Abstract
Overconsumption of added sugars is a key contributor to the growing obesity, prediabetes, and type 2 diabetes pandemics. The nutrition therapy guidance of the American Diabetes Association recognizes that using low- and no-calorie sweeteners (LNCS) to reduce consumption of added sugars can reduce low–nutrient-density sources of calories and carbohydrate to beneficially affect glycemia, weight, and cardiometabolic health. This article provides information for primary care providers, diabetes care and education specialists, and other diabetes clinicians on the safety of LNCS and summarizes research evidence on the role of LNCS in glycemic and weight management. It also provides practical strategies for counseling individuals about how to integrate LNCS into their healthy eating pattern.
The increasing number of adults and children/adolescents who are overweight and obese in the United States is a national health concern. Numerous studies have shown that overweight and obesity are significant risk factors for several interrelated health conditions, including prediabetes, type 2 diabetes, cardiovascular and cerebrovascular disease, hypertension, stroke, and other significant health conditions of increasing concern (1,2), such as nonalcoholic steatohepatitis and nonalcoholic fatty liver disease (3). Excessive weight is a concern in individuals with type 1 or type 2 diabetes and is a leading risk factor for prediabetes (4) because it decreases insulin sensitivity, which creates additional challenges in achieving and maintaining management of glycemia and other cardiometabolic health metrics (5).
Given the growing pandemics of type 1 and type 2 diabetes, prediabetes, and obesity and their associated costs (6), it is imperative that primary care providers (PCPs), diabetes care and education specialists, and other diabetes clinicians provide people who have or are at risk for developing diabetes with practical strategies for weight management and healthier eating. For many people, the most challenging part of their diabetes care plan is knowing what to eat and adhering to a healthy eating plan over time (7). Some individuals can achieve some success by reducing consumption of added sugars by choosing foods and beverages sweetened with low- and no-calorie sweeteners (LNCS) and using their preferred type and forms of table-top LNCS to sweeten foods and beverages. LNCS, the term used throughout this publication, are also referred to as low-calorie sweeteners, nonnutritive sweeteners, sugar substitutes, and high-intensity sweeteners (8). As sweetening ingredients, LNCS add no or negligible calories to foods and beverages.
This article reviews evidence supporting the safety and efficacy of LNCS in glycemic and weight management. It also provides practical strategies for clinicians to help people with diabetes and prediabetes effectively use LNCS to replace full-calorie sources of added sugars to assist with weight management and glycemic goals.
Scope of the Problem
The National Center for Health Statistics reports that the prevalence of obesity was 42.4% in 2017–2018 (9). The prevalence of obesity among children and adolescents is estimated to be 18.5% (10).
Overconsumption of various sources of added sugars is one contributor to the growing obesity pandemic. Several recent meta-analyses confirm the strong relationship between the consumption of added sugars, including sugar-sweetened beverages (SSBs), and the onset of obesity and development of type 2 diabetes (11–19).
In a study by Schulze et al. (16), which followed >50,000 women for 8 years, investigators found that women who consumed more than one SSB per day had an 83% greater risk of developing type 2 diabetes than those who consumed less than one SSB per month. It has been speculated that high amounts of added sugars (particularly high-fructose corn syrup) are rapidly absorbed, and the excessive glycemic load may increase the risk of cardiovascular disease and type 2 diabetes independent of obesity (18). As recently reported by O’Connor et al. (20), higher intakes of added sugars from nonalcoholic beverages and full-calorie sweeteners added to tea, coffee, and cereal are associated with deleterious glycemia and inflammatory markers.
According to the 2015–2020 U.S. Dietary Guidelines Advisory Committee report, added sugars contribute, on average, 270 kcal/day, or ∼13% of total daily calories. This amount is the equivalent of 17 teaspoons per day, which is two times the recommended intake (21). The estimated proportion of the U.S. population who met the guidance in the 2015–2020 Dietary Guidelines for Americans to consume <10% of energy from added sugars has increased from 30% in 2007–2010 to 37% in 2013–2016. Based on evidence explored by the 2020–2025 Dietary Guidelines Advisory Committee, the report recommends limiting added sugars to an even lower amount: ≤6% of total calories at most energy levels based on newer evidence about the negative health impacts of added sugars and to allow people to meet their nutrient needs from nutrient-dense foods (21). The report also states that added sugars could be reduced by consuming low- or no-sugar-added versions of foods and beverages that make positive nutrient contributions. This recommendation to further reduce added sugars aligns more closely with the added sugars recommendation of the World Health Organization (WHO), last issued in 2015 (22). At that time, the WHO strongly recommended that adults and children should reduce added sugars to <10%, with a conditional recommendation to further reduce added sugars to 5% of total calories.
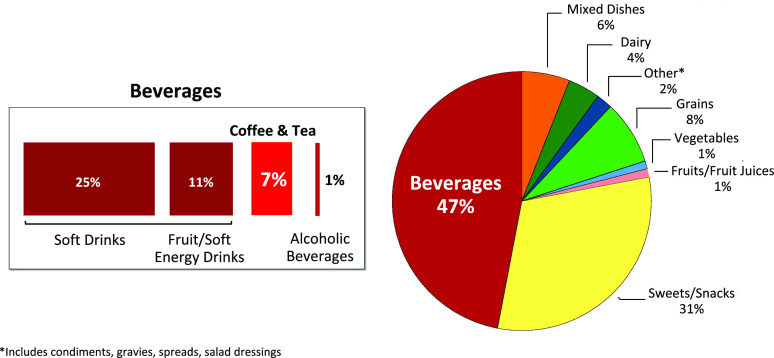
Although consumption of SSBs has slowly declined during the past several years (23), they remain the single largest source of added sugars (47%) in the U.S. diet. Approximately 7% of added sugars from beverages are attributed to a variety of table-top sugars such as granulated sugar and honey that are added to coffee and tea (21). Additional sources of added sugars are found in snacks and sweets (31%), grains (8%), and condiments, gravies, spreads, and salad dressings (2%) (Figure 1) (21).
FIGURE 1.
Sources of added sugars in the U.S. diet (21).
LNCS Available for Use in the U.S. Marketplace
Global regulatory authorities, including the U.S. Food and Drug Administration (FDA), Joint U.N. Food and Agriculture Organization/WHO Expert Committee on Food Additives, European Food Safety Authority, and Health Canada, have, over many years, determined the safety of LNCS using similar rigorous regulatory review protocols. The FDA regulates LNCS either through the Food Additive approval process or the Generally Recognized as Safe (GRAS) process (24–26). LNCS are deemed safe for their permitted uses and allowed for use by the general population, including people with diabetes, children, and pregnant and lactating women. One type of LNCS, aspartame, should be limited by individuals with a rare inherited metabolic disorder known as phenylketonuria because of its phenylalanine content.
As a result of increasing consumer demand for more natural products over the past decade, several plant-derived LNCS have entered the marketplace, including those derived from stevia and monk fruit. The dominant ingredients in the LNCS naturals category are derived from one or a combination of steviol glycosides. All are allowed on the U.S. marketplace as GRAS ingredients (27,28).
LNCS ingredients, either traditional or natural, are used in one of two ways: to replace added sugars in an array of commercially manufactured foods and beverages or as table-top sweetener substitutes for full-calorie sweeteners such as granulated sugar, honey, and brown sugar. Table 1 presents a list of added sugars used in foods and beverages.
TABLE 1.
Added Sugars Commonly Used as Ingredients in Foods and Beverages
|
|
|
When used as substitutes for added sugars, LNCS are most commonly used to sweeten coffee and other hot or cold beverages, hot or cold cereals, and yogurt and fruit; they are also used for cooking and baking. For these purposes, LNCS are available in various forms. Granulated LNCS are provided in individual packets for table-top use and in jars or pouches for use in beverages, baking, and cooking. “Squeeze and stir” liquid LNCS can be added to cold beverages and cereals without the need to dissolve. LNCS blends, with granulated sugar or granulated and brown sugar, can be used for an array of baking and cooking when the functional properties of sugar are needed but the benefit of fewer calories and carbohydrates per serving can be gained.
Evidence on Effectiveness of LNCS on Glycemic and Weight Management
Findings from Expert Consensus
Randomized controlled trials (RCTs) and meta-analyses/systematic reviews have demonstrated the impact of LNCS use on glucose metabolism (29–35) and weight management (36–39). A summary of findings from these studies is presented in Tables 2 and 3.
TABLE 2.
Findings from Key Studies of the Effects of LNCS on Glycemic Management (Glucose Metabolism)
| Glucose Metabolism Study | Study Design/Population | Findings |
|---|---|---|
| Jensen et al., 2020 (29) |
|
|
| Toora et al., 2018 (30) |
|
|
| Nichol et al., 2018 (31) |
|
|
| Grotz et al., 2017 (32) |
|
|
| Campos et al., 2015 (33) |
|
|
| Ma et al., 2009 (34) |
|
|
| Grotz et al., 2003 (35) |
|
|
TABLE 3.
Findings from Key Studies of the Effects of LNCS on Weight Management
| Weight Management Study | Study Design/Population | Findings |
|---|---|---|
| Laviada-Molina et al., 2020 (36) |
|
|
| Peters et al., 2016 (37) |
|
|
| Piernas et al., 2013 (38) |
|
|
| Tate et al., 2012 (39) |
|
|
In 2018, an international panel of health care professionals, nutrition researchers, and food toxicologists evaluated the substantial body of evidence relevant to the associations between use of LNCS and weight and glycemic management (8). The following summarizes the panel’s key findings, which are consistent with other recent international consensus statements (40,41):
LNCS reduce calorie intake, can enhance adherence to nutrition recommendations, and assist in weight and glycemic management when substituted for added sugars in an individual’s eating plan.
LNCS do not adversely affect blood glucose levels (A1C or fasting or postprandial glucose) or insulin regulation in individuals with or without diabetes.
There is a need to research and develop evidence-based strategies to communicate facts to consumers, health professionals, and policy makers.
Experts agree that, with the reduction of added sugars being recommended globally to lower the risk and prevalence of obesity, LNCS are a strategy to consider.
Efforts should be made to understand and, where possible, reconcile policy discrepancies between organizations and reduce regulatory hurdles that impede product development and reformulation designed to reduce sugars and calories.
Although water is considered by some to be an optimal beverage choice, a recent American Heart Association science advisory that was supported by the American Diabetes Association recognized that children with well-managed diabetes may be able to prevent excessive glucose excursions by substituting beverages with LNCS for SSBs when needed (42). The authors of this science advisory also determined that use of beverages with LNCS may also be an effective replacement strategy for adults who are habitual consumers of SSBs. Carbonated soft drinks with LNCS were found in one study to assist adults in controlling calorie intake, weight loss, and weight maintenance (43).
Limitations of Observational Studies on LNCS
A common thread throughout all current recommendations on LNCS is recognition of the limitations inherent to using meta-analyses of observational study designs to assess the efficacy of LNCS in weight management (8,40,41,44). Unlike RCTs, which directly assess the effects of an identifiable intervention (e.g., use of LNCS) versus a comparator (i.e., control) intervention within a well-defined study population, observational studies cannot determine causal relationships between intervention and outcome. For example, whereas an observational study may show an association between use of LNCS and weight gain, it is not possible to determine whether individuals gained weight because they were consuming LNCS or whether they were consuming these products because they were overweight or were managing type 2 diabetes. Other limitations of these studies include small sample sizes, short study durations, and lack of participants’ dietary history and information on other factors that can affect clinical outcomes. These limitations are compounded by reliance on meta-analyses/systematic reviews that are heavily weighted with observational studies (11,44–47). Many of these reports provide little or no information about the characteristics of included studies such as study designs, comparators assessed, effect sizes, and study quality (48).
Consideration of results from RCTs, reported individually or within well-designed meta-analyses, is the most appropriate approach for assessing the impact of LNCS relevant to glycemic control and weight management.
So, Why the Controversy About LNCS?
Despite the robust body of evidence supporting the benefits of use of LNCS in glycemic and weight management, these findings are often overshadowed by media headlines and stories based on unsubstantiated data or observational studies, which, as discussed earlier, are inherently flawed and inconclusive. For example, the recent study by Dalenberg et al. reported that “consumption of sucralose in the presence of a carbohydrate rapidly impairs glucose metabolism and results in longer-term decreases in brain but not perceptual, sensitivity to sweet taste, suggesting dysregulation of gut-brain control of glucose metabolism” (49). Adhering to established ethics for reporting medical research, the investigators appropriately listed the limitations of their study, which included:
Small sample size: included only 13 people in the experimental group
Short study duration: lasted only 2 weeks
Nutrition data self-reported and collected only at baseline, allowing for the possibility that other components of the diet and diet-related behavior could have affected the findings
Questionable clinical significance: no group differences observed in glucose response
However, rather than provide an objective review of the study findings, the Washington Post instead published a uniformed article titled, “A common artificial sweetener might be making you fatter and sicker, a new study says: Sucralose in conjunction with carbohydrates may blunt the body’s ability to metabolize sugar appropriately” (50). Although the article contained numerous references to observational studies “associating” use of LNCS to various adverse outcomes, it failed to report the limitations of the study that significantly diminished the credibility of its findings.
Sensationalized headlines have created unwarranted public alarm and confusion about the safety of LNCS over the course of many years. Conversely, a recent well-designed meta-analysis/systematic review by Laviada-Molina et al. (36), which demonstrated significant benefits of using LNCS in weight management, received no media coverage.
In short, meta-analyses and systematic reviews that include both RCTs and observational studies provide limited guidance. For example, a recent systematic review and meta-analysis by Azad et al. (50) reported no statistically or clinically relevant differences between subjects who consumed LNCS and those who regularly consumed sugar. Yet, the authors emphasized that many of the studies they included were of low quality and that the findings of the observational studies regarding the health effects of using LNCS should be interpreted with caution.
Importantly, the findings from Azad et al. (51) are in stark contrast to those reported in a meta-analysis by Rogers et al. (52), which included only RCTs in the analysis. In the study by Rogers et al., investigators concluded that the preponderance of evidence from all human RCTs indicates that LNCS do not increase energy intake or body weight and that the balance of evidence indicates that use of LNCS as a replacement for added sugars in children and adults leads to reduced body weight, and this reduction is also apparent when beverages containing LNCS are compared with water (52).
Practical Strategies to Reduce Consumption of Added Sugars
Motivations that Influence Use of LNCS
To our knowledge, no academic studies have been conducted to assess consumer motivations for using LNCS or preferences for specific LNCS. However, results from a 2020 survey of people who use LNCS (MarketLab, Philadelphia, PA; unpublished observations) provide some insights regarding consumer attitudes and behaviors. Conducted by an independent market research firm (MarketLab, unpublished observations), the survey included a national cohort of 919 respondents that was equally balanced in terms of sex, education, and income level.
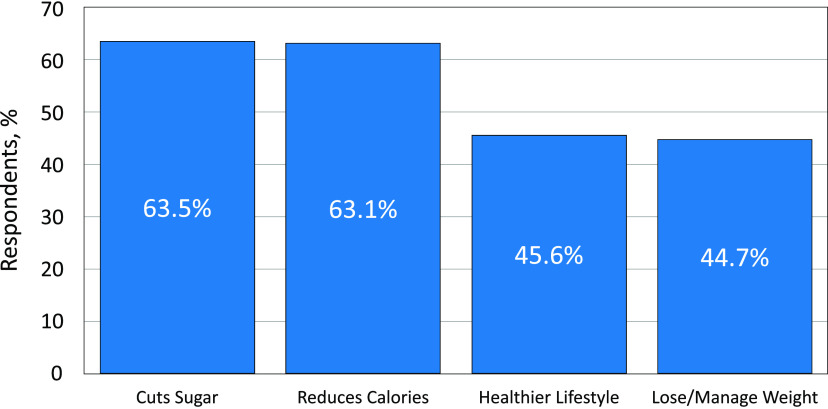
As shown in Figure 2, the most commonly reported reasons for using LNCS were to reduce the intake of added sugars and to reduce overall calorie consumption. We hypothesize that “reduces calories” is a motivation for weight loss and management. When counseling individuals, clinicians who deliver diabetes care should leverage the motivators for leading a healthier lifestyle and losing/managing weight to emphasize that reducing added sugars in both ready-to-eat and home-prepared foods and beverages can help people achieve the associated health benefits.
FIGURE 2.
Most common reasons for using LNCS reported in a 2020 survey of 919 users of these products (MarketLab, unpublished observations).
Importance of Taste in Successful Transition to Using LNCS
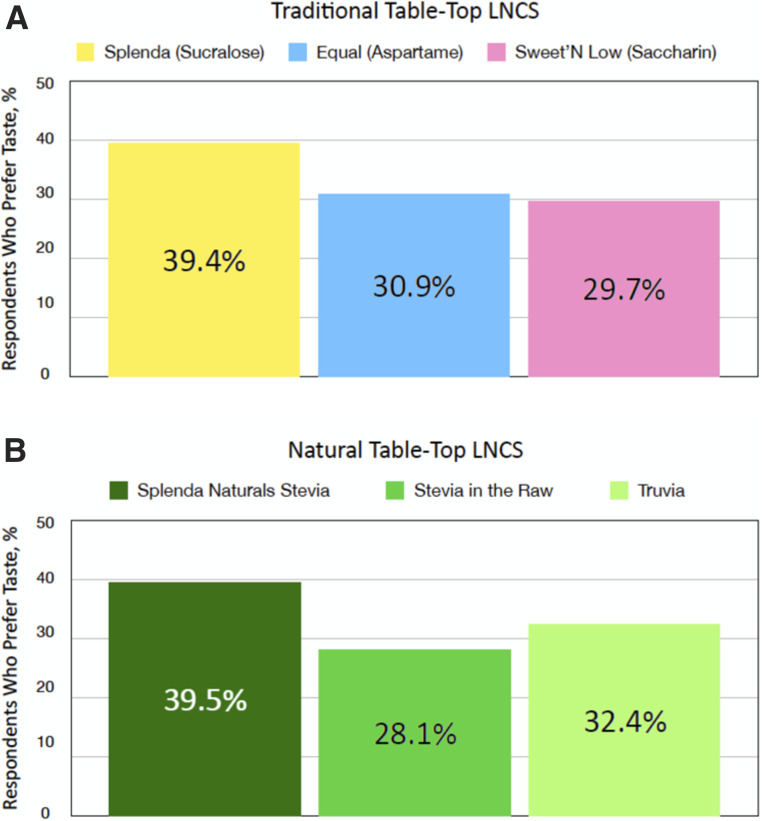
In the previously mentioned 2020 survey (MarketLab, unpublished observations), 514 users of LNCS were asked to identify the brand of LNCS they consistently use. As presented in Figure 3, survey respondents showed a stronger preference for Splenda (sucralose) compared with Equal and Sweet’N Low, the other traditional table-top sweeteners, as evidenced by a higher percentage of respondents who report consistent use of the brand. Splenda Naturals Stevia was consistently rated highest in the natural LNCS category.
FIGURE 3.
Consumer taste preferences for users of traditional (n = 514) (A) and natural (n = 512) (B) table-top sweeteners by brand name (MarketLab, unpublished observations).
These finding are important when counseling individuals because numerous studies have shown that the taste of food plays an important role in food choices, eating behaviors, and food intake (53–56) and that the more distant a recommended change is from the person’s actual eating habits, the more difficult it is to gain sustained adherence to the recommended change (57,58). In a national survey of 2,967 U.S. adults (54), respondents were asked to rate the factors they felt were most influential in their food choices. On a 5-point Likert scale (from 1 = least to 5 = most), the mean score for importance of taste was 4.7, followed by cost (4.1), nutrition (3.9), convenience (3.8), and weight control (3.4). The investigators concluded that their results suggest that nutritional concerns per se are of less importance to most people than taste and cost. Therefore, product recommendations should focus on promoting healthy eating habits that are aligned with the consumer goals of having foods that are “tasty and inexpensive” (54).
Starting the Conversation
Although patients may generally understand the importance of limiting their intake of added sugars, many may not realize the quantity of added sugars they consume on a daily basis. As previously noted, U.S. adults consume, on average, 17 teaspoons of added sugars per day, which is nearly two times the recommended maximum daily intake. Therefore, a starting point for discussion could be to raise patients’ awareness that their daily added sugars intake is likely much higher than they realize. The reason may be, in part, that they do not recognize that added sugars in foods and beverages are represented on food packaging nutrition labels by numerous ingredients and names, as listed in Table 1. It can be valuable to make this list a teaching tool to raise patients’ awareness about the many sources of added sugars. With this knowledge in hand, encourage patients to read the ingredient lists on the foods and beverages they consider purchasing and to consider not buying those that contain large amounts of added sugars.
The next step might be to discuss the current recommendations for daily intake of added sugars. The current guidance from the 2015–2020 Dietary Guidelines for Americans (21), to limit intake of added sugars from all foods and beverages to <10% of total daily calories, translates to ≤9 teaspoons for men and ≤6 teaspoons for women. Table 4 illustrates how substituting LNCS for full-calorie sweeteners can help patients achieve these recommendations.
TABLE 4.
Impact of Substituting LNCS for Added Sugars in Sweetened Beverages
| Sweetened Beverage, 12 oz | Sweetened With Sugar | Sweetened With LNCS | ||
|---|---|---|---|---|
| Calories (Teaspoons of Sugar) | Carbohydrates, g | Calories, (Packets of LNCS)* | Carbohydrates, g | |
| Iced tea | 128 (8) | 32 | 0 (4) | <1 |
| Coffee | 43 (3) | 12 | 0 (1) | <1 |
Per FDA guidance, all products with <5 calories per serving are listed as having 0 calories.
Successful weight loss and long-term weight management require making sustainable changes in eating habits and food choices. However, PCPs have limited time to spend offering nutrition guidance to their patients to reduce added sugars with the use of LNCS. To assist, Table 5 offers suggested open-ended questions that diabetes care clinicians can use to start a conversation with patients to assess their knowledge and, based on their readiness to change, help them set goals to reduce added sugars and consider the use of LNCS (both as table-top sweeteners and in products sweetened with LNCS). A crucial conceptual point to cover when encouraging patients to use LNCS to reduce added sugars is to avoid compensating for the reduction of calories with increased intake of calorie-containing foods and beverages.
TABLE 5.
Goals for Reducing Intake of Added Sugars
| Goal: Assess total consumption of added sugars and types of foods and beverages. |
| Questions to ask: |
| 1. List all of the beverages you drink (and the amounts) on a given day from the time you wake up until you go to sleep. (Follow-up: What do you add to hot and cold beverages such as coffee and tea?) |
| 2. How many times a day (or week) do you eat sweets? (Follow-up: What types of sweets and in what amounts?) |
| 3. Can you tell me what a few of the names are for added sugars on food and beverage ingredient labels? (Table 1 provides a list. Make this a handout and have a couple of representative products with nutrition facts and ingredient lists available to illustrate further.) |
| Goal: Assess knowledge and use of LNCS. |
| Questions to ask: |
| 1. What are your thoughts about using LNCS (sugar substitutes) instead of sugar or other calorie-containing sweeteners? (If the response does not accurately reflect the science, attempt to offer accurate information.) |
| 2. What are a few ways you could use LNCS to reduce the amount of sugars you eat and drink? (Use content in Table 4 to illustrate the calories and grams of carbohydrate saved when using LNCS rather than added sugars in beverages.) If the patient states that he or she does not use LNCS because they are not natural, you may note that there are now a variety of natural LNCS that may suit their product and taste preferences. |
| 3. Tell me where you would find LNCS (sugar substitutes) in the supermarket? |
| 4. What is the best way for you to find LNCS (sugar substitutes) that taste most like sugar? |
| Goal: Set a few small changes to reduce added sugars before the next appointment. |
| Question to ask: |
| 1. What are two or three small changes you are willing and able to make to reduce the amount of added sugars you eat and drink? |
Note: It is crucial to have patients write out or state their goals. PCPs should make a copy for or record their goals in their electronic health record. At the next appointment, ask about how successful they were with their goals. Having you spend a few minutes on this topic conveys an imperative to patients and sets expectations. Asking about their progress at the follow-up appointment increases this imperative.
When counseling patients, it is essential to provide guidance that is achievable and sustainable, empowering people with prediabetes and diabetes (type 1 or type 2) to adopt healthier food choices without compromising their taste preferences. It is also important to provide specific product recommendations when counseling individuals, particularly for people who have little or no previous experience using products made with LNCS (57,58). In their recent cross-sectional study of 91 people with type 2 diabetes, Jaworski et al. (59) reported that lack of knowledge about recommended products and their availability was the most common problem reported by study participants. For this reason, we suggest that diabetes care providers try the available table-top LNCS. The most commonly used table-top LNCS in the United States today are listed in Table 6.
TABLE 6.
Most Common Brand-Name Table-Top LNCS
| LNCS Ingredient | Brand Name |
|---|---|
| Aspartame | Equal NutraSweet |
| Saccharin | Sweet’N Low |
| Sucralose | Splenda |
| Steviol glycosides | Splenda Stevia Sweet Leaf Stevia in the Raw Truvia Whole Earth |
Another important strategy is to present simple options for substituting LNCS for added sugars in common foods. Following are ideas to encourage switching from using added sugars to using LNCS:
Use LNCS to sweeten hot or iced coffee or tea.
Instead of SSBs, use a diet beverage or drink still or sparkling water instead. To increase the palatability of water, flavor it with a splash of fruit juice or a few squeezes of lemon or lime and then add an LNCS to sweeten. (Consider that some patients may find that drinking carbonated beverages is satisfying and quenches their desire for a sweet taste.)
Use LNCS to sweeten fruit (e.g., grapefruit, strawberries, or other berries).
Put LNCS in the sugar bowl instead of sugar.
Use LNCS instead of sugar when making sweets, treats, and desserts.
Use LNCS in homemade salad dressings, marinades, and sauces.
The impact of taste cannot be overemphasized. Diabetes care providers should consider the taste preference survey results when recommending LNCS options (Figure 3).
Summary
Individuals who are overweight or obese are at significant risk for developing prediabetes, coronary heart disease, hypertension, stroke, nonalcoholic steatohepatitis, and other health conditions (1–3). These risks are elevated among overweight or obese individuals with type 1 or type 2 diabetes, who are further challenged to maintain their glucose control because of decreased insulin sensitivity (5). Overconsumption of added sugars is a driver of overweight and obesity (21). Given the growing pandemics of diabetes and prediabetes, accompanied by the increasing prevalence of overweight and obesity (9), there is a clear need for effective strategies that promote healthier eating habits and alternatives to overconsumption of added sugars.
Based on evidence from recent RCTs (30–33,35,39,58), experts on LNCS have published consensus statements (8,40,41,59) that recognize the potential of LNCS to reduce calorie intake and assist in weight loss and weight management when consciously substituted for added sugars (30–33,35,58). Importantly, these benefits can be realized without adversely affecting blood glucose levels (A1C or fasting or postprandial blood glucose) or insulin regulation in individuals with diabetes (36,37,39).
Diabetes clinicians can play a significant role in assisting patients to reduce their intake of added sugars. In this article, we have outlined practical strategies clinicians can implement to help their patients obtain evidence-based information about LNCS. When encouraging lifestyle behavior modification to change food choices and eating habits, it is crucial to meet patients where they are, with an understanding of their current food choices, eating habits, food security, home and work situations, and other factors. In addition, if weight loss is being encouraged to prevent or delay prediabetes or type 2 diabetes, it is important to identify and leverage each patient’s motivations for weight management. This strategy also provides an opportunity to dispel any myths and misinformation reported in the media and reinforces the message that LNCS are both safe and effective as a component of weight management efforts.
It is important to recognize the role of taste in choosing foods and to make specific product recommendations that consider taste as a key consumer factor. Therefore, clinicians should consider the preference data discussed earlier as a first option for patients. It is also important to present various options and forms of LNCS and to encourage experimentation with these alternatives.
Because most people require frequent, consistent nutrition counseling and support over time to make and adhere to behavioral lifestyle changes that assist with weight loss maintenance, it is suggested that clinicians refer people with or at high risk for prediabetes to a National Diabetes Prevention Program, Medicare Diabetes Prevention Program, or similar program (7,59). Additionally, diabetes self-management education and support and medical nutrition therapy should be provided at regular intervals through the course of patients’ disease (60). These services are covered by Medicare and many private payors (7,59).
Perhaps most important is to establish an honest, collaborative, and person-centered relationship with patients to facilitate shared decision-making in setting practical, individualized, and achievable goals that address their preferences, circumstances, and capabilities.
Article Information
Funding
Funding for the development of this manuscript was provided by Heartland Food Products Group.
Duality of Interest
H.W. has received consulting and speaker fees from Heartland Food Products Group, Tate & Lyle, and several trade associations. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
H.W. and S.V.E. wrote, reviewed, and approved the manuscript for submission. H.W. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the content.
References
- 1.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 2.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA 1999;282:1530–1538 [DOI] [PubMed] [Google Scholar]
- 3.Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes 2018;11:533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naser KA, Gruber A, Thomson GA. The emerging pandemic of obesity and diabetes: are we doing enough to prevent a disaster? Int J Clin Pract 2006;60:1093–1097 [DOI] [PubMed] [Google Scholar]
- 5.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2020: Estimates of Diabetes and Its Burden in the United States. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 29 February 2020
- 7.American Diabetes Association 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 8.Ashwell M, Gibson S, Bellisle F, et al. Expert consensus on low-calorie sweeteners: facts, research gaps and suggested actions. Nutr Res Rev 2020;33:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, National Center for Health Statistics Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Available from https://www.cdc.gov/nchs/products/databriefs/db360.htm. Accessed 3 March 2020
- 10.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016: U.S. National Center for Health Statistics Data Brief, No. 28. Available from https://www.cdc.gov/nchs/data/databriefs/db288.pdf. Accessed 23 June 2020
- 11.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 2007;97:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev 2009;10:68–75 [DOI] [PubMed] [Google Scholar]
- 14.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13–27 [DOI] [PubMed] [Google Scholar]
- 15.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013;14:606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–934 [DOI] [PubMed] [Google Scholar]
- 17.Greenwood DC, Threapleton DE, Evans CEL, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr 2014;112:725–734 [DOI] [PubMed] [Google Scholar]
- 18.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S, McKee M, Galea G, Stuckler D. Relationship of soft drink consumption to global overweight, obesity, and diabetes: a cross-national analysis of 75 countries. Am J Public Health 2013;103:2071–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor L, Imamura F, Brage S, Griffin SJ, Wareham NJ, Forouhi NG. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin Nutr 2018;37:1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietary Guidelines Advisory Committee Scientific report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Part D. Chapter 6: Cross-cutting topics of public health importance. Available from https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines. Accessed 23 June 2020
- 22.World Health Organization Guideline: sugars intake for adults and children. Available from https://www.who.int/publications/i/item/9789241549028. Accessed 23 June 2020 [PubMed]
- 23.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rulis AM, Levitt JA. FDA’s food ingredient approval process: safety assurance based on scientific assessment. Regul Toxicol Pharmacol 2009;53:20–31 [DOI] [PubMed] [Google Scholar]
- 25.Roberts A. The safety and regulatory process for low calorie sweeteners in the United States. Physiol Behav 2016;164:439–444 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available from https://www.who.int/foodsafety/areas_work/chemical-risks/jecfa/en. Accessed 4 April 2020
- 27.Perrier JD, Mihalov JJ, Carlson SJ. FDA regulatory approach to steviol glycosides. Food Chem Toxicol 2018;122:132–142 [DOI] [PubMed] [Google Scholar]
- 28.Samuel P, Ayoob KT, Magnuson BA, et al. Stevia leaf to stevia sweetener: exploring its science, benefits, and future potential. J Nutr 2018;148:1186S–1205S [DOI] [PubMed] [Google Scholar]
- 29.Jensen PN, Howard BV, Best LG, et al. Associations of diet soda and non-caloric artificial sweetener use with markers of glucose and insulin homeostasis and incident diabetes: the Strong Heart Family Study. Eur J Clin Nutr 2020;74:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toora BD, Seema S, Manju M, Mishra S. Effect of artificial sweeteners on the blood glucose concentration. Journal of Medical Academics 2018;1:81–85 [Google Scholar]
- 31.Nichol AD, Holle MJ, An R. Glycemic impact of non-nutritive sweeteners: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 2018;72:796–804 [DOI] [PubMed] [Google Scholar]
- 32.Grotz VL, Pi-Sunyer X, Porte D Jr, Roberts A, Richard Trout J. A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul Toxicol Pharmacol 2017;88:22–33 [DOI] [PubMed] [Google Scholar]
- 33.Campos V, Despland C, Brandejsky V, et al. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obes (Silver Spring) 2015;23:2335–2339 [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009;296:G735–G739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grotz VL, Henry RR, McGill JB, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 2003;103:1607–1612 [DOI] [PubMed] [Google Scholar]
- 36.Laviada-Molina H, Molina-Segui F, Pérez-Gaxiola G, et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev 2020;21:e13020. [DOI] [PubMed] [Google Scholar]
- 37.Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity (Silver Spring) 2016;24:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piernas C, Tate DF, Wang X, Popkin BM. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2013;97:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tate DF, Turner-McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serra-Majem L, Raposo A, Aranceta-Bartrina J, et al. Ibero-American consensus on low- and no-calorie sweeteners: safety, nutritional aspects and benefits in food and beverages. Nutrients 2018;10:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson S, Drewnowski A, Hill J, et al. Consensus statement on benefits of low-calorie sweeteners. Nutr Bull 2014;39:386–389 [Google Scholar]
- 42.Johnson RK, Lichtenstein AH, Anderson CAM, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126–e140 [DOI] [PubMed] [Google Scholar]
- 43.Catenacci VA, Pan Z, Thomas JG, et al. Low/no calorie sweetened beverage consumption in the National Weight Control Registry. Obesity (Silver Spring) 2014;22:2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sievenpiper JL, Khan TA, Ha V, Viguiliouk E, Auyeung R. The importance of study design in the assessment of nonnutritive sweeteners and cardiometabolic health. CMAJ 2017;189:E1424–E1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sports Med 2016;50:496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PLoS One 2016;11:e0161264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowler SP, Williams K, Hazuda HP. Diet soda intake is associated with long-term increases in waist circumference in a biethnic cohort of older adults: the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 2015;63:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosdøl A, Vist GE, Svendsen C, et al. Hypotheses and evidence related to intense sweeteners and effects on appetite and body weight changes: a scoping review of reviews. PLoS One 2018;13:e0199558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalenberg JR, Patel BP, Denis R, et al. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab 2020;31:493–502.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Washington Post A common artificial sweetener might be making you fatter and sicker, a new study says: sucralose in conjunction with carbohydrates may blunt the body’s ability to metabolize sugar appropriately. Available from https://www.washingtonpost.com/business/2020/03/10/common-artificial-sweetener-might-be-making-you-fatter-sicker-new-study-says. Accessed 12 April 2020
- 51.Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017;189:E929–E939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers PJ, Hogenkamp PS, de Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes 2016;40:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kourouniotis S, Keast RSJ, Riddell LJ, Lacy K, Thorpe MG, Cicerale S. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 2016;103:1–7 [DOI] [PubMed] [Google Scholar]
- 54.Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc 1998;98:1118–1126 [DOI] [PubMed] [Google Scholar]
- 55.Ebrahim Z, Villiers A, Ahmed T. Factors influencing adherence to dietary guidelines: a qualitative study on the experiences of patients with type 2 diabetes attending a clinic in Cape Town. Journal of Endocrinology. Metabolism and Diabetes of South Africa 2014;19:76–84 [Google Scholar]
- 56.Neumark-Sztainer D, Story M, Perry C, Casey MA. Factors influencing food choices of adolescents: findings from focus-group discussions with adolescents. J Am Diet Assoc 1999;99:929–937 [DOI] [PubMed] [Google Scholar]
- 57.Jaworski M, Panczyk M, Cedro M, Kucharska A. Adherence to diet recommendations in diabetes mellitus: disease acceptance as a potential mediator. Patient Prefer Adherence 2018;12:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J, Chang J, Checklin HL, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 2010;104:803–806 [DOI] [PubMed] [Google Scholar]
- 59.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Educ 2020;46:350–369 [DOI] [PubMed] [Google Scholar]