repeated measures was used to compare mean A1C values from baseline to follow-up post-initiation for the overall sample, as well as for within-group comparisons by prior treatment with either MDI or CSII with a tubed pump and by baseline A1C category (<9 or ≥9%).
A t test for independent means was used to compare change in mean A1C values between prior MDI and CSII treatment groups. The percentages of patients at various levels of glycemic control at baseline and follow-up were assessed by stratifying A1C values into categories of <7% (ADA treatment target) (19), 7 to <8%, 8 to <9%, and ≥9%. Additionally, glycemic control was assessed according to the Healthcare Effectiveness Data and Information Set (HEDIS) performance measurement criteria of A1C <8% (“adequate control”), 8–9% (“moderate control”), and >9% (“poor control”). McNemar’s test was used for comparison of HEDIS “poorly controlled” (A1C >9%) versus “not poorly controlled” (includes adequate and moderate control, A1C ≤9%) classification at baseline and 12 months post-initiation of the tubeless insulin pump. The proportion of patients shifting between A1C categories at 12 months was evaluated by Wilcoxon signed-rank test.
Change in body weight was evaluated by a dependent t test for repeated measures.
Statistical significance was defined as P <0.05 for all comparisons. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Microsoft Excel (Microsoft Corp., Redmond, WA).
Results
Patient Demographics and Clinical Characteristics
A total of 156 patients with type 1 diabetes who met the inclusion criteria were identified across the study sites. Baseline characteristics are summarized in Table 1. The majority of patients (63%) used MDI as their prior treatment modality. The group transitioning from MDI had a higher A1C at baseline than the group transitioning from CSII with a tubed insulin pump. Approximately half of eligible patients had A1C data available at the interim time points of 3 months (54%, n = 85) and 6 months (50%, n = 78).
TABLE 1.
Baseline Characteristics
| Parameter | Patients (N = 156) |
|---|---|
| Age at tubeless pump start, years | 43.7 ± 14.0 |
| Female | 86 (55) |
| Race/ethnicity | |
| Caucasian/non-Hispanic | 114 (73) |
| Unknown | 28 (18) |
| Black or African American | 13 (8) |
| Asian | 1 (1) |
| Prior treatment modality | |
| MDI | 99 (63) |
| CSII | 57 (37) |
| A1C, % | |
| Overall | 8.1 ± 1.5 |
| Prior MDI treatment (n = 99) | 8.2 ± 1.6 |
| Prior CSII treatment (n = 57) | 8.0 ± 1.4 |
| BMI, kg/m2* | 26.9 ± 4.6 |
Results are mean ± SD or n (%).
Missing data for n = 1.
Glycemic Outcomes
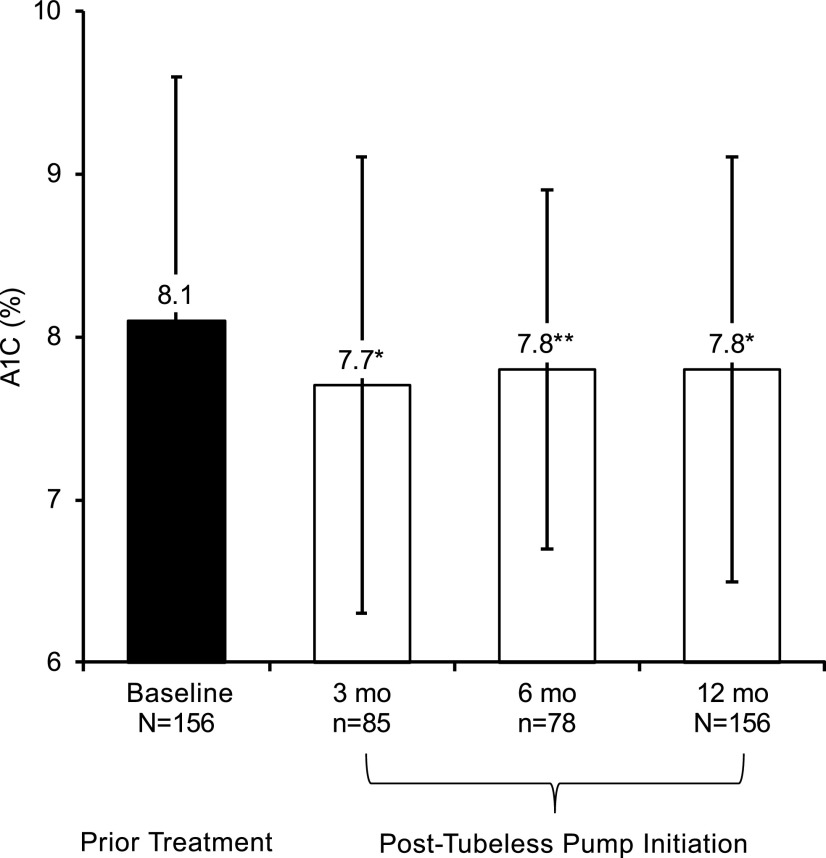
Results from the full cohort of 156 patients (including both prior therapy modalities together) demonstrated a clinically significant reduction in A1C 12 months after initiating tubeless insulin pump therapy (Figure 1), with a change of −0.3% (95% CI −0.5 to −0.1%, P = 0.007), from 8.1 ± 1.5 to 7.8 ± 1.3%. Improvement in A1C was also observed for the subsamples with data available at the 3- and 6-month interim time points: a change of −0.4% (n = 85, 95% CI −0.7 to −0.1%, P = 0.008), from 8.1 ± 1.6 to 7.7 ± 1.4%, and −0.3% (n = 78, 95% CI −0.6 to −0.05%, P = 0.021), from 8.1 ± 1.4 to 7.8 ± 1.1%, respectively.
FIGURE 1.
A1C post-tubeless insulin pump initiation compared with prior treatment in the full cohort of 156 adults with type 1 diabetes. Mean A1C is plotted at baseline with prior treatment, as well as for the 3-, 6-, and 12-month follow-up time points after tubeless insulin pump initiation. A1C at baseline was 8.1 ± 1.6% for the subsample with data at the 3-month follow-up time point (n = 85) and 8.1 ± 1.4% for the subsample with data at the 6-month follow-up time point (n = 78) (not shown). The change from baseline at each point of follow-up was found to be significant by dependent t test for repeated measures (*P <0.01, **P = 0.02). The error bars indicate SD.
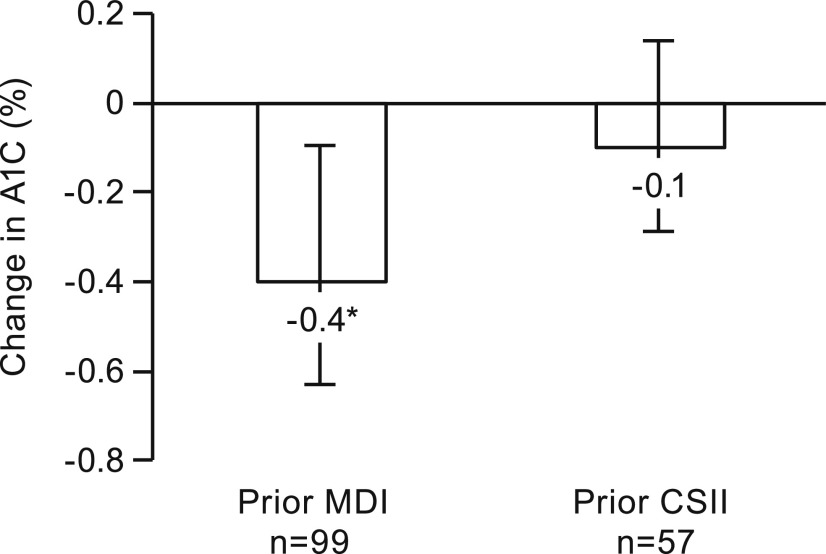
Patients transitioning to the tubeless insulin pump from MDI (n = 99) experienced a significant decrease in A1C of −0.4% (from 8.2 ± 1.6 to 7.8 ± 1.2%, P = 0.009) after 12 months (Figure 2). There was no significant change in A1C for prior CSII users (n = 57), who experienced a mean decrease in A1C of −0.1% (from 8.0 ± 1.4 to 7.9 ± 1.5%, P = 0.5). Although the mean change in A1C for prior MDI users was greater than the corresponding change for prior CSII users, the difference between the two groups did not reach statistical significance (P = 0.1).
FIGURE 2.
Change in A1C at 12 months post-tubeless insulin pump initiation stratified by prior treatment in adults with type 1 diabetes. The change in A1C was significant for those previously treated with MDI (n = 99, *P = 0.009) and was not significant for those previously treated with CSII with a tubed insulin pump (n = 57, P = 0.5). The error bars indicate 95% CIs.
Patients with baseline A1C ≥9% (n = 40, 65% prior MDI users) demonstrated a significant decrease in A1C 12 months after transitioning to the tubeless insulin pump: −1.2 ± 1.7% (P <0.001) (Table 2). Patients with baseline A1C <9% (n = 116, 63% prior MDI users) maintained a similar level of glycemic control between baseline and 12 months after transitioning to the tubeless insulin pump (from 7.4 ± 0.7 to 7.5 ± 0.9%, P = 0.4).
TABLE 2.
Change in A1C at 12 Months Post-Tubeless Insulin Pump Initiation Stratified by Baseline A1C
| Baseline A1C Range | Baseline A1C, % | A1C 12 Months Post-Tubeless Pump Initiation, % | Change in A1C, % |
|---|---|---|---|
| <9.0% (n = 116) | 7.4 ± 0.7 | 7.5 ± 0.9 | 0.1 ± 0.7 |
| ≥9.0% (n = 40) | 10.1 ± 1.3 | 8.9 ± 1.7 | −1.2 ± 1.7* |
Data are mean ± SD.
P <0.001 by dependent t test for repeated measures.
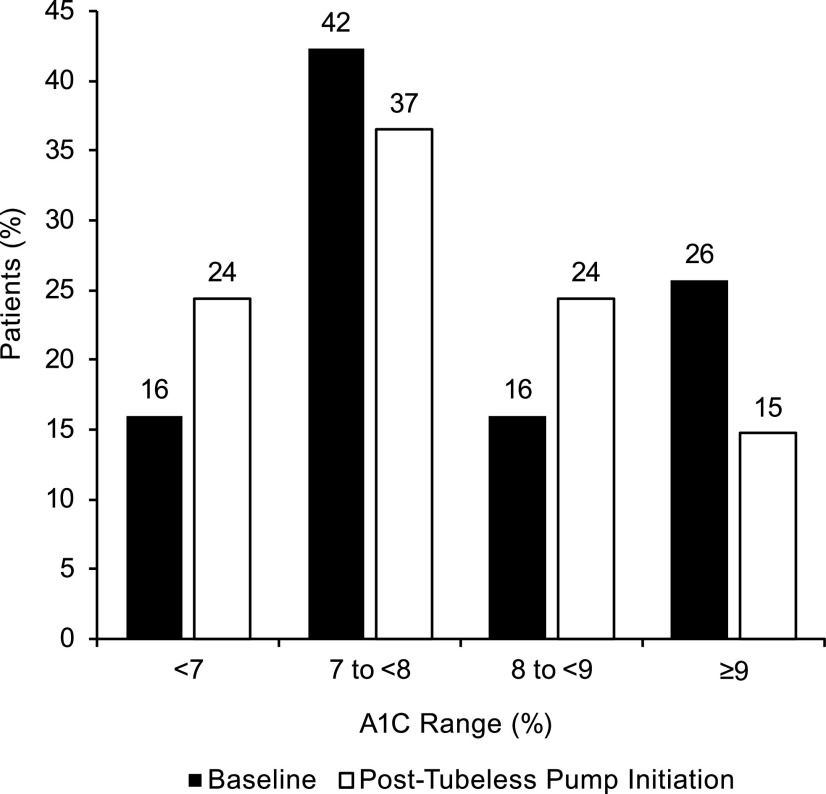
As shown in Figure 3, there was a significant improvement in glycemic control following tubeless pump initiation, as assessed by the shift from higher to lower A1C category (P = 0.006). The percentage of patients meeting the ADA treatment target of A1C <7% increased from 16% at baseline to 24% at 12 months post-tubeless pump initiation. This result was similar for the subgroups of prior MDI users (16% at baseline to 24% at follow-up, n = 99) and prior CSII users (16% at baseline to 25% at follow-up, n = 57). An improvement was also observed when using HEDIS performance criteria categories of <8, 8–9, and >9%, with a significant shift from higher to lower category (P = 0.04). The percentage of patients achieving “adequate control” (A1C <8%) increased from 58 to 61%. The percentage classified as “poorly controlled” (A1C >9%) significantly decreased from 24% at baseline to 15% at 12 months after tubeless insulin pump initiation (P = 0.004). This result was similar for the subgroups of prior MDI users (25% at baseline to 14% at follow-up) and prior CSII users (21% at baseline to 16% at follow-up).
FIGURE 3.
Proportion of patients in each A1C category at baseline on prior treatment and 12 months post-tubeless insulin pump initiation. There was a statistically significant shift from higher to lower category from baseline to post-tubeless insulin pump initiation (P = 0.006).
Body Weight Outcomes
There was no clinically significant increase in body weight 12 months following transition to the tubeless insulin pump (Table 3). Weight was stable for patients transitioning from MDI or CSII with a tubed insulin pump (P >0.05 for all comparisons).
TABLE 3.
Body Weight at 12 Months Post-Tubeless Insulin Pump Initiation Compared with Prior Treatment Overall and Stratified by Prior Treatment
| Baseline Regimen | Baseline Weight, kg | Weight 12 Months Post–Tubeless Pump, kg | P |
|---|---|---|---|
| Overall (N = 154) | 79.2 ± 16.4 | 79.8 ± 16.7 | 0.17 |
| Prior MDI (n = 97) | 77.8 ± 16.3 | 77.9 ± 16.2 | 0.75 |
| Prior CSII (n = 57) | 81.6 ± 16.6 | 82.9 ± 17.3 | 0.09 |
Data are mean ± SD. Missing data for n = 2 prior MDI users. Calculated by dependent t test for repeated measures.
Discussion
This multicenter, retrospective study demonstrated significant improvement in A1C in adults with type 1 diabetes 12 months after transitioning to the tubeless insulin pump. Improvement in A1C was also observed at the 3- and 6-month interim time points. The subgroup transitioning from MDI had a significant improvement in A1C, whereas the smaller subgroup transitioning from CSII with a tubed pump maintained comparable glycemic control between baseline and follow-up. The greatest decrease was noted in the subgroup with baseline A1C ≥9%, whereas A1C levels were stable between baseline and follow-up in the subgroup with baseline A1C <9%. The significant reductions in A1C observed in this study were achieved without an increase in body weight.
Real-world population data in routine clinical practice settings can provide important insight into factors affecting treatment choice and clinical outcomes in people with type 1 diabetes. Tubeless insulin pump therapy is a differentiated form of CSII that has been associated with improvements in glycemic control over the short term (3–7 months) across all ages (1,28) and over the longer term in youth (27), as well as with improvements in quality of life in adults (8,26). However, there are limited data on long-term (1 year or more) follow-up of glycemic outcomes associated with tubeless insulin pump use in adults (10). This report adds to the body of evidence on the long-term effects of tubeless pump insulin therapy on glycemic outcomes in the adult population with type 1 diabetes in a real-world clinical setting.
In this study, the mean A1C of the full cohort (including both prior treatment modalities together) was significantly lower after 3, 6, and 12 months following transition to tubeless pump therapy. After 12 months of use, a clinically significant 0.3% reduction in A1C was observed, resulting in a mean A1C of 7.8 ± 1.3%. The A1C observed at the 12-month follow-up in this study is consistent with an analysis from the T1D Exchange Clinic Registry, which reported that CSII users have a mean A1C of 8.6 ± 1.6% for those aged 18–25 years, 7.7 ± 1.2% for those aged 26–49 years, and 7.6 ± 1.1% for those aged ≥50 years (an estimated average A1C of 8.0% for adult CSII users overall based on the sample size of each age-group) (18).
At 12 months, 24% of tubeless pump users in our study achieved the ADA treatment goal of A1C <7.0%, a notable increase of 8% from the proportion at goal prior to tubeless pump initiation. As a comparator, the T1D Exchange Clinic Registry reported that 21% of their adult participants achieved this treatment goal (18). Our results are consistent with previously published data showing improved glycemic control after initiating tubeless insulin pump therapy (1,8,10,28). For example, a large survey study of adults with type 1 diabetes in the United States found that 64% of tubeless insulin pump users reported an improvement in A1C compared with prior therapy, with 43% reporting an improvement in A1C ≥0.5%, and only a small percentage (12%) reporting an increase in A1C (8).
Patients with A1C ≥9% demonstrated the greatest reduction in A1C following transition to tubeless pump therapy, while a relatively stable level of glycemic control was maintained in patients with A1C <9% at baseline. Those with A1C ≥9% at baseline had a mean A1C of 10.1%, which was significantly reduced by 1.2% to 8.9% after 12 months of tubeless pump therapy. Overall, the number of patients in the A1C ≥9% category was nearly halved, from 40 at baseline to 23 at follow-up. This finding reinforces the benefit of offering other options for insulin delivery to people with type 1 diabetes who are not meeting glycemic goals with their current therapy (29,30). Patients with higher A1C are at a greater risk for both short- and long-term health complications, and higher A1C is also correlated with greater health care spending (31–35). Therefore, it is important for both clinical and economic reasons to ensure that these patients have the tools they need to succeed in lowering their A1C. As evidenced in this study, one such tool for consideration is the tubeless insulin pump, which may be particularly beneficial for those who are struggling with a high A1C while using MDI therapy.
Multiple studies have demonstrated that CSII therapy is associated with improved glycemic outcomes compared with MDI therapy (5,10,14,17,36,37). Our findings were consistent with these existing reports, as the subgroup of patients transitioning to the tubeless insulin pump from MDI in our study experienced a significant reduction in A1C of −0.4% after 12 months of use. A previous U.K. study of patients transitioning to tubeless insulin pump therapy from MDI had a similar result, with a decrease in A1C from 8.4 to 8.0% after 1 year of use (10). Conversely, patients who were already treated with CSII with a tubed pump prior to initiating the tubeless insulin pump were not necessarily expected to have a significant improvement in A1C, as they were already receiving the benefits of CSII therapy at baseline (10). In keeping with this expectation, the mean A1C change for prior CSII users in our study was not statistically significant.
Although those using CSII with a tubed pump may not see a benefit in A1C when transitioning to a tubeless pump, it is possible that they could experience benefits in other areas, such as quality of life. Patient satisfaction and quality of life were not assessed in the current analysis; however, a previous study demonstrated that tubeless pump users perceived substantial benefits in quality of life from use of the device (8). Within that cohort, 43.7% had previously used CSII with a tubed pump, although the results were not stratified by prior therapy. Another study explored reasons for transitioning to tubeless pump therapy, which included wanting better control of diabetes and wanting to lower A1C, as well as not wanting to be connected to tubing for prior tubed insulin pump users (26). Future work could investigate patient preferences regarding the decision to elect transition from tubed pump therapy to tubeless pump therapy, for example, to improve their quality of life, and whether such expectations were met after the transition.
Weight was stable for patients transitioning from both MDI and CSII. These findings are in line with a recent observational study in two large cohorts of adults with type 1 diabetes from real-world multidisciplinary diabetes centers that found clinically significant reductions in A1C in patients with suboptimal glycemic control without a negative impact on weight 1 year after transitioning from MDI to CSII (29).
Strengths of the current study include the multisite, real-world setting, which allowed for the collection of data from patients using the tubeless insulin pump in a routine clinical setting across multiple diabetes centers. Additionally, an advantage of the current study was the follow-up period of 12 months, which allowed for assessment of the durability of changes in glycemic control following tubeless insulin pump initiation beyond the initial transition period.
Principal study limitations relate to selection bias resulting from the retrospective nature of the analysis, lack of a control group, exclusion of patients who did not have 12-month A1C data, and lack of data regarding patient satisfaction and quality of life. Data were not available for most participants on modality of glucose measurement (continuous glucose monitor [CGM] or blood glucose meter), so we were not able to account for CGM use as a factor that may affect glycemic outcomes. Although the number of prior MDI users was substantial, there was a smaller population of prior CSII users, which may have affected our ability to assess significant changes in this population. Furthermore, we were unable to assess the influence of prior therapy among those with a baseline A1C ≥9% because there were not sufficient prior CSII users with high baseline A1C within the study population (n = 14).
Additional work is needed to better understand the effects on glycemic control of initiating tubeless pump therapy in those previously treated with CSII with a tubed pump. Future studies may also evaluate additional outcomes such as total daily insulin use, frequency of acute complications, quality of life measures, and patient preference for the tubeless insulin pump versus prior therapy.
Conclusion
This multisite, retrospective study of adults with type 1 diabetes electing to transition to the tubeless insulin pump demonstrated improved glycemic control over a 12-month follow-up period compared with prior therapy. The subgroup transitioning from MDI had a significant improvement in A1C, whereas the smaller subgroup transitioning from CSII with a tubed pump maintained comparable glycemic control between baseline and follow-up. The greatest decrease was noted in the subgroup with baseline A1C ≥9%, whereas A1C remained stable between baseline and follow-up in the subgroup with baseline A1C <9%. Future evaluations using mixed methods–based approaches may provide additional insight into patient preference for the tubeless insulin pump in the current era of emerging advanced diabetes technologies.
Article Information
Acknowledgments
The authors thank the study participants and dedicated staff at the participating research centers, including Lisa Volkening, Nisha Naik, Zijing Guo, and Luisa Masclans of the Joslin Diabetes Center; Heather Remtema of the Henry Ford Health System; and staff at Atlanta Diabetes Associates. We appreciate the efforts of the research and development and clinical teams at Insulet Corp. We also thank Clarity Pharma Research for its role in conducting the study.
Funding
This research was sponsored by Insulet Corp. S.N.M. was supported by National Institutes of Health grant P30DK036836. B.B. was supported by research and grant support from his employer, Atlanta Diabetes Associates.
Duality of Interest
D.K. is a speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Dexcom, Insulet Corp., Lilly, Novo Nordisk, and Xeris; is a consultant for Abbott, Dexcom, Sanofi, and Xeris; and has done research with Abbott, Dexcom, Novo Nordisk, and Sanofi. B.B. is a stockholder of Aseko; a consultant for Adocia, Astra Zeneca, Janssen, Lilly, Mannkind, Medtronic, Novo Nordisk, and Sanofi; and a speaker for Astra Zeneca, Lilly/Boehringer Ingelheim, Janssen, Medtronic, Novo Nordisk, Sanofi, and Senseonics. J.E.L. and K.D. were employees of Insulet Corp. when this work was performed. L.M.H., B.D., and T.T.L. are full-time employees of Insulet Corp. L.M.L. is a consultant for AstraZeneca, Boehringer Ingelheim, Convatec, Dexcom, Eli Lilly, Insulet Corp., Insulogic, Janssen, Novo Nordisk, Roche, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
S.N.M., J.E.L., B.D., T.T.L., and L.M.L. contributed to the conception or design of the work. S.N.M., L.J.T., D.K., B.B., B.D., and L.M.L. contributed to the acquisition of data. S.N.M., B.B., J.E.L., L.M.H., K.D., T.T.L., and L.M.L. contributed to the analysis of data. S.N.M., L.J.T., D.K., B.B., J.E.L., L.M.H., K.D., T.T.L., and L.M.L. contributed to the interpretation of data. S.N.M., L.M.H., K.D., and J.E.L. drafted the manuscript, and all authors critically revised the manuscript. T.T.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Data from this manuscript were presented in part at the American Diabetes Association’s 79th Scientific Sessions in San Francisco, CA, 7–11 June 2019.
Footnotes
Author J.E.L. is currently affiliated with Onduo, Newton, MA.
Author K.D. is currently affiliated with Takeda, Lexington, MA.
References
- 1.Layne JE, Parkin CG, Zisser H. Efficacy of the Omnipod Insulin Management System on glycemic control in patients with type 1 diabetes previously treated with multiple daily injections or continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2016;10:1130–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 3.Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941–951 [DOI] [PubMed] [Google Scholar]
- 4.Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev 2006;22:232–237 [DOI] [PubMed] [Google Scholar]
- 5.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010:CD005103. [DOI] [PubMed] [Google Scholar]
- 6.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab 2009;94:729–740 [DOI] [PubMed] [Google Scholar]
- 7.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079–1087 [DOI] [PubMed] [Google Scholar]
- 8.Polonsky WH, Hessler D, Layne JE, Zisser H. Impact of the Omnipod Insulin Management System on quality of life: a survey of current users. Diabetes Technol Ther 2016;18:664–670 [DOI] [PubMed] [Google Scholar]
- 9.Barnard K, Skinner TC. Qualitative study into quality of life issues surrounding insulin pump use in type 1 diabetes. Pract Diabetes Int 2007;24:143–148 [Google Scholar]
- 10.Leelarathna L, Roberts SA, Hindle A, et al. Comparison of different insulin pump makes under routine care conditions in adults with type 1 diabetes. Diabet Med 2017;34:1372–1379 [DOI] [PubMed] [Google Scholar]
- 11.Beato-Víbora P, Yeoh E, Rogers H, Hopkins D, Amiel SA, Choudhary P. Sustained benefit of continuous subcutaneous insulin infusion on glycaemic control and hypoglycaemia in adults with type 1 diabetes. Diabet Med 2015;32:1453–1459 [DOI] [PubMed] [Google Scholar]
- 12.Joubert M, Morera J, Vicente A, Rod A, Parienti JJ, Reznik Y. Cross-sectional survey and retrospective analysis of a large cohort of adults with type 1 diabetes with long-term continuous subcutaneous insulin infusion treatment. J Diabetes Sci Technol 2014;8:1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orr CJ, Hopman W, Yen JL, Houlden RL. Long-term efficacy of insulin pump therapy on glycemic control in adults with type 1 diabetes mellitus. Diabetes Technol Ther 2015;17:49–54 [DOI] [PubMed] [Google Scholar]
- 14.Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine 2017;55:77–84 [DOI] [PubMed] [Google Scholar]
- 15.Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology Insulin Pump Management Task Force. Endocr Pract 2014;20:463–489 [DOI] [PubMed] [Google Scholar]
- 16.Pickup J. Insulin pumps. Int J Clin Pract Suppl 2011;64(Suppl.):16–19 [DOI] [PubMed] [Google Scholar]
- 17.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 18.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 [DOI] [PubMed] [Google Scholar]
- 20.Insulet Corp User Guide: Omnipod Insulin Management System. Acton, MA, Insulet Corp., 2017 [Google Scholar]
- 21.Zisser HC. The OmniPod Insulin Management System: the latest innovation in insulin pump therapy. Diabetes Ther 2010;1:10–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zisser H. Quantifying the impact of a short-interval interruption of insulin-pump infusion sets on glycemic excursions. Diabetes Care 2008;31:238–239 [DOI] [PubMed] [Google Scholar]
- 23.Reidy C, Bracher M, Foster C, Vassilev I, Rogers A. The process of incorporating insulin pumps into the everyday lives of people with type 1 diabetes: a critical interpretive synthesis. Health Expect 2018;21:714–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebenthal Y, Lazar L, Benzaquen H, Shalitin S, Phillip M. Patient perceptions of using the OmniPod system compared with conventional insulin pumps in young adults with type 1 diabetes. Diabetes Technol Ther 2012;14:411–417 [DOI] [PubMed] [Google Scholar]
- 25.Zisser H, Jovanovic L. OmniPod Insulin Management System: patient perceptions, preference, and glycemic control. Diabetes Care 2006;29:2175. [DOI] [PubMed] [Google Scholar]
- 26.Layne JE, Huyett LM, Ly TT. Glycemic control and factors impacting treatment choice in tubeless insulin pump users: a survey of the T1D Exchange Glu online community. J Diabetes Sci Technol 2019;13:1180–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danne T, Schwandt A, Biester T, et al.; DPV Initiative . Long-term study of tubeless insulin pump therapy compared to multiple daily injections in youth with type 1 diabetes: data from the German/Austrian DPV registry. Pediatr Diabetes 2018;19:979–984 [DOI] [PubMed] [Google Scholar]
- 28.Beck RW, Riddlesworth TD, Ruedy KJ, et al.; DIAMOND Study Group . Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:700–708 [DOI] [PubMed] [Google Scholar]
- 29.Mehta SN, Andersen HU, Abrahamson MJ, et al. Changes in HbA1c and weight following transition to continuous subcutaneous insulin infusion therapy in adults with type 1 diabetes. J Diabetes Sci Technol 2017;11:83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004;27:2590–2596 [DOI] [PubMed] [Google Scholar]
- 31.Mata-Cases M, Rodríguez-Sánchez B, Mauricio D, et al. The association between poor glycemic control and health care costs in people with diabetes: a population-based study. Diabetes Care 2020;43:751–758 [DOI] [PubMed] [Google Scholar]
- 32.Herman WH, Braffett BH, Kuo S, et al. What are the clinical, quality-of-life, and cost consequences of 30 years of excellent vs. poor glycemic control in type 1 diabetes? J Diabetes Complications 2018;32:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm 2010;16:264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmer TP, O’Connor PJ, Manning WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes Care 1997;20:1847–1853 [DOI] [PubMed] [Google Scholar]
- 35.Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 36.Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002;324:705. [DOI] [PMC free article] [PubMed] [Google Scholar]